An Effective Approach towards the Immobilization of PtSn Nanoparticles on Noncovalent Modified Multi-Walled Carbon Nanotubes for Ethanol Electrooxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
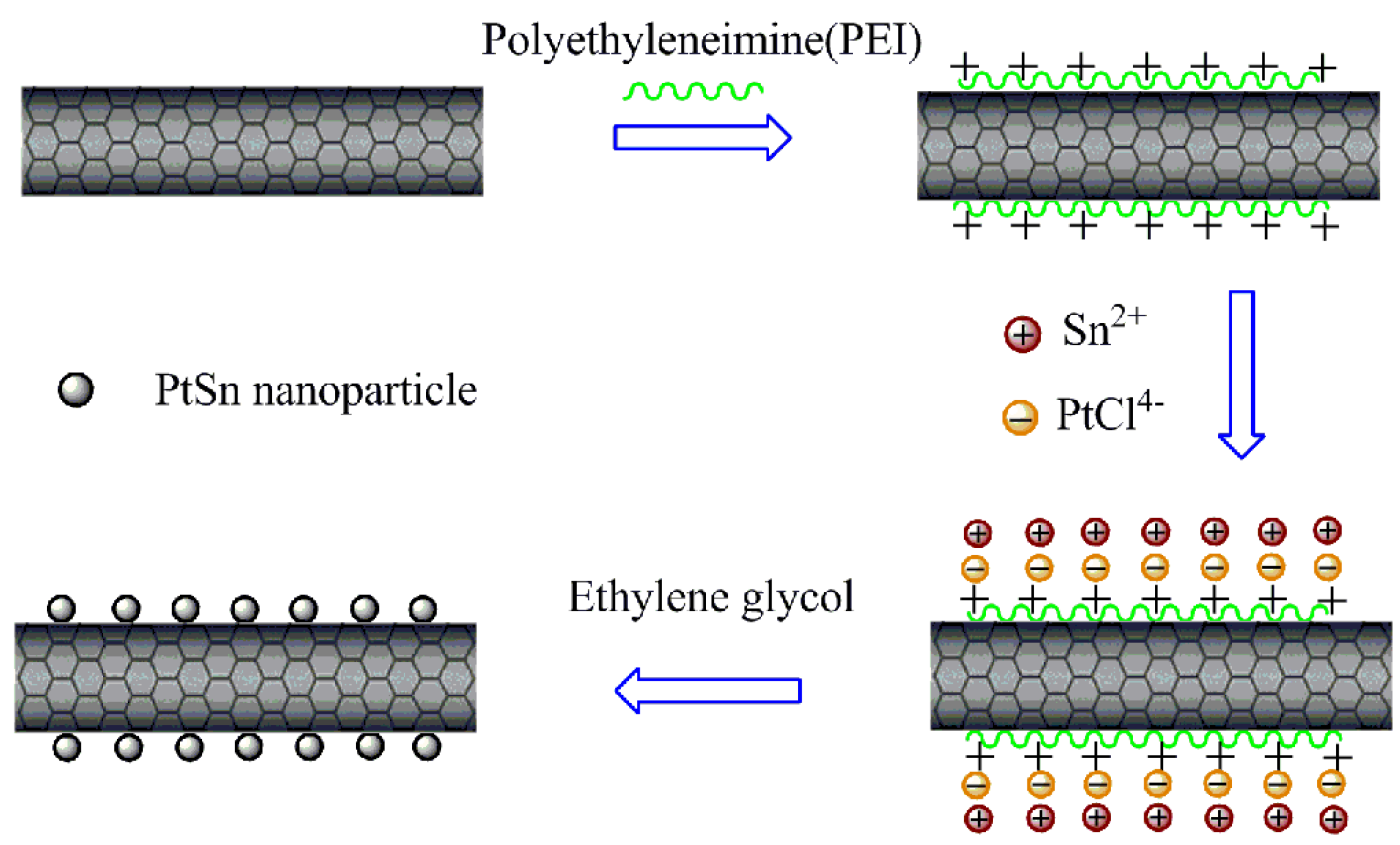
2.2. Functionalization of Multi-Walled Carbon Nanotubes s with Polyethyleneimine
2.3. Synthesis of PEI-MWCNTs Supported Pt and PtSn Electrocatalysts
2.4. Physicochemical Characterization
2.5. Electrochemical Analysis
3. Results and Discussion
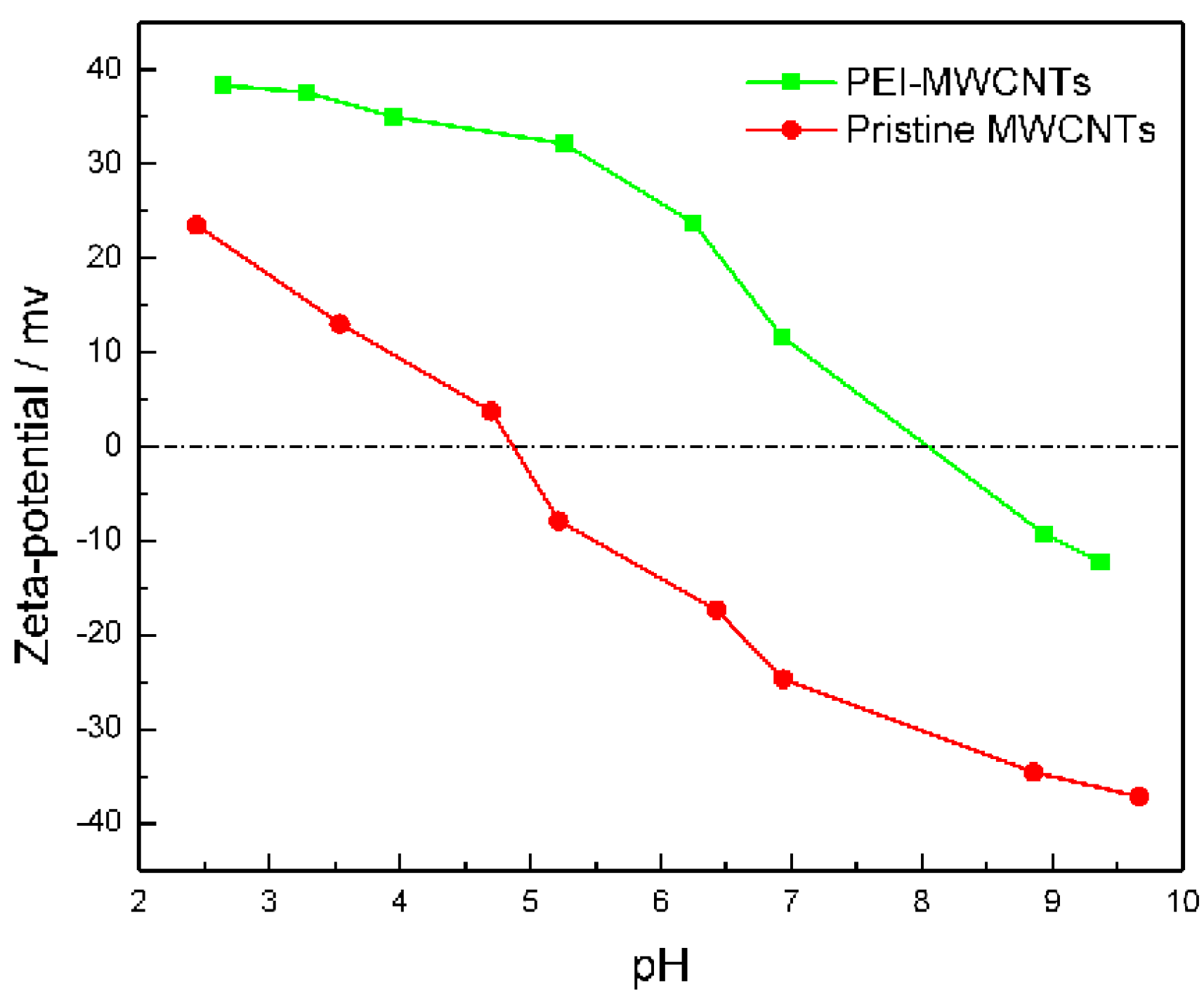
3.1. Zeta Potential Measurement of PEI-MWCNTs Nanocomposite
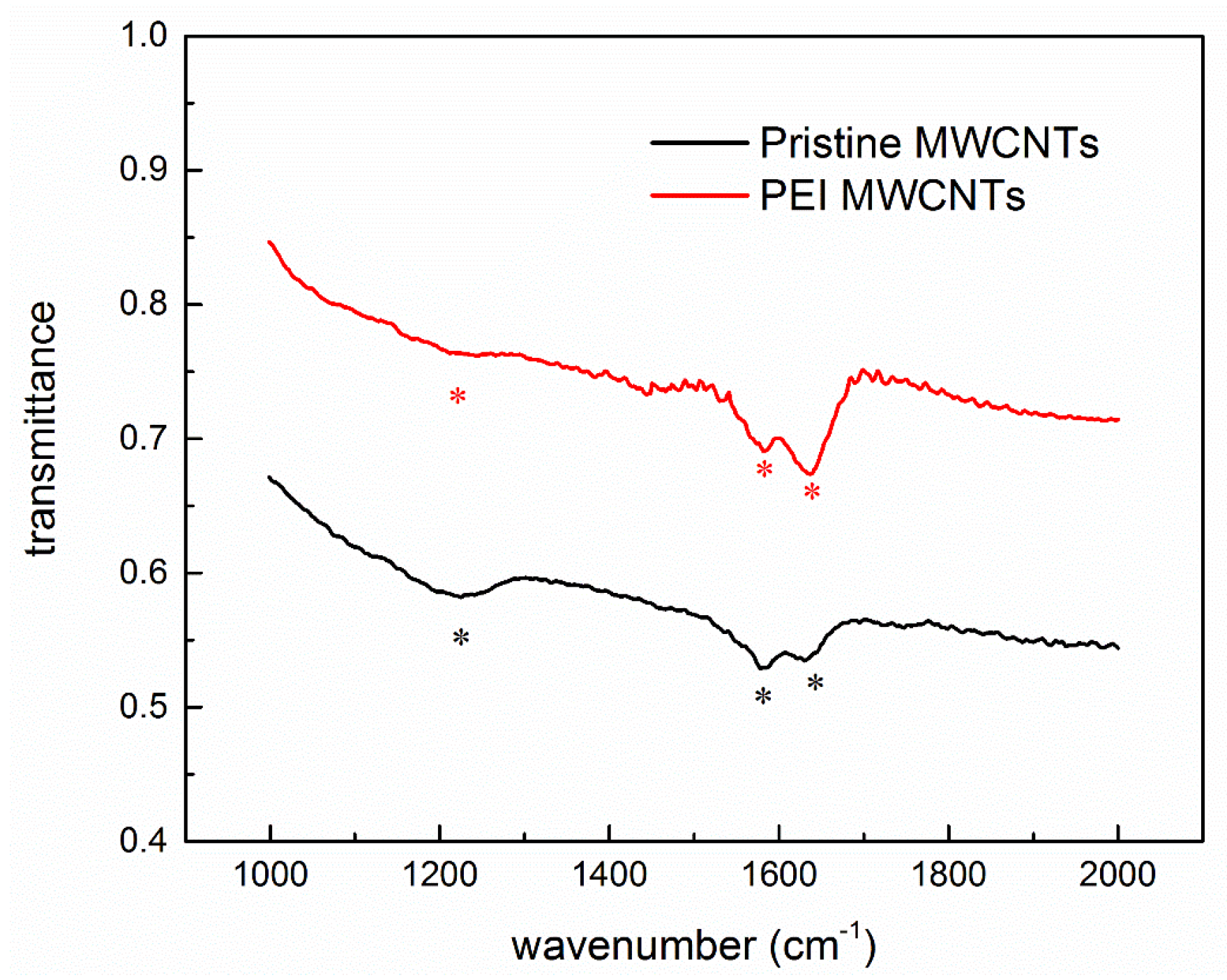
3.2. Fourier Transform Infrared Spectroscopy Spectra of Polyethyleneimine-Functionalized Multi-Walled Carbon Nanotubes
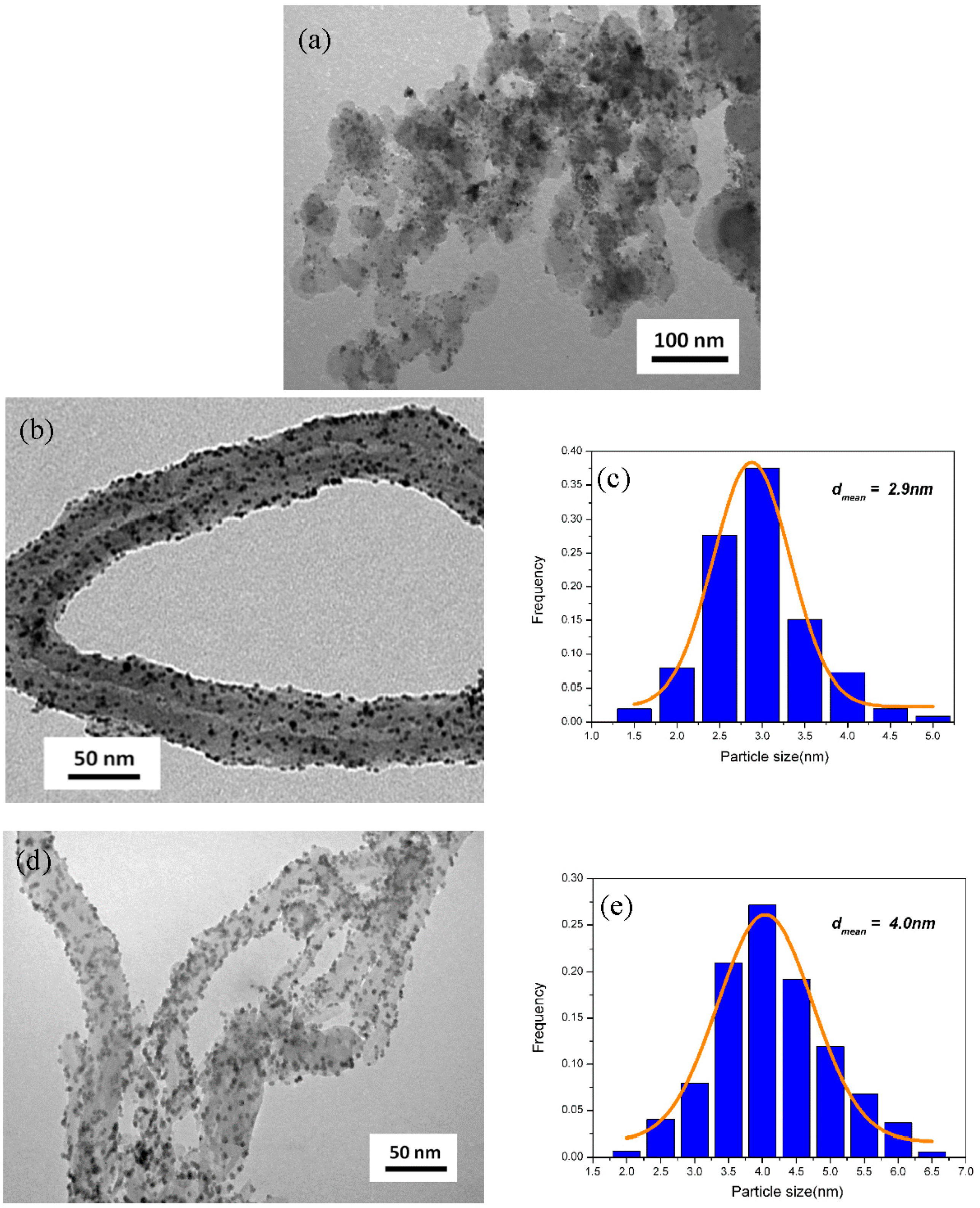
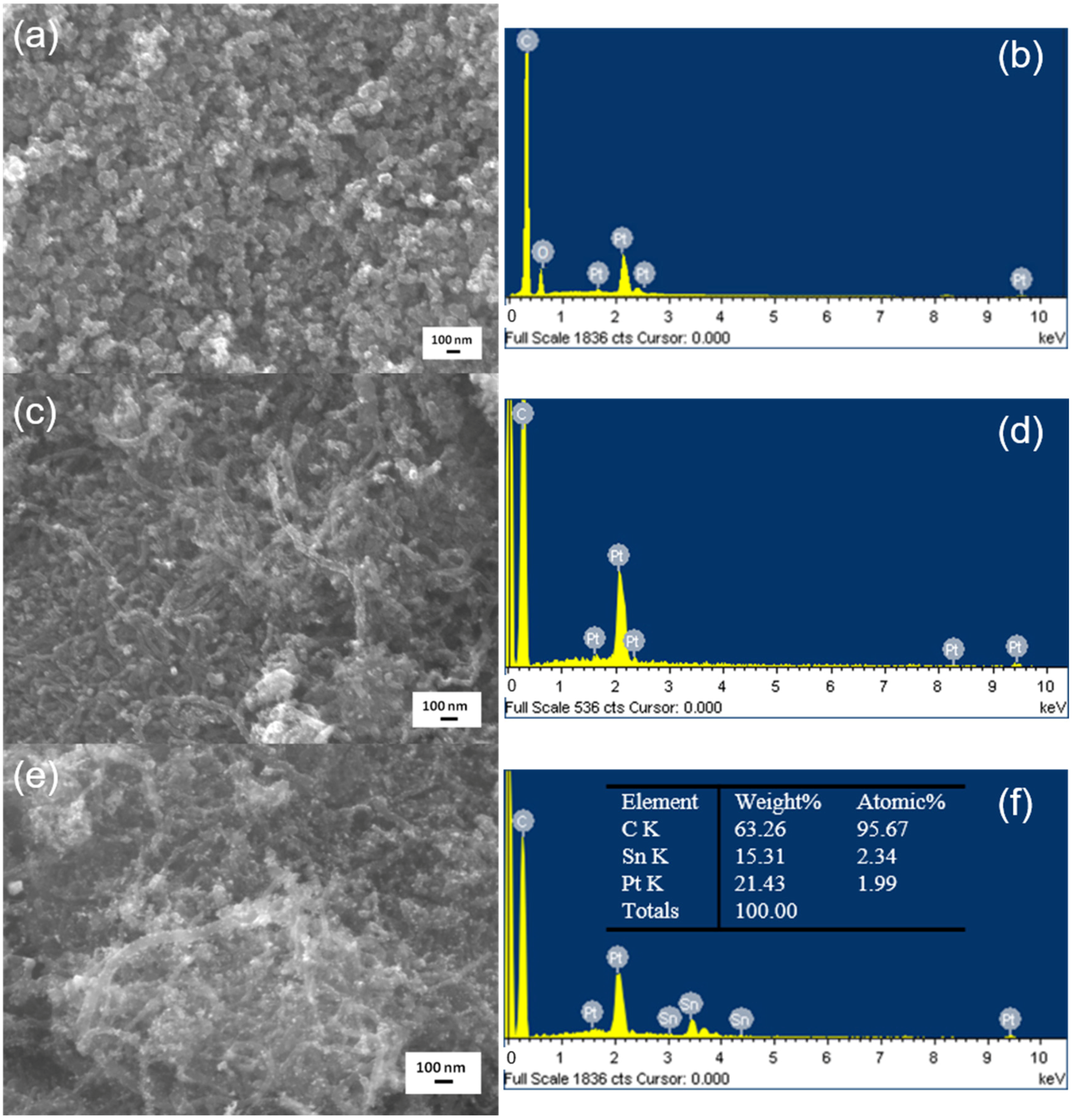
3.3. Morphology and Composition of the As-Prepared Catalysts
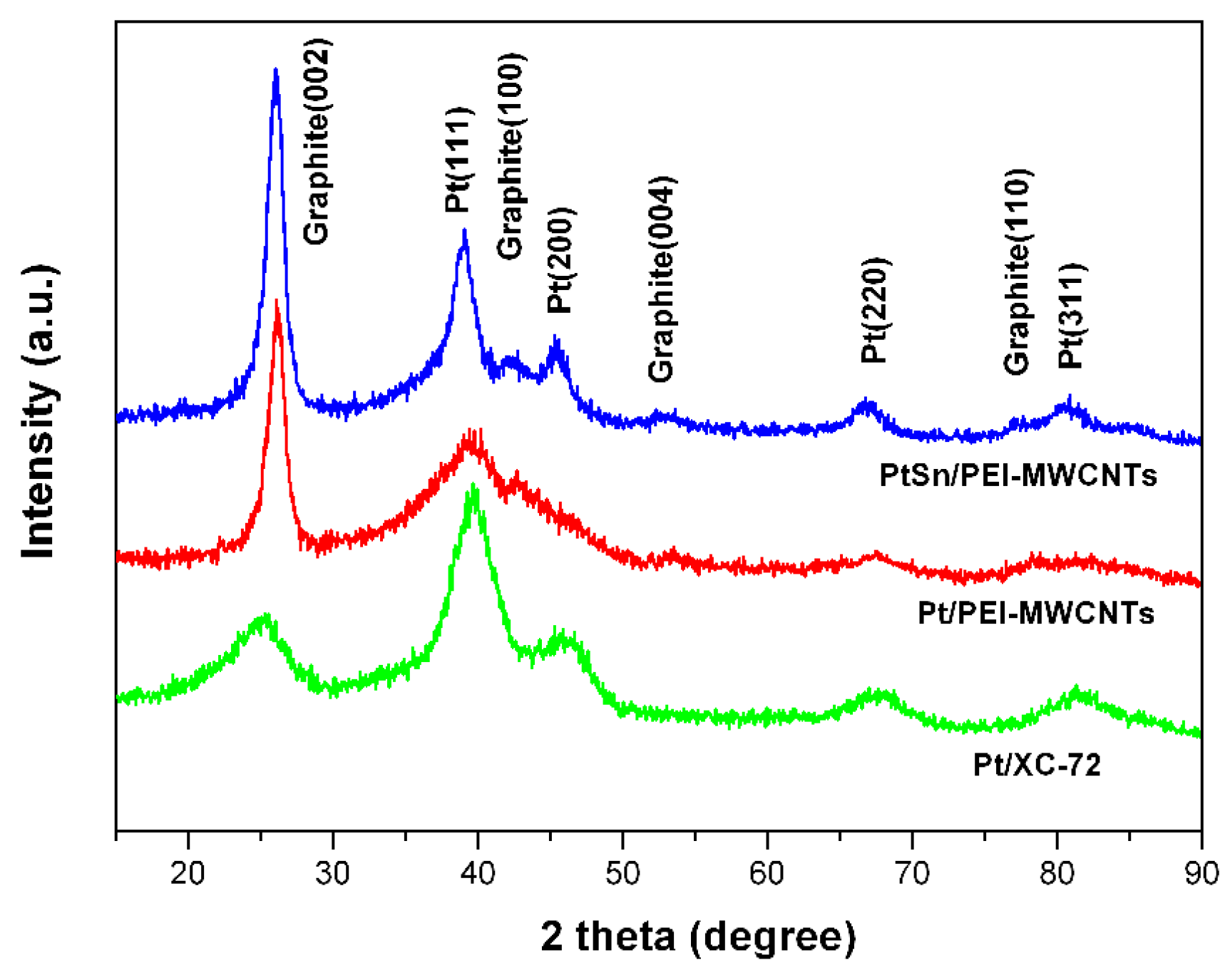
3.4. X-ray Diffraction Analysis
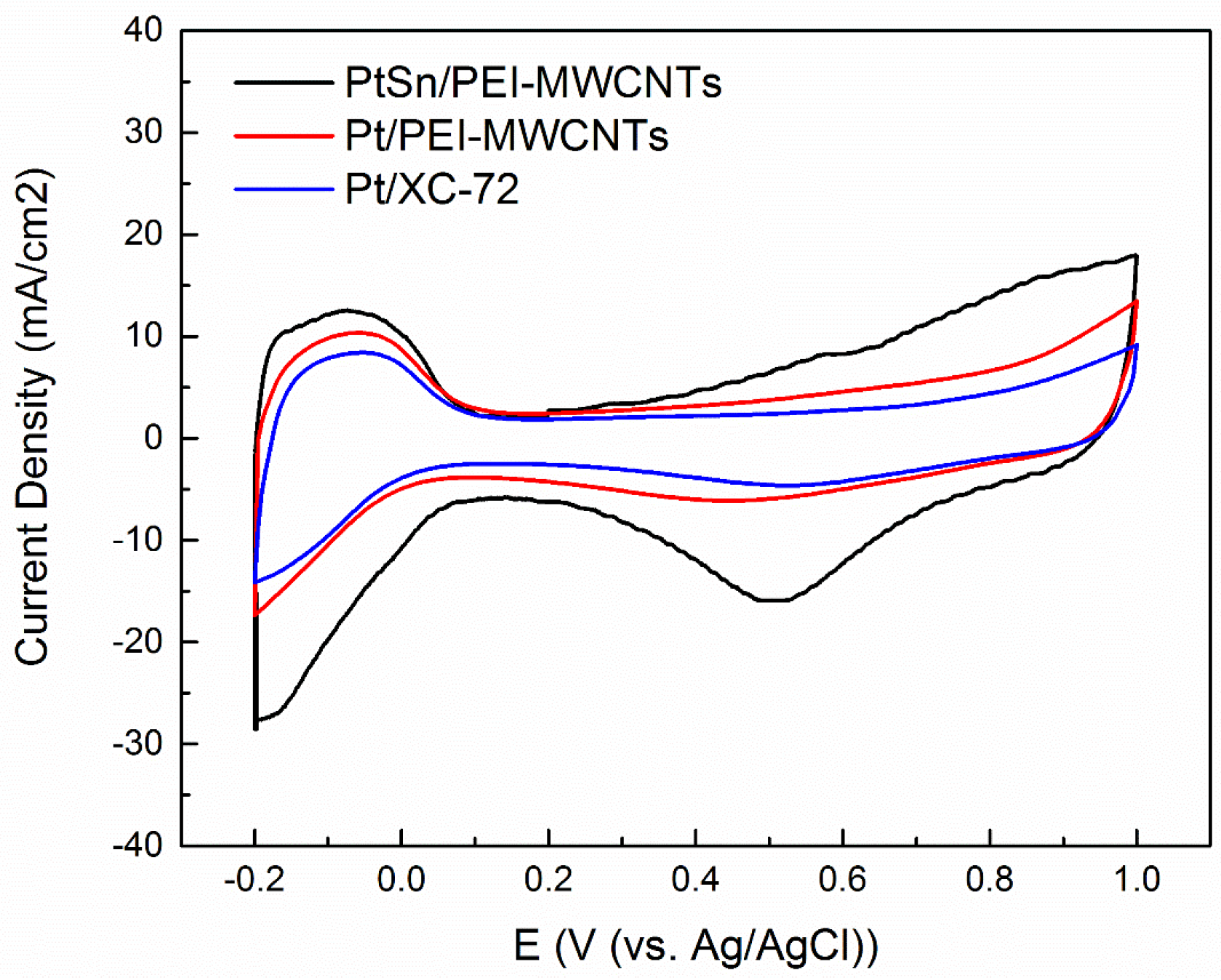
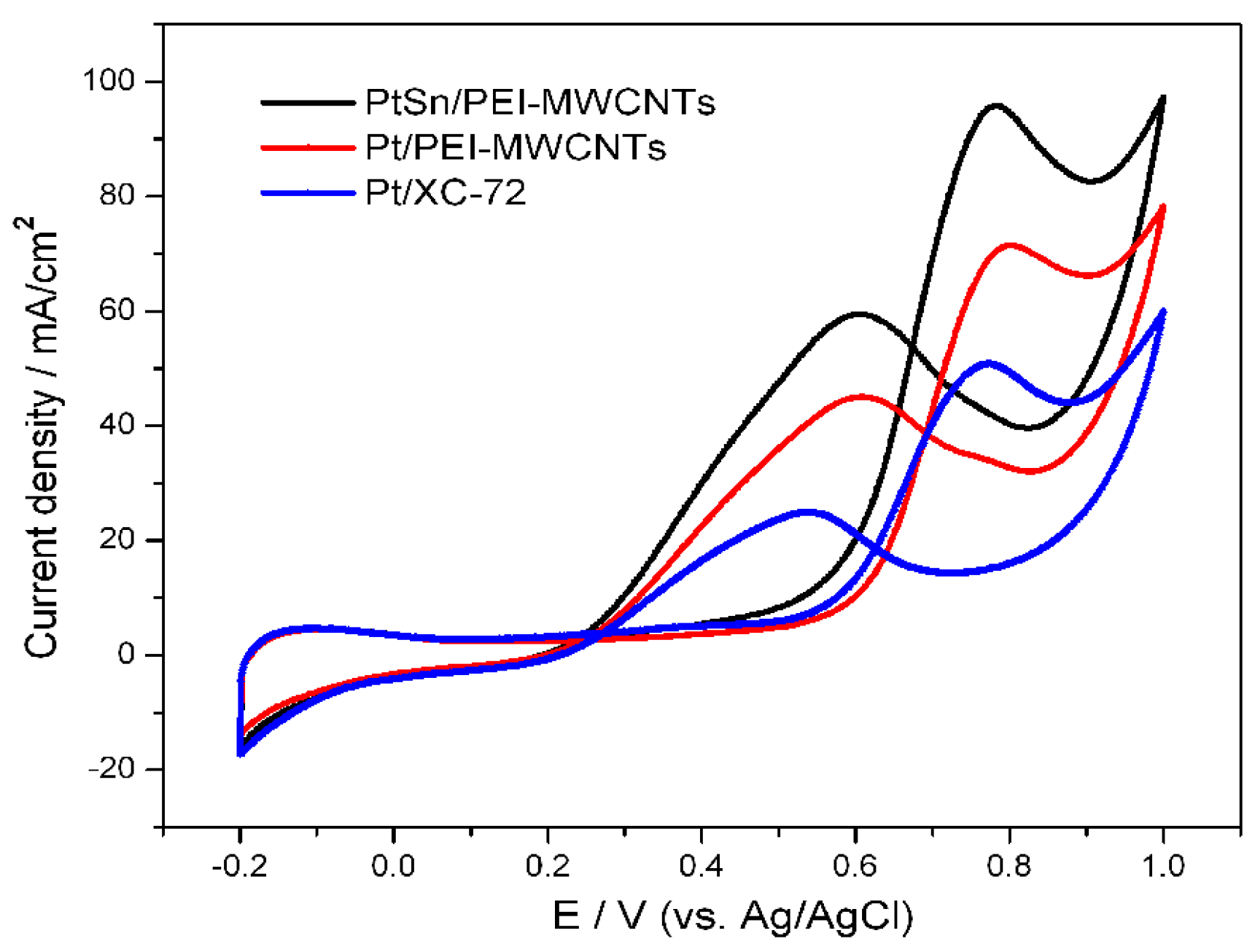
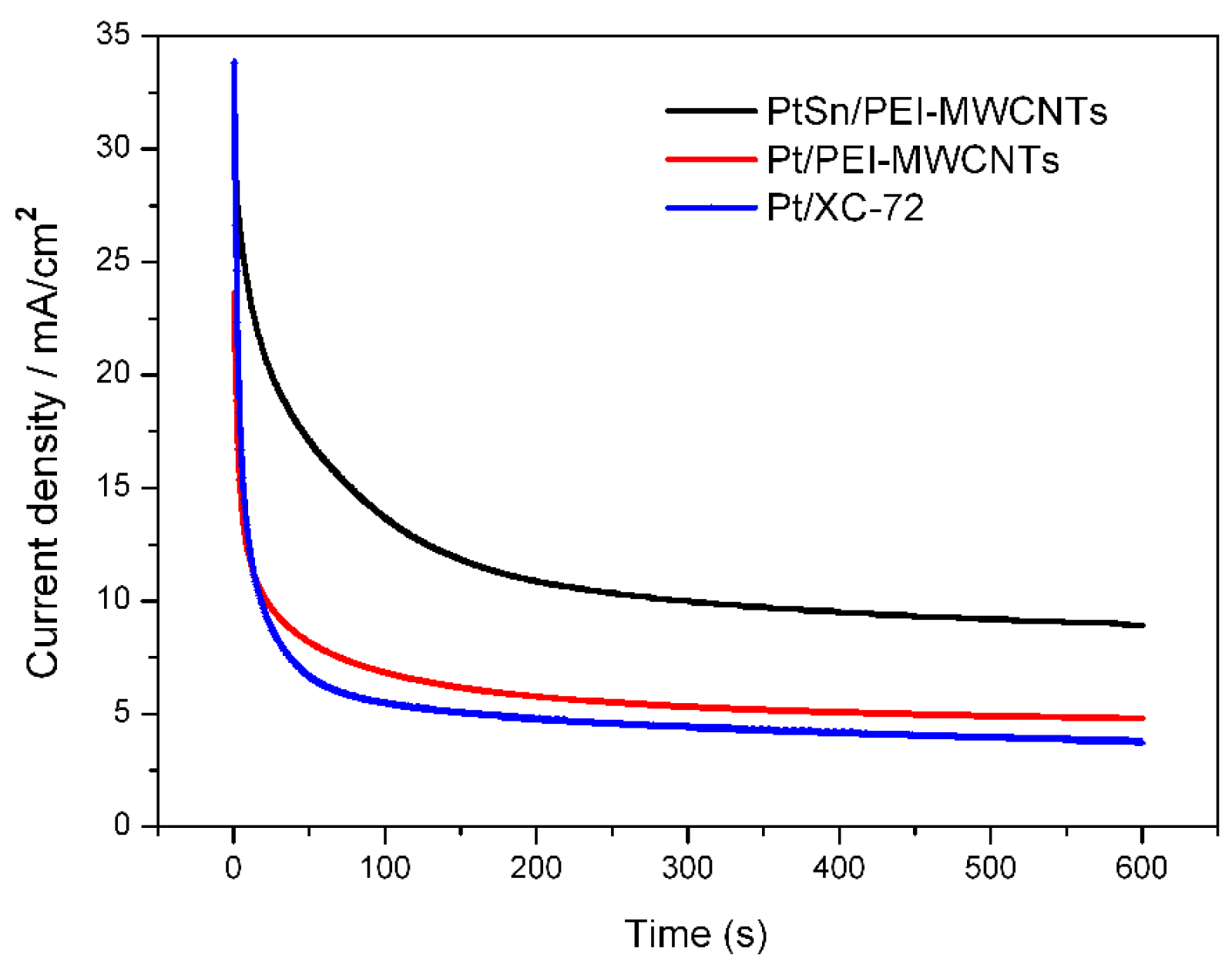
3.5. Electrochemical Activity of the Catalysts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, L.; Sun, G.; Li, H.; Xin, Q. Carbon-supported irsn catalysts for direct ethanol fuel cell. Fuel Cells Bull. 2007, 2007, 12–16. [Google Scholar] [CrossRef]
- Godoi, D.R.M.; Perez, J.; Villullas, H.M. Alloys and oxides on carbon-supported Pt–Sn electrocatalysts for ethanol oxidation. J. Power Sources 2010, 195, 3394–3401. [Google Scholar] [CrossRef]
- Song, H.; Qiu, X.; Li, F. Promotion of carbon nanotube-supported Pt catalyst for methanol and ethanol electro-oxidation by ZrO2 in acidic media. Appl. Catal. A Gen. 2009, 364, 1–7. [Google Scholar] [CrossRef]
- Kamarudin, M.; Kamarudin, S.; Masdar, M.; Daud, W. Review: Direct ethanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Badwal, S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-based electrocatalysts for alcohol oxidation in half cells and in direct alcohol fuel cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Jafri, R.I.; Ramaprabhu, S. Multi walled carbon nanotubes based micro direct ethanol fuel cell using printed circuit board technology. Int. J. Hydrog. Energy 2010, 35, 1339–1346. [Google Scholar] [CrossRef]
- Guo, D.-J.; Qiu, X.-P.; Chen, L.-Q.; Zhu, W.-T. Multi-walled carbon nanotubes modified by sulfated Tio2—A promising support for Pt catalyst in a direct ethanol fuel cell. Carbon 2009, 47, 1680–1685. [Google Scholar] [CrossRef]
- Gao, G.; Yang, G.; Xu, M.; Wang, C.; Xu, C.; Li, H. Simple synthesis of Pt nanoparticles on noncovalent functional mwnt surfaces: Application in ethanol electrocatalysis. J. Power Sources 2007, 173, 178–182. [Google Scholar] [CrossRef]
- Pang, H.L.; Lu, J.P.; Chen, J.H.; Huang, C.T.; Liu, B.; Zhang, X.H. Preparation of SnO2-CNTs supported Pt catalysts and their electrocatalytic properties for ethanol oxidation. Electrochim. Acta 2009, 54, 2610–2615. [Google Scholar] [CrossRef]
- Guha, A.; Lu, W.; Zawodzinski, T.A.; Schiraldi, D.A. Surface-modified carbons as platinum catalyst support for PEM fuel cells. Carbon 2007, 45, 1506–1517. [Google Scholar] [CrossRef]
- Du, H.; Li, B.; Kang, F.; Fu, R.; Zeng, Y. Carbon aerogel supported Pt–Ru catalysts for using as the anode of direct methanol fuel cells. Carbon 2007, 45, 429–435. [Google Scholar] [CrossRef]
- Choi, W.C.; Woo, S.I.; Jeon, M.K.; Sohn, J.M.; Kim, M.R.; Jeon, H.J. Platinum nanoclusters studded in the microporous nanowalls of ordered mesoporous carbon. Adv. Mater. 2005, 17, 446–451. [Google Scholar] [CrossRef]
- Muneendra, P.A.; Santhosh, C.; Grace, A.N. Carbon nanotubes and polyaniline supported Pt nanoparticles for methanol oxidation towards dmfc applications. Appl. Nanosci. 2012, 2, 457–466. [Google Scholar] [CrossRef]
- Wang, L.; Imura, M.; Yamauchi, Y. Tailored design of architecturally controlled Pt nanoparticles with huge surface areas toward superior unsupported Pt electrocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-B.-R.; Baeck, S.H.; Jaramillo, T.F.; Bent, S.F. Growth of Pt nanowires by atomic layer deposition on highly ordered pyrolytic graphite. Nano Lett. 2013, 13, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kylián, O.; Valeš, V.; Polonskyi, O.; Pešička, J.; Čechvala, J.; Solař, P.; Choukourov, A.; Slavínská, D.; Biederman, H. Deposition of Pt nanoclusters by means of gas aggregation cluster source. Mater. Lett. 2012, 79, 229–231. [Google Scholar] [CrossRef]
- Li, X.L.; Liu, Y.Q.; Fu, L.; Cao, L.C.; Wei, D.C.; Wang, Y. Efficient synthesis of carbon nanotube–nanoparticle hybrids. Adv. Funct. Mater. 2006, 16, 2431–2437. [Google Scholar] [CrossRef]
- Du, N.; Zhang, H.; Wu, P.; Yu, J.; Yang, D. A general approach for uniform coating of a metal layer on MWCNTs via layer-by-layer assembly. J. Phys. Chem. C 2009, 113, 17387–17391. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, S.P.; Wang, X. Polyelectrolyte functionalized carbon nanotubes as a support for noble metal electrocatalysts and their activity for methanol oxidation. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, W.; Zhu, H.; Wang, X.; Yang, F.; Zhang, B.; Yang, X. In situ pei and formic acid directed formation of Pt nps/mwnts hybrid material with excellent electrocatalytic activity. Talanta 2009, 79, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, T.; Wang, L.; Guo, S.; Dong, S. A general route to prepare one- and three-dimensional carbon nanotube/metal nanoparticle composite nanostructures. Langmuir 2007, 23, 6352–6357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jiang, S.P. Highly effective and co-tolerant PtRu electrocatalysts supported on poly (ethyleneimine) functionalized carbon nanotubes for direct methanol fuel cells. Electrochim. Acta 2013, 99, 124–132. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Chirea, M.; Altantzis, T.; Pastoriza-Santos, I.; Perez-Juste, J.; Silva, F.; Bals, S.; Liz-Marzan, L.M. Dimethylformamide-mediated synthesis of water-soluble platinum nanodendrites for ethanol oxidation electrocatalysis. Nanoscale 2013, 5, 4776–4784. [Google Scholar] [CrossRef] [PubMed]
- Lamy, C.; Belgsir, E.; Leger, J. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (dafc). J. Appl. Electrochem. 2001, 31, 799–809. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Tsiakaras, P. Low and non-platinum electrocatalysts for pemfcs: Current status, challenges and prospects. Appl. Catal. B Environ. 2012, 127, 371–388. [Google Scholar] [CrossRef]
- Purgato, F.L.S.; Olivi, P.; Léger, J.M.; De Andrade, A.R.; Tremiliosi-Filho, G.; Gonzalez, E.R.; Lamy, C.; Kokoh, K.B. Activity of platinum–tin catalysts prepared by the pechini–adams method for the electrooxidation of ethanol. J. Electroanal. Chem. 2009, 628, 81–89. [Google Scholar] [CrossRef]
- Hitmi, H.; Belgsir, E.M.; Léger, J.M.; Lamy, C.; Lezna, R.O. A kinetic analysis of the electro-oxidation of ethanol at a platinum electrode in acid medium. Electrochim. Acta 1994, 39, 407–415. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Q.; Chen, Y.; Yan, Y.; Zhou, C.; Guo, Z.; Jia, X.; Yang, Y. Microwave-assisted synthesis of PtRu/CNT and PtSn/CNT catalysts and their applications in the aerobic oxidation of benzyl alcohol in base-free aqueous solutions. Catal. Sci. Technol. 2013, 3, 328–338. [Google Scholar] [CrossRef]
- Purgato, F.L.S.; Pronier, S.; Olivi, P.; De Andrade, A.R.; Léger, J.M.; Tremiliosi-Filho, G.; Kokoh, K.B. Direct ethanol fuel cell: Electrochemical performance at 90 °C on Pt and PtSn/C electrocatalysts. J. Power Sources 2012, 198, 95–99. [Google Scholar] [CrossRef]
- Beyhan, S.; Coutanceau, C.; Léger, J.-M.; Napporn, T.W.; Kadırgan, F. Promising anode candidates for direct ethanol fuel cell: Carbon supported PtSn-based trimetallic catalysts prepared by bönnemann method. Int. J. Hydrog. Energy 2013, 38, 6830–6841. [Google Scholar] [CrossRef]
- Zhou, W.J.; Song, S.Q.; Li, W.Z.; Zhou, Z.H.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Tsiakaras, P. Direct ethanol fuel cells based on PtSn anodes: The effect of Sn content on the fuel cell performance. J. Power Sources 2005, 140, 50–58. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Z.; Song, S.; Li, W.; Sun, G.; Tsiakaras, P.; Xin, Q. Pt based anode catalysts for direct ethanol fuel cells. Appl. Catal. B Environ. 2003, 46, 273–285. [Google Scholar] [CrossRef]
- Spinacé, E.V.; Linardi, M.; Neto, A.O. Co-catalytic effect of nickel in the electro-oxidation of ethanol on binary Pt–Sn electrocatalysts. Electrochem. Commun. 2005, 7, 365–369. [Google Scholar] [CrossRef]
- Tsiakaras, P. Ptm/c (M = Sn, Ru, Pd, W) based anode direct ethanol–pemfcs: Structural characteristics and cell performance. J. Power Sources 2007, 171, 107–112. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ragupathy, D.; Gopalan, A.; Lee, K.-P. Development of novel catalysts by γ-irradiation-induced distribution of Pt-Sn nanoparticles onto multiwalled carbon nanotubes. J. Nanoelectron. Optoelectron. 2012, 7, 444–448. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Modified carbon nanotubes: An effective way to selective attachment of gold nanoparticles. Carbon 2003, 41, 2923–2929. [Google Scholar] [CrossRef]
- Dai, J.; Bruening, M.L. Catalytic nanoparticles formed by reduction of metal ions in multilayered polyelectrolyte films. Nano Lett. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Wu, H.-X.; Cao, W.-M.; Li, Y.; Liu, G.; Wen, Y.; Yang, H.-F.; Yang, S.-P. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta 2010, 55, 3734–3740. [Google Scholar] [CrossRef]
- Zou, G.; Yang, H.; Jain, M.; Zhou, H.; Williams, D.; Zhou, M.; McCleskey, T.; Burrell, A.; Jia, Q. Vertical connection of carbon nanotubes to silicon at room temperature using a chemical route. Carbon 2009, 47, 933–937. [Google Scholar] [CrossRef]
- Jia, N.; Lian, Q.; Shen, H.; Wang, C.; Li, X.; Yang, Z. Intracellular delivery of quantum dots tagged antisense oligodeoxynucleotides by functionalized multiwalled carbon nanotubes. Nano Lett. 2007, 7, 2976–2980. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhu, H.; Thrasher, J.S.; Street, S.C. Synthesis and electrocatalytic activity of photoreduced platinum nanoparticles in a poly(ethylenimine) matrix. ACS Appl. Mater. Interfaces 2009, 1, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Du, Y.; Wang, E. Polyethyleneimine-functionalized platinum nanoparticles with high electrochemiluminescence activity and their applications to amplified analysis of biomolecules. Chem. Asian J. 2008, 3, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Pickup, P.G. Decoration of carbon-supported Pt catalysts with Sn to promote electro-oxidation of ethanol. J. Power Sources 2007, 173, 121–129. [Google Scholar] [CrossRef]
- Zhou, W.J.; Song, S.Q.; Li, W.Z.; Sun, G.Q.; Xin, Q.; Kontou, S.; Poulianitis, K.; Tsiakaras, P. Pt-based anode catalysts for direct ethanol fuel cells. Solid State Ion. 2004, 175, 797–803. [Google Scholar] [CrossRef]
- Lee, E.; Park, I.-S.; Manthiram, A. Synthesis and characterization of Pt−Sn−Pd/c catalysts for ethanol electro-oxidation reaction. J. Phys. Chem. C 2010, 114, 10634–10640. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-B.; Yin, G.-P.; Zhang, J.; Sun, Y.-C.; Shi, P.-F. Investigation of ethanol electrooxidation on a Pt–Ru–Ni/c catalyst for a direct ethanol fuel cell. J. Power Sources 2006, 160, 37–43. [Google Scholar] [CrossRef]
- Delime, F.; Leger, J.M.; Lamy, C. Enhancement of the electrooxidation of ethanol on a PT–PEM electrode modified by tin. Part I: Half cell study. J. Appl. Electrochem. 1999, 29, 1249–1254. [Google Scholar] [CrossRef]
- Vigier, F.; Coutanceau, C.; Hahn, F.; Belgsir, E.M.; Lamy, C. On the mechanism of ethanol electro-oxidation on Pt and PtSn catalysts: Electrochemical and in situ ir reflectance spectroscopy studies. J. Electroanal. Chem. 2004, 563, 81–89. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Cen, Y.; Sisson, R.D.; Liang, J. An Effective Approach towards the Immobilization of PtSn Nanoparticles on Noncovalent Modified Multi-Walled Carbon Nanotubes for Ethanol Electrooxidation. Energies 2016, 9, 165. https://doi.org/10.3390/en9030165
Geng X, Cen Y, Sisson RD, Liang J. An Effective Approach towards the Immobilization of PtSn Nanoparticles on Noncovalent Modified Multi-Walled Carbon Nanotubes for Ethanol Electrooxidation. Energies. 2016; 9(3):165. https://doi.org/10.3390/en9030165
Chicago/Turabian StyleGeng, Xi, Yinjie Cen, Richard D. Sisson, and Jianyu Liang. 2016. "An Effective Approach towards the Immobilization of PtSn Nanoparticles on Noncovalent Modified Multi-Walled Carbon Nanotubes for Ethanol Electrooxidation" Energies 9, no. 3: 165. https://doi.org/10.3390/en9030165





