Spray Formation of a Liquid Carbon Dioxide-Water Mixture at Elevated Pressures
Abstract
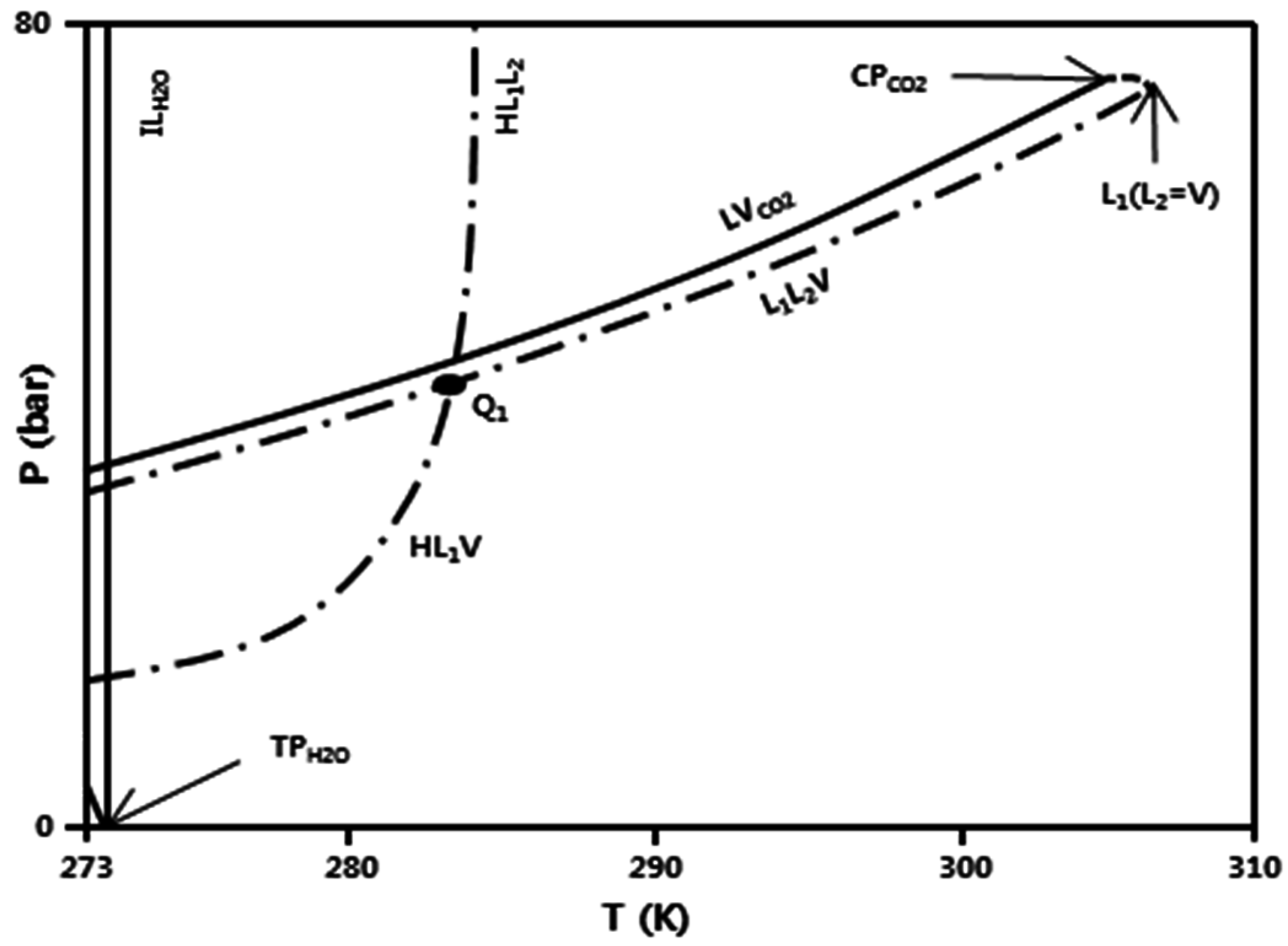
:1. Introduction
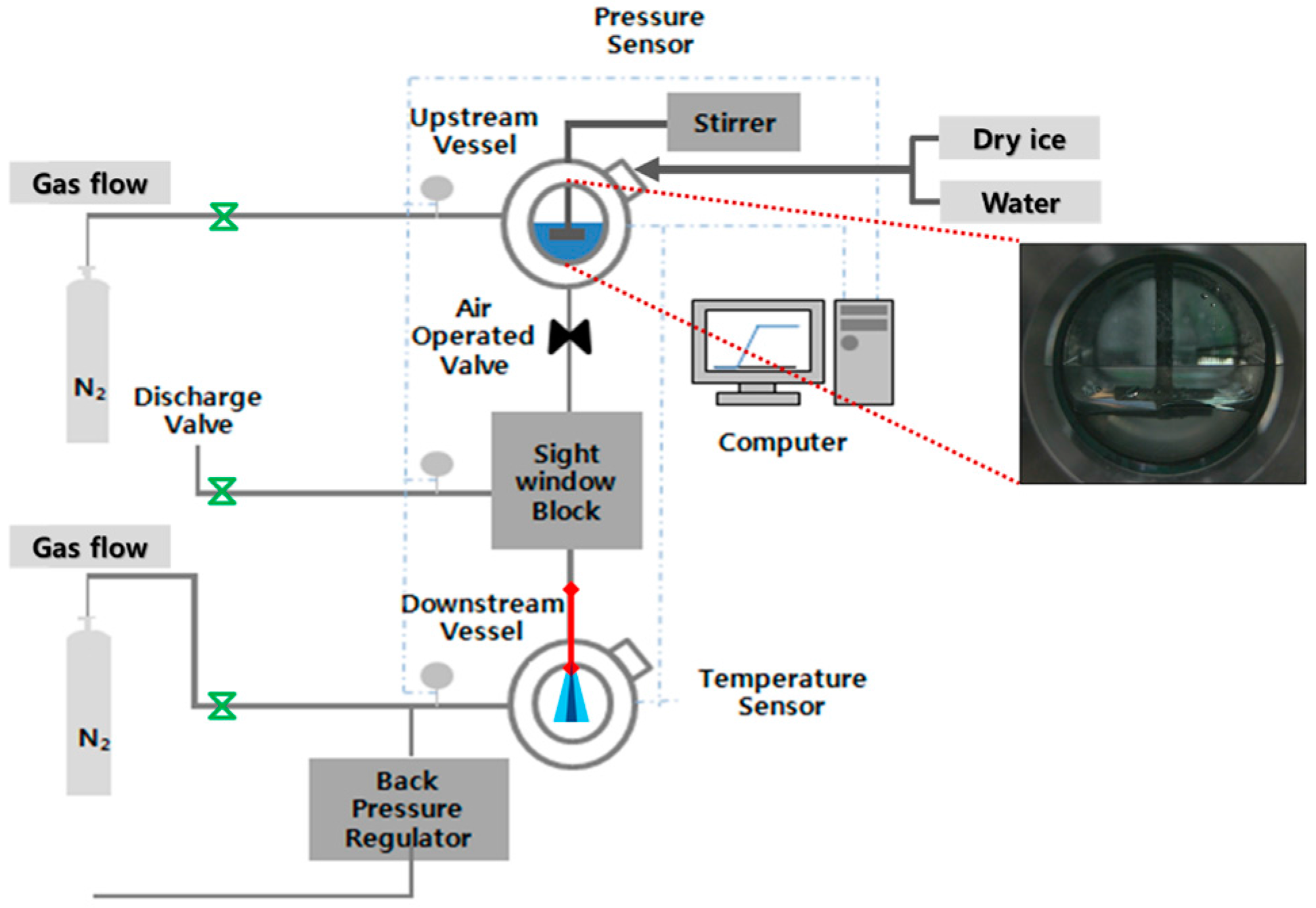
2. Experimental Setup
3. Results and Discussion
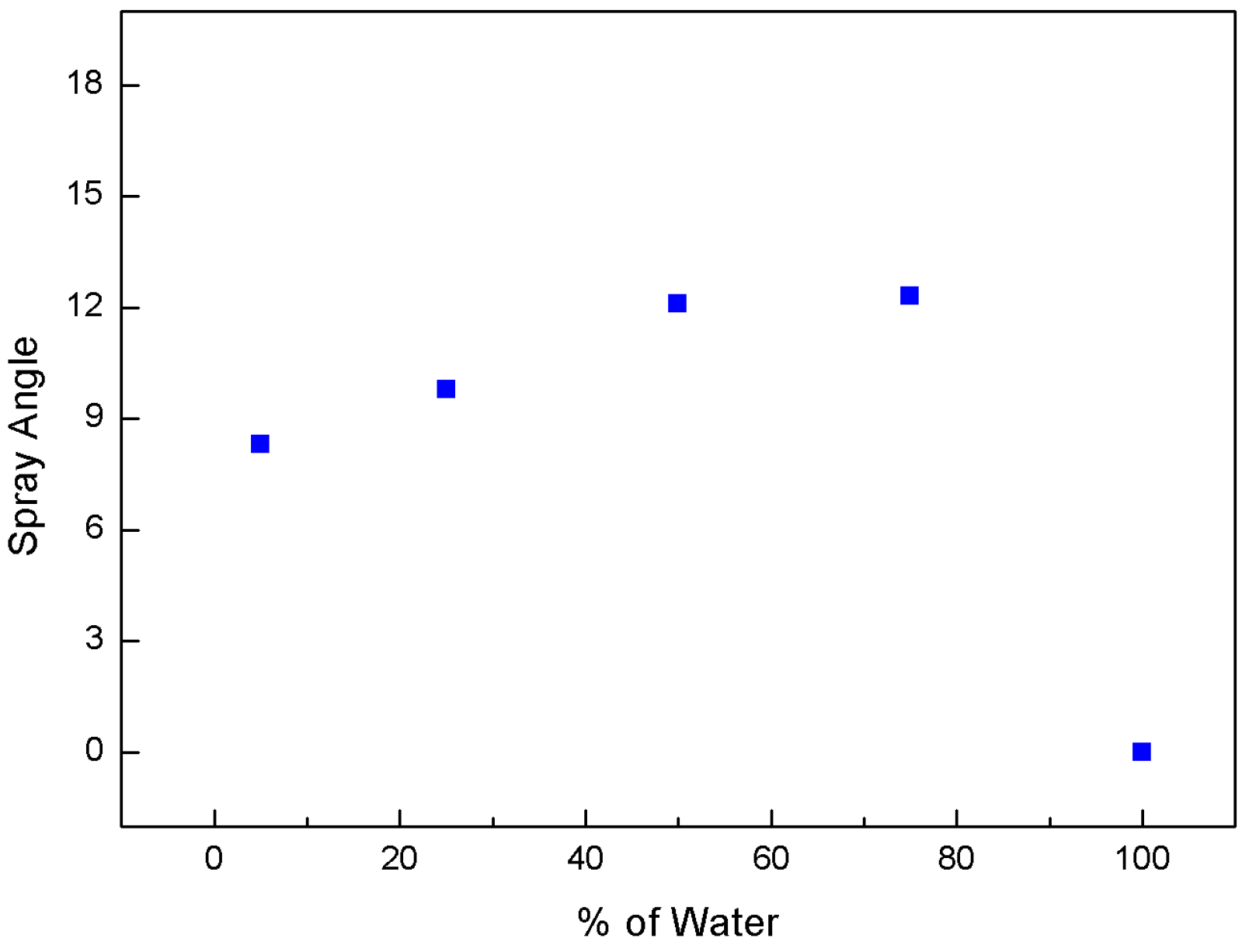
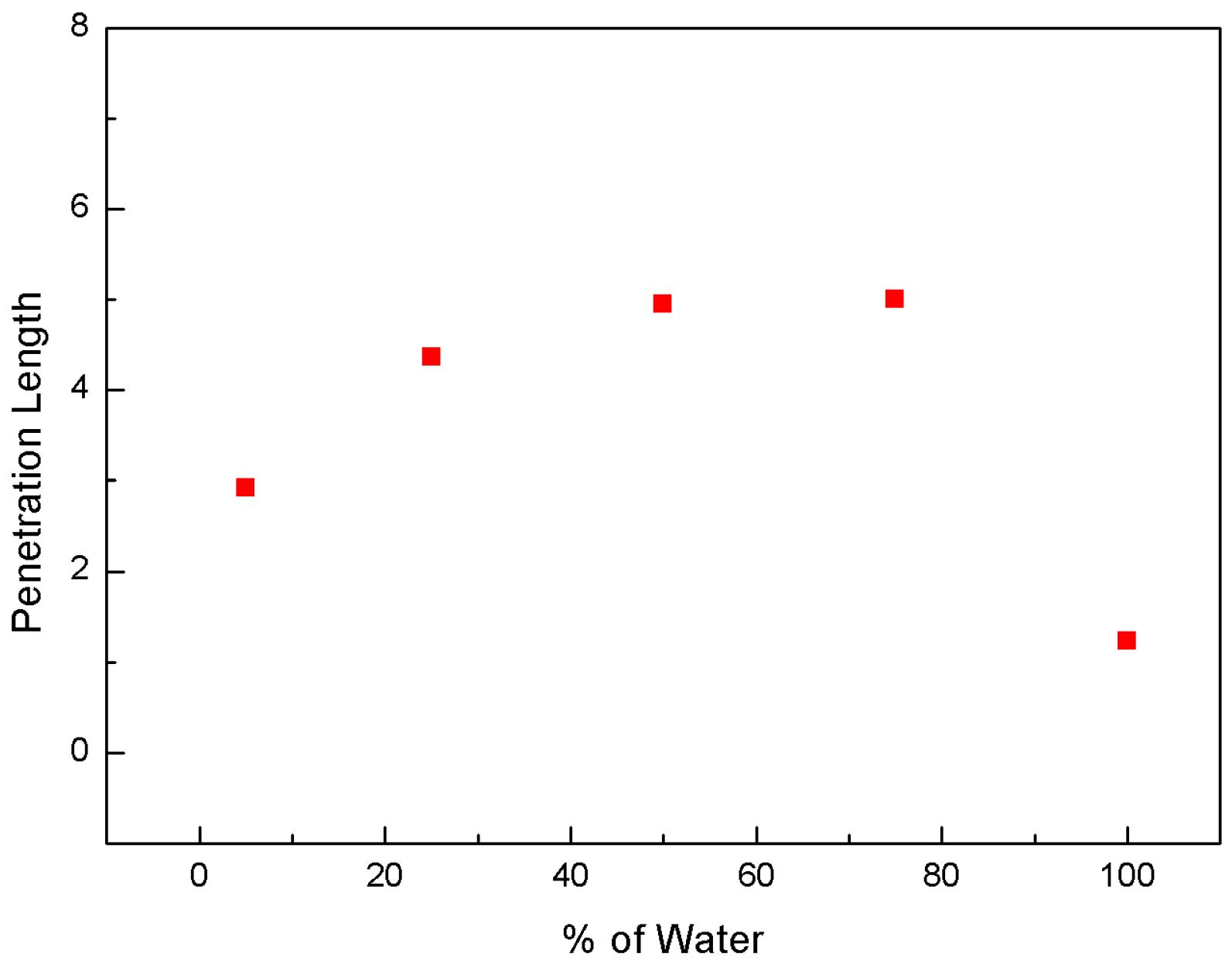
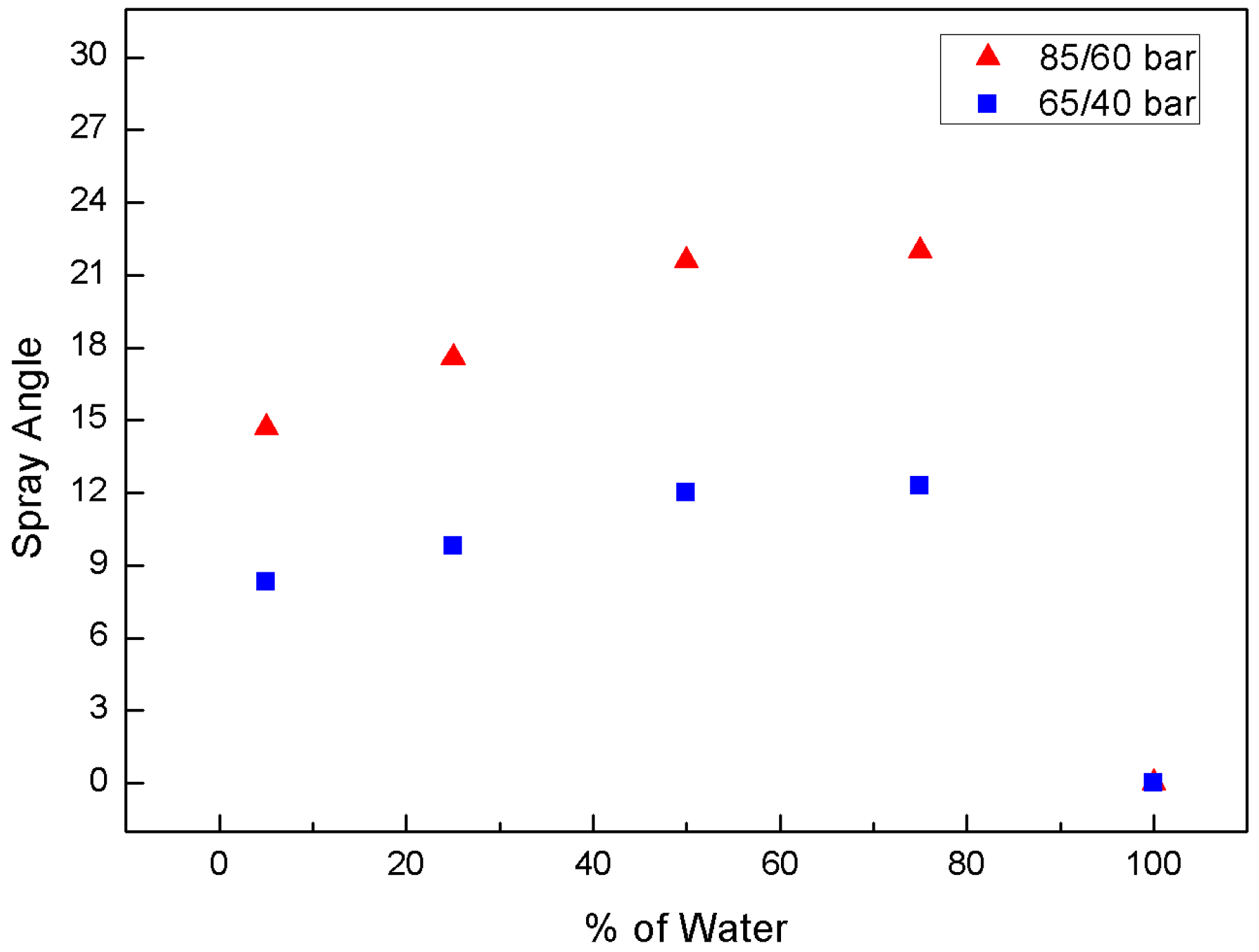
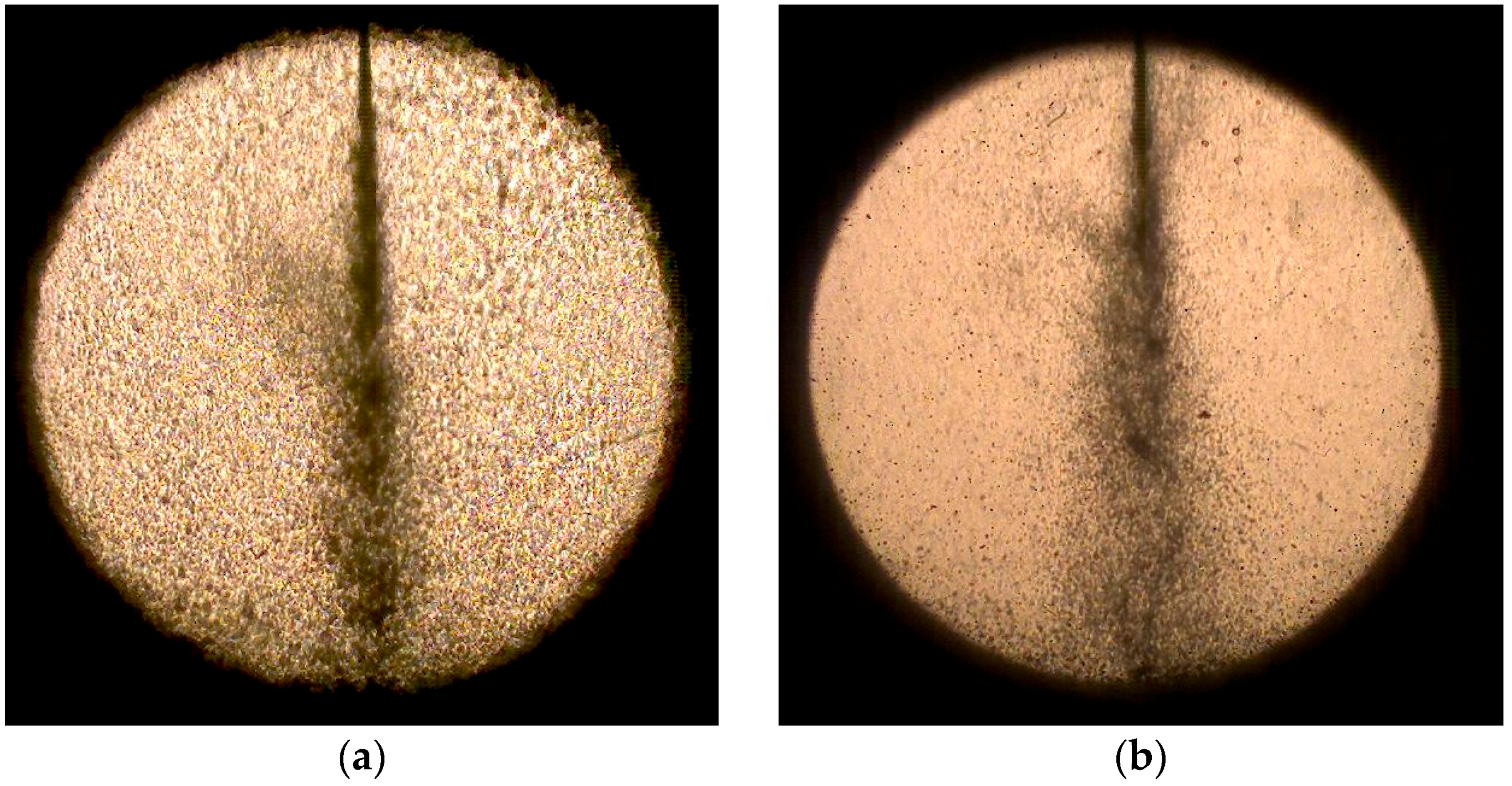
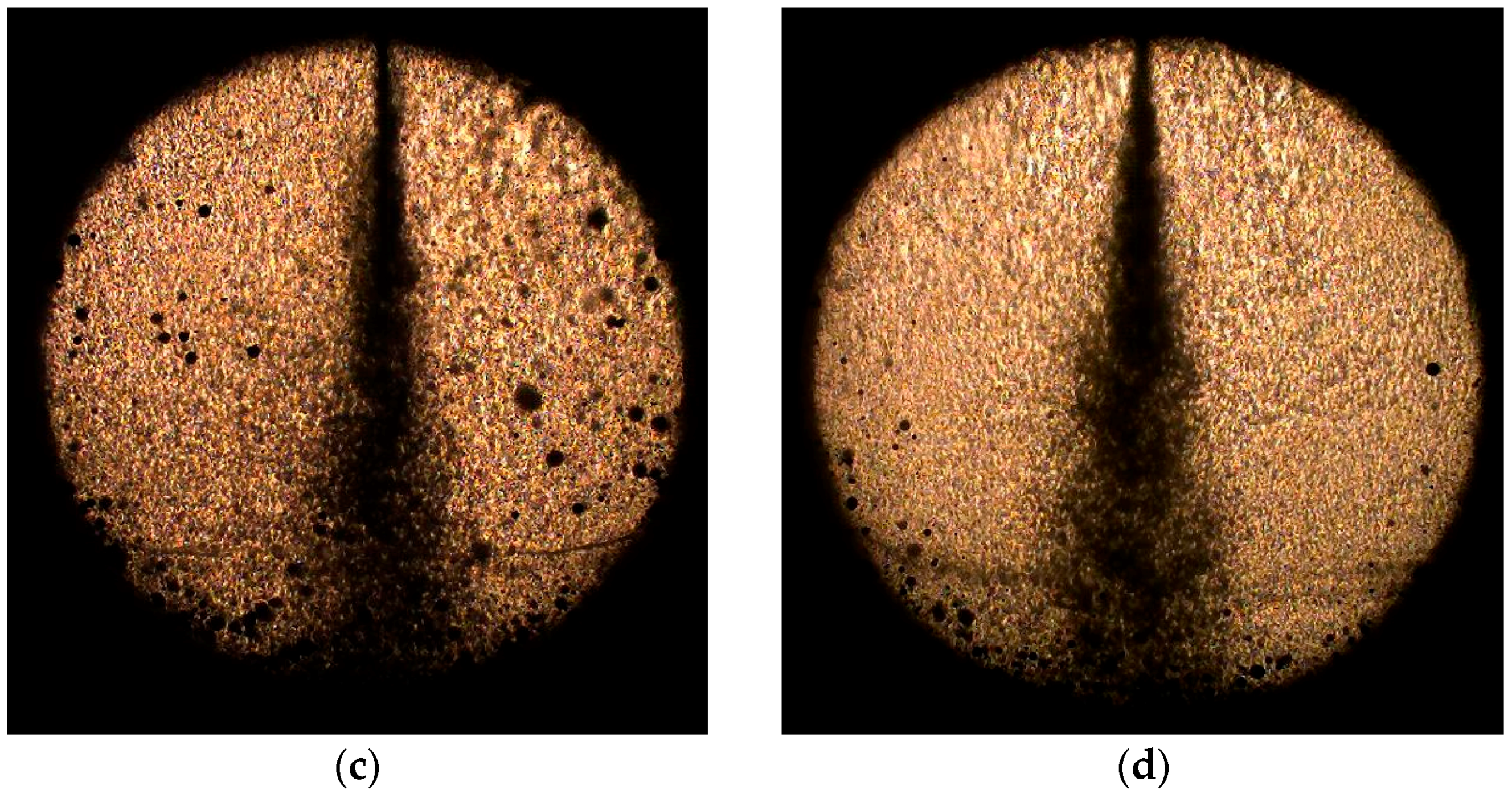
3.1. Effect of Water-Blending Ratio in Water-LCO2 Mixture
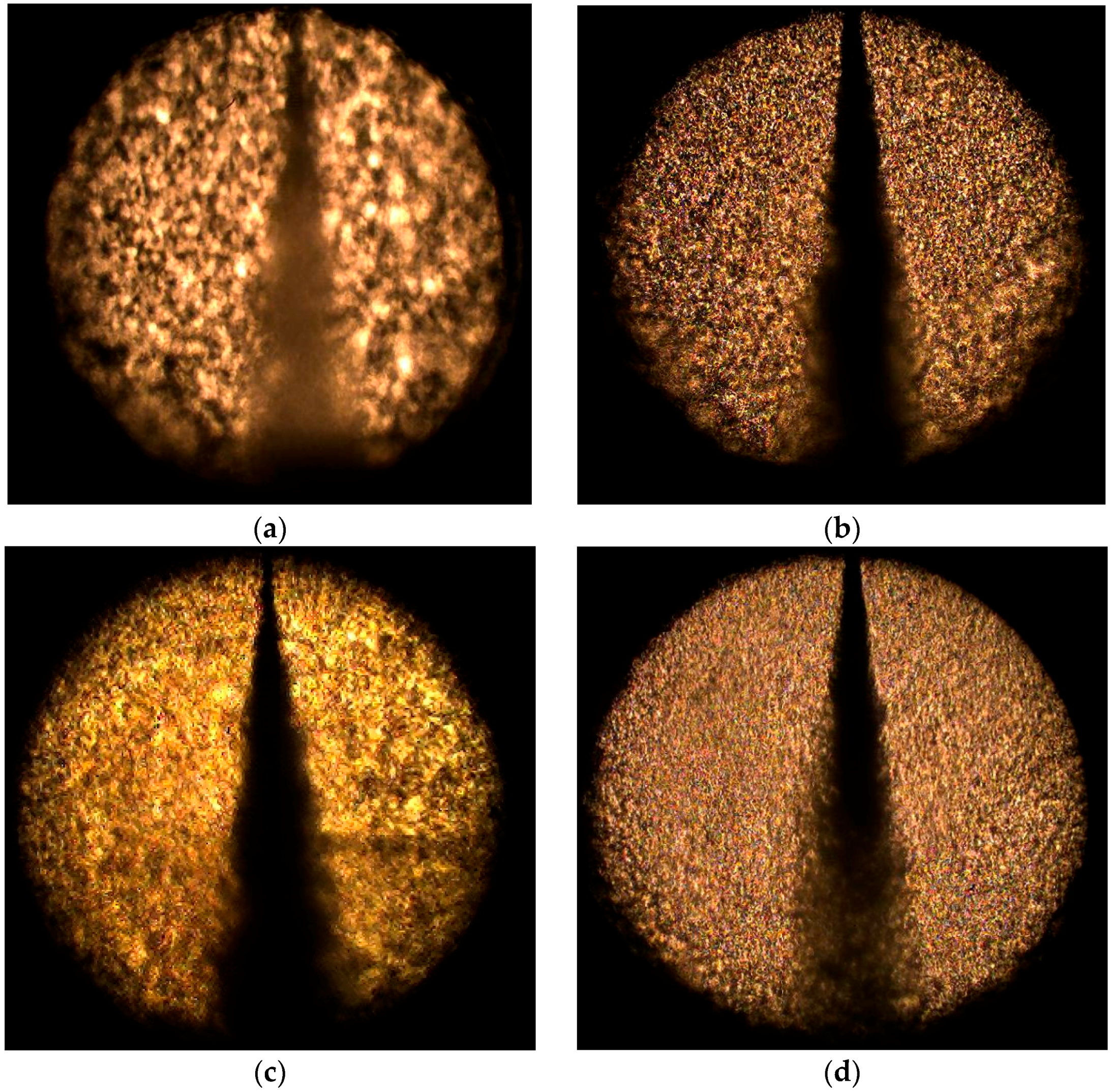
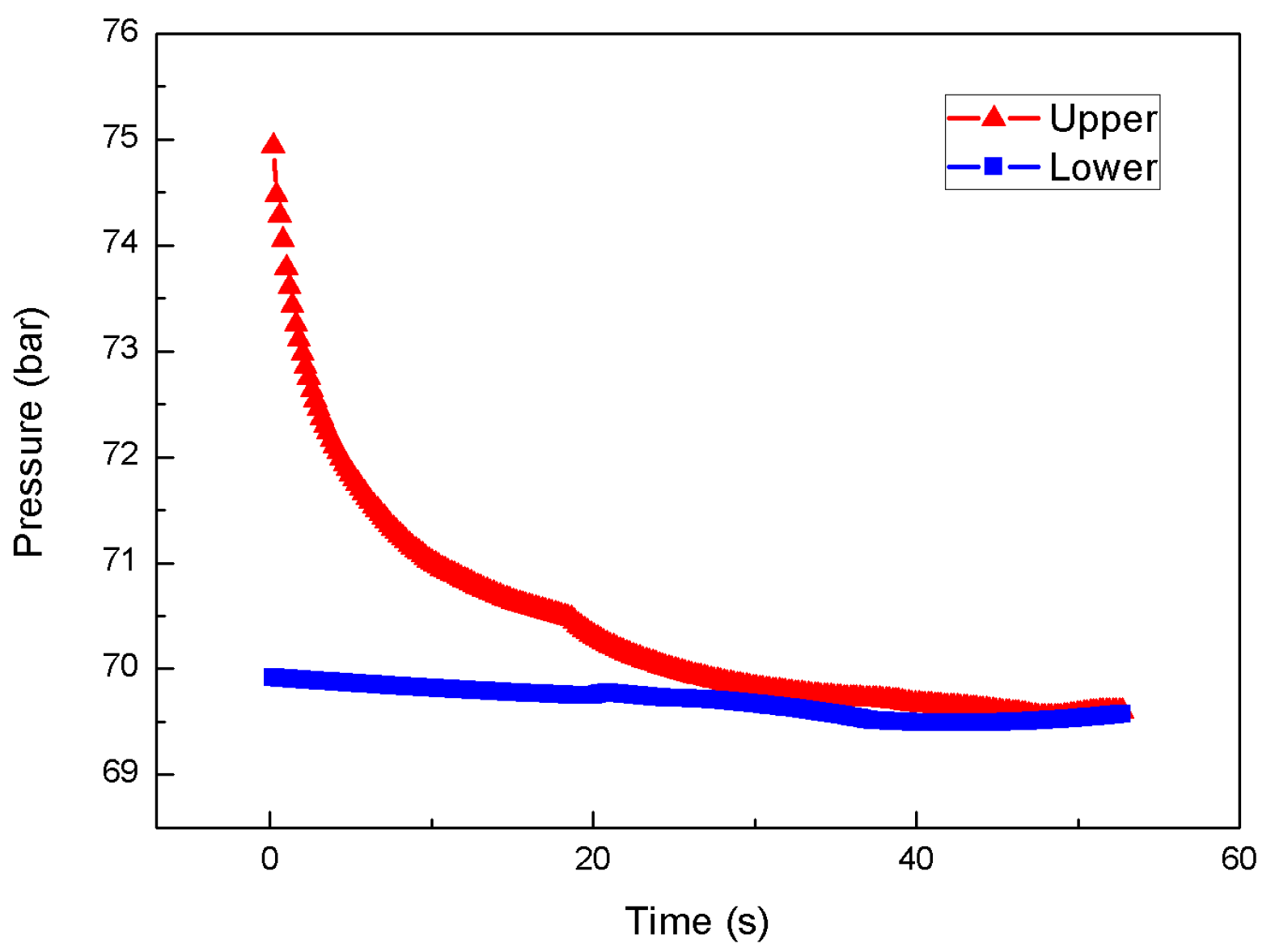
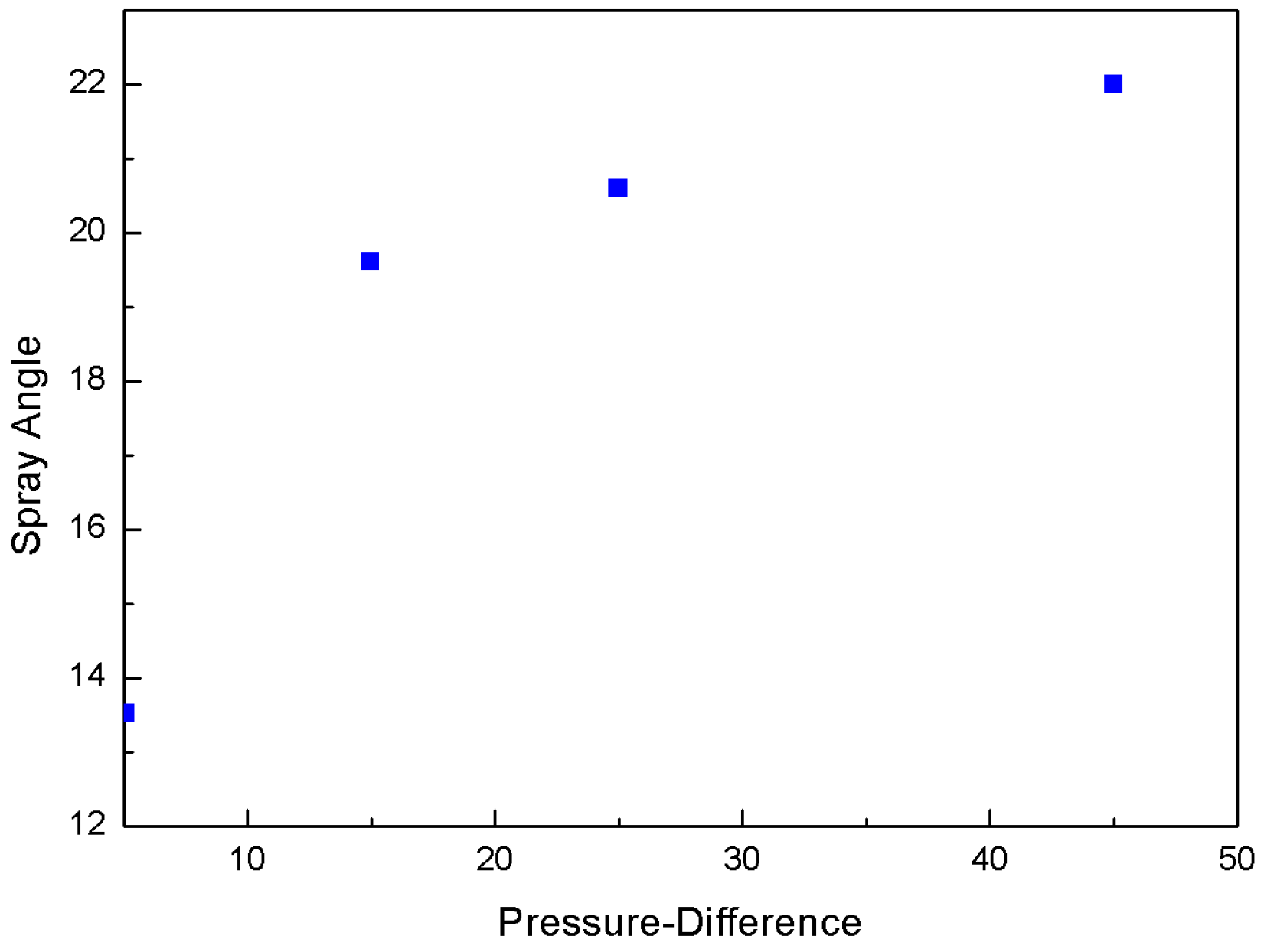
3.2. Effect of Injection Pressure
3.3. Effect of Chamber Pressure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jakobs, T.; Djordjevic, N.; Fleck, S.; Mancini, M.; Weber, R.; Kolb, T. Gasification of high viscous slurry R&D on atomization and numerical simulation. Appl. Energy 2012, 93, 449–456. [Google Scholar]
- Yu, T.U.; Kang, S.W.; Beer, J.M.; Teare, J.D.; Sarofim, A.F. Fundamental Aspects of coal-Water Fuel Droplet Combustion and Secondary Atomization of Coal-Water Mixtures, Final Report; Cambridge: London, UK, 1987; Volume II. [Google Scholar]
- Kim, K.W.; Kim, H.D.; Kim, C.Y.; Song, J.H. Flash spray characteristics of a coal-liquid carbon dioxide slurry. Korean J. Chem. Eng. 2016, 33, 1612–1619. [Google Scholar] [CrossRef]
- Lin, T.C.; Shen, Y.J.; Wang, M.R. Effects of superheat on characteristics of flashing spray and snow particles produced by expanding liquid carbon dioxide. J. Aerosol Sci. 2013, 61, 27–35. [Google Scholar] [CrossRef]
- Zeng, Y.; Lee, C.F.F. An atomization model for flash boiling sprays. Combust. Sci. Technol. 2001, 169, 45. [Google Scholar] [CrossRef]
- Liu, Y.H.; Calvert, G.; Hare, C.; Ghadiri, M.; Matsusaka, S. Size measurement of dry ice particles produced from liquid carbon dioxide. J. Aerosol Sci. 2012, 48, 1. [Google Scholar] [CrossRef] [Green Version]
- Engelmeier, L.; Pollak, S.; Kilzer, A.; Weidner, E. Liquid carbon dioxide jets for cutting applications. J. Supercrit. Fluids 2012, 69, 29–33. [Google Scholar] [CrossRef]
- Reverchon, E. Supercritical-Assisted atomization to produce micro- and/or nanoparticles of controlled size and distribution. Ind. Eng. Chem. Res. 2002, 41, 2405–2411. [Google Scholar] [CrossRef]
- Caputo, G.; Adami, R.; Reverchon, E. Analysis of dissolved-gas atomization: Supercritical CO2 dissolved in water. Ind. Eng. Chem. Res. 2010, 49, 9454–9461. [Google Scholar] [CrossRef]
- Sher, E.; Elata, C. Spray formation from pressure cans by flashing. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 237–242. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modeling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Wendland, M.; Hasse, H.; Maurer, G. Experimental pressure-temperature data on three- and four-phase equilibria of fluid, hydrate, and ice phases in the system carbon dioxide-water. J. Chem. Eng. Data 1999, 44, 901–906. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.Y. An experimental investigation of the flash atomization mechanism. At. Sprays 1994, 4, 159–179. [Google Scholar] [CrossRef]
- Dechelette, A.; Campanella, O.; Corvalan, C.; Sojka, P.E. An experimental investigation on the breakup of surfactant-laden non-Newtonian jets. Chem. Eng. Sci. 2011, 66, 6367–6374. [Google Scholar] [CrossRef]
- Xiao, J.; Qiao, X.; Huang, Z.; Fang, J. Study of droplet size and velocity of fuel containing CO2 spray by means of PDA. Chin. Sci. Bull. 2004, 49, 1195–1199. [Google Scholar] [CrossRef]
- Johnson, J.E.; Yoon, S.H.; Naber, J.D.; Lee, S.Y.; Hunter, G.; Truemner, R.; Harcombe, T. Characteristics of 3000 bar diesel spray injection under non-vaporizing and vaporizing conditions. In Proceedings of the 12th Triennial International Conference on Liquid Atomization and Spray Systems (ICLASS), Heidelberg, Germany, 2–6 September 2012.
- Risberg, M.; Marklund, M. Visualizations of gas-assisted atomization of black liquor and syrup/water mixtures at elevated ambient pressures. At. Sprays 2009, 19, 957–967. [Google Scholar] [CrossRef]


| Fluids | Water | CO2 |
|---|---|---|
| Vapor pressure at 298 K (kPa) | 3 | 6400 |
| Density (mol/L) at 298 K and 64 bar | 55.5 | 16.2 (liquid) |
| Viscosity (mPa·s) at 298 K and 64 bar | 0.89 | 0.057 (liquid) |
| Parameters | Description |
|---|---|
| chamber shape | Cylinder |
| chamber diameter/length | 5 cm/13.4 cm |
| chamber volume | 260 cm3 |
| capillary nozzle inner diameter | 0.4 mm |
| capillary nozzle length | 300 mm |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, C.; Lim, H.; Song, J. Spray Formation of a Liquid Carbon Dioxide-Water Mixture at Elevated Pressures. Energies 2016, 9, 948. https://doi.org/10.3390/en9110948
Kim H, Kim C, Lim H, Song J. Spray Formation of a Liquid Carbon Dioxide-Water Mixture at Elevated Pressures. Energies. 2016; 9(11):948. https://doi.org/10.3390/en9110948
Chicago/Turabian StyleKim, Hakduck, Changyeon Kim, Heechang Lim, and Juhun Song. 2016. "Spray Formation of a Liquid Carbon Dioxide-Water Mixture at Elevated Pressures" Energies 9, no. 11: 948. https://doi.org/10.3390/en9110948






