Plasma Glow Discharge as a Tool for Surface Modification of Catalytic Solid Oxides: A Case Study of La0.6Sr0.4Co0.2Fe0.8O3−δ Perovskite
Abstract
:1. Introduction
2. Results and Discussion
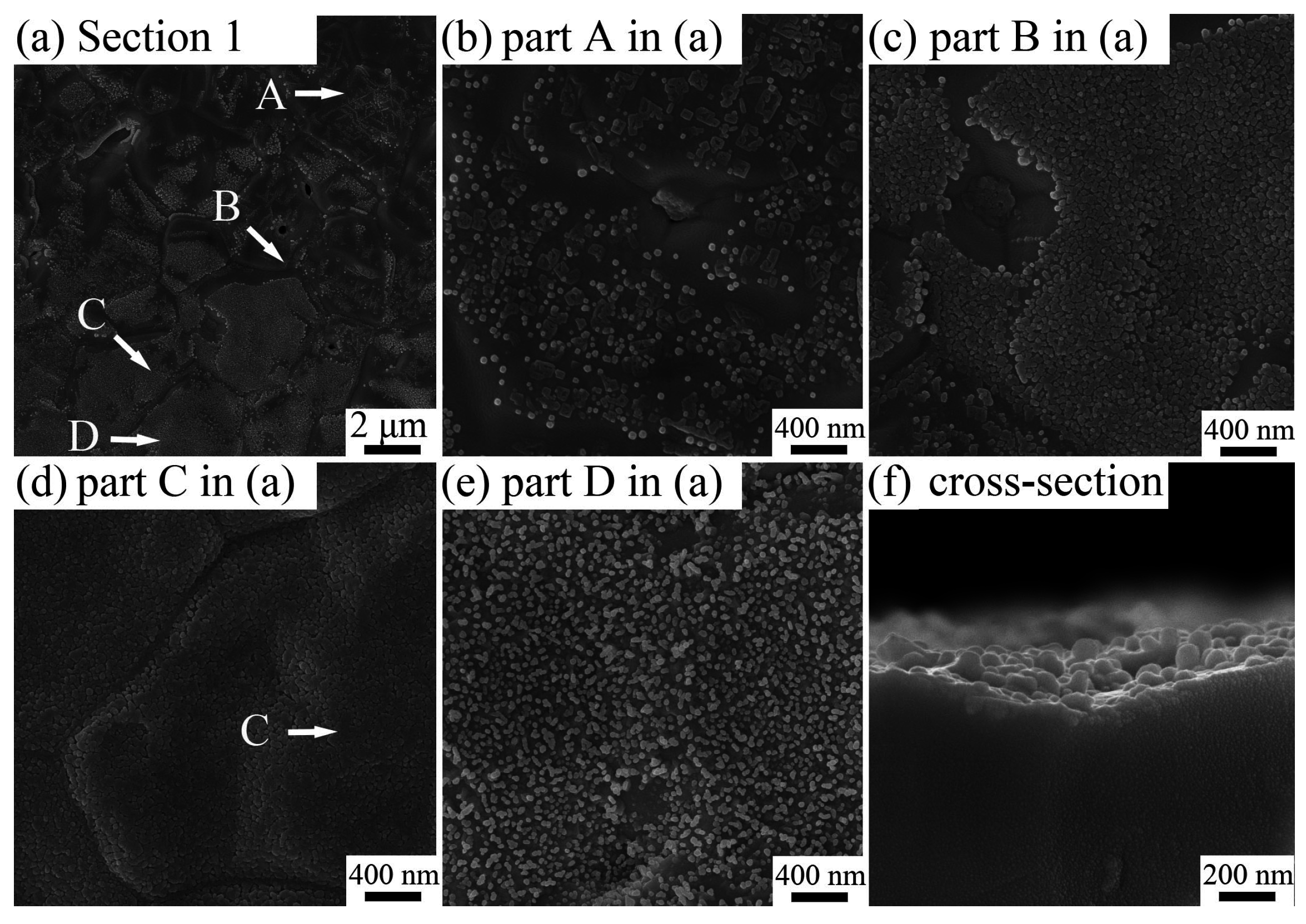
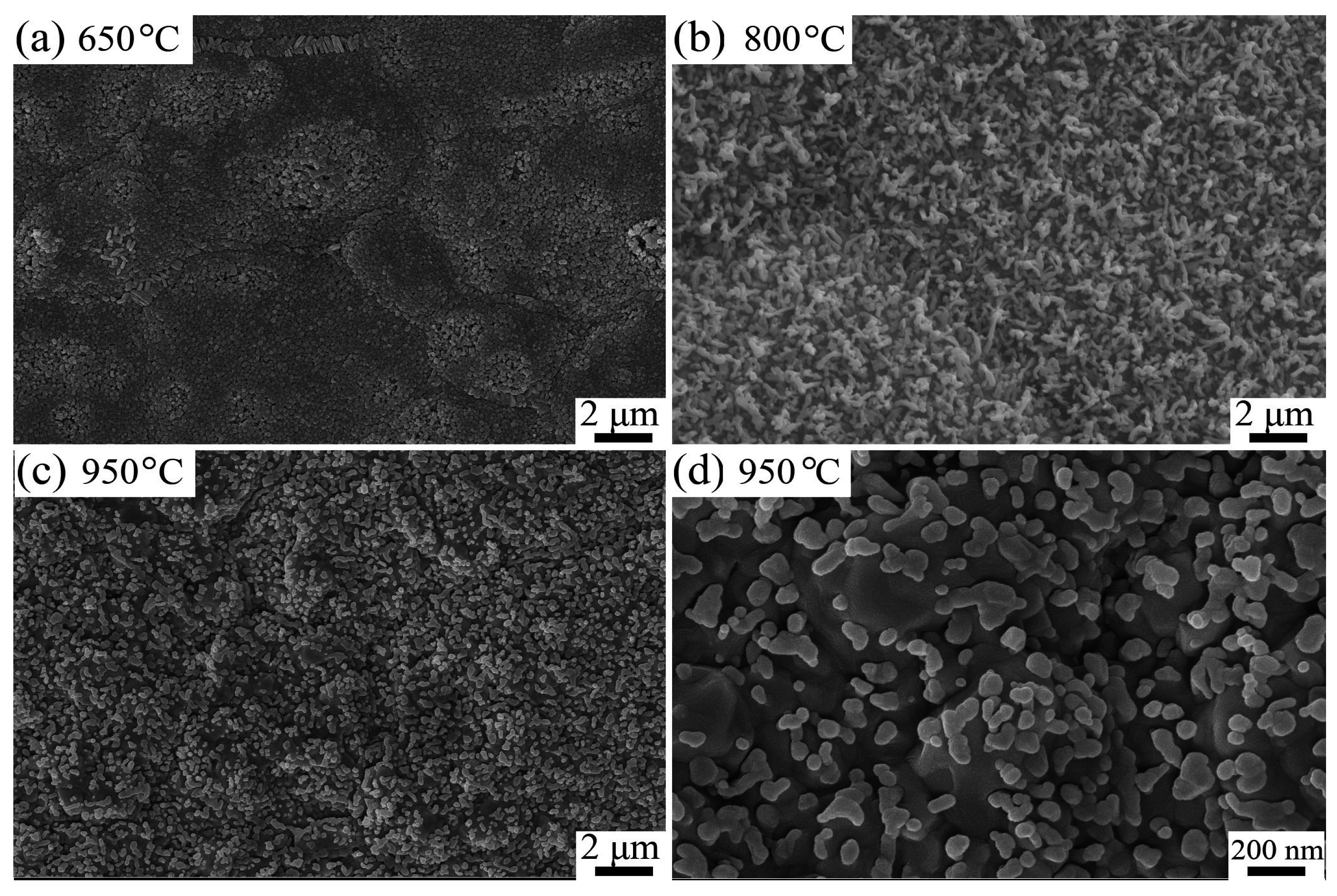
2.1. Effects of Depositing Time and Temperature
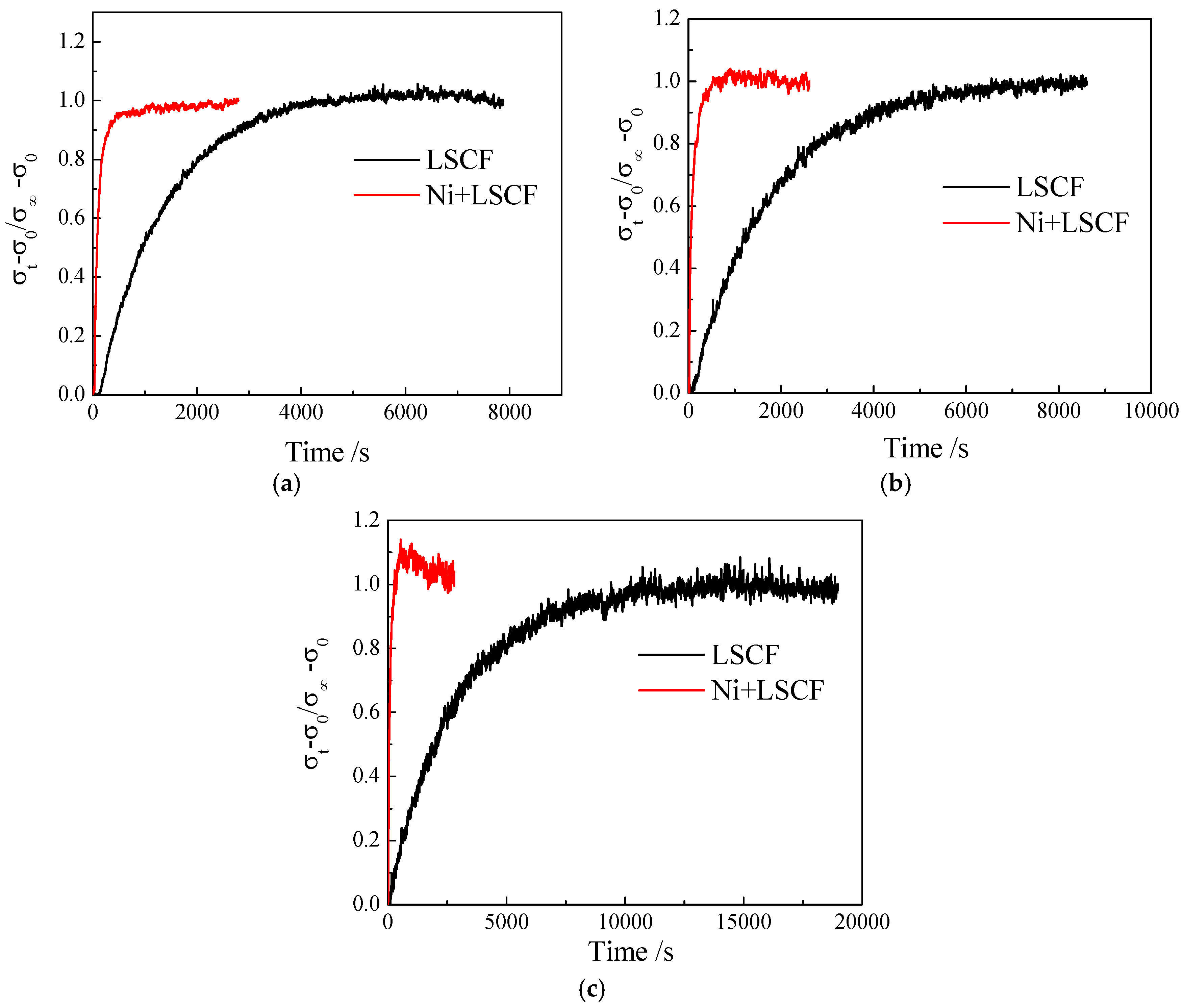
2.2. Promotion in Catalytic Activity
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arnab, C.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar]
- Babaei, A.; Zhang, L.; Liu, E.; Jiang, S.P. Performance and stability of La0.8Sr0.2MnO3 cathode promoted with palladium based catalysts in solid oxide fuel cells. J. Alloy. Compd. 2011, 509, 4781–4787. [Google Scholar] [CrossRef]
- Kiebach, R.; Knöfel, C.; Bozza, F.; Klemensø, T.; Chatzichristodoulou, C. Infiltration of ionic-, electronic- and mixed-conducting nano particles into La0.75Sr0.25MnO3–Y0.16Zr0.84O2 cathodes—A comparative study of performance enhancement and stability at different temperatures. J. Power Sources 2013, 228, 170–177. [Google Scholar] [CrossRef]
- Zou, J.; Park, J.; Yoon, H.; Sammes, N.M.; Chung, J. Effects of transition metal ion dopants on the performance of Ca2.9Bi0.1Co4O9−δ cathode. J. Alloy. Compd. 2013, 558, 188–194. [Google Scholar] [CrossRef]
- Gan, Y.; Qin, Q.; Chen, S.; Wang, Y.; Dong, D.; Xie, K.; Wu, Y. Composite cathode La0.4Sr0.4TiO3−δ–Ce0.8Sm0.2O2−δ impregnated with Ni for high-temperature steam electrolysis. J. Power Sources 2014, 245, 245–255. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Chi, B.; Pu, J.; Li, J.; Jiang, S.P. A stability study of impregnated LSCF–GDC composite cathodes of solid oxide fuel cells. J. Alloy. Compd. 2013, 578, 37–43. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, X.; Ye, X.; Li, J.; Wang, S.; Zhan, Z.L. Metal-supported solid oxide fuel cells with impregnated SrFe0.75Mo0.25O3 cathodes. J. Power Sources 2014, 247, 556–561. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.; Yan, D.; Pu, J.; Chi, B.; Jiang, S.P.; Li, J. Performance of large-scale anode-supported solid oxide fuel cells with impregnated La0.6Sr0.4Co0.2Fe0.8O3−δ+Y2O3 stabilized ZrO2 composite cathodes. J. Power Sources 2010, 195, 5201–5205. [Google Scholar] [CrossRef]
- Jung, S.; Lu, C.; He, H.; Ahn, K.; Gorte, R.J.; Vohs, J.M. Influence of composition and Cu impregnation method on the performance of Cu/CeO2/YSZ SOFC anodes. J. Power Sources 2006, 154, 42–50. [Google Scholar] [CrossRef]
- Vohs, J.M.; Gorte, R.J. High-performance SOFC cathodes prepared by infiltration. Adv. Mater. 2009, 21, 943–956. [Google Scholar] [CrossRef]
- Jiang, S.P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges. Int. J. Hydrog. Energy 2012, 37, 449–470. [Google Scholar] [CrossRef]
- Jiang, Z.; Xia, C.; Chen, F. Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique. Electrochim. Acta 2010, 55, 3595–3605. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, B.; Pu, J.; Li, J. Performance degradation of impregnated La0.6Sr0.4Co0.2Fe0.8O3+Y2O3 stabilized ZrO2 composite cathodes of intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2012, 37, 4388–4393. [Google Scholar] [CrossRef]
- Shah, M.; Voorhees, P.W.; Barnett, S.A. Time-dependent performance changes in LSCF-infiltrated SOFC cathodes: The role of nano-particle coarsening. Solid State Ion. 2011, 187, 64–67. [Google Scholar] [CrossRef]
- Ruiz-Trejo, E.; Atkinson, A.; Brandon, N.P. Metallizing porous scaffolds as an alternative fabrication method for solid oxide fuel cell anodes. J. Power Sources 2015, 280, 81–89. [Google Scholar] [CrossRef]
- Kim, J.; Jang, D.; Kim, M.; Choi, H.; Shim, J. Nano-granulization of gadolinia-doped ceria electrolyte surface by aerosol-assisted chemical vapor deposition for low-temperature solid oxide fuel cells. J. Power Sources 2016, 301, 72–77. [Google Scholar] [CrossRef]
- Ju, Y.; Jun, A.; Inoishi, A.; Ida, S.; Lim, T.; Kim, G.; Ishihara, T. Growth of thin-film layered perovskite cathodes by pulsed laser deposition and their electrochemical studies in IT-SOFCs. J. Electrochem. Soc. 2014, 161, F698–F702. [Google Scholar] [CrossRef]
- Gong, Y.H.; Patel, R.; Liang, X.; Palacio, D.; Song, X.; Goodenough, J.B.; Huang, K. Atomic layer deposition functionalized composite SOFC cathode La0.6Sr0.4Fe0.8Co0.2O3−δ-Gd0.2Ce0.8O1.9: Enhanced long-term stability. Chem. Mater. 2013, 25, 4224–4231. [Google Scholar] [CrossRef]
- Choi, H.; Bae, K.; Jang, D.; Park, S.; Han, G.; Shim, J. Surface modification of La0.6Sr0.4Co0.2Fe0.8O3−δ cathode by atomic layer deposition of La0.6Sr0.4CoO3−δ for high-performance solid oxide fuel cells. ECS Trans. 2015, 68, 729–733. [Google Scholar] [CrossRef]
- Cong, T.; Yan, M.; Liu, R. Multiphase films bearing Ti2AlN by pulse plasma hollow cathode nitriding. Surf. Eng. 2013, 29, 336–341. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Lu, Q.; Sun, L.; Crumlin, E.; Yildiz, B. Improved chemical and electrochemical stability of perovskite oxides with less reducible cations at the surface. Nat. Mater. 2016, 15, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Brinkman, K.; Xia, C. Copper oxide as a synergistic catalyst for the oxygen reduction reaction on La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite structured electrocatalyst. J. Power Sources 2016, 329, 281–289. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Den Otter, M.; Boukamp, B.A. Oxygen transport in La0.6Sr0.4Co1-yFeyO3−δ. J. Solid State Electrochem. 2004, 8, 599–605. [Google Scholar] [CrossRef]
- Smith, F.; Um, W.; Taylor, C.; Kim, D.; Schweiger, M.; Kruger, A. Computational investigation of technetium(IV) incorporation into inverse spinels: Magnetite (Fe3O4) and trevorite (NiFe2O4). Environ. Sci. Technol. 2016, 50, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Wang, Z.; Chen, L.; Wu, R.; Peng, R.; Lu, Y. Structural, electrical, and electrochemical properties of cobalt-doped NiFe2O4 as a potential cathode material for solid oxide fuel cells. Int. J. Hydrog. Energy 2013, 38, 14329–14336. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Li, M.; Ren, Y.; Zhu, Z.; Zhu, S.; Chen, F.; Zhang, Y.; Xia, C. La0.4Bi0.4Sr0.2FeO3−δ as cobalt-free cathode for intermediate-temperature solid oxide fuel cell. Electrochim. Acta 2016, 191, 651–660. [Google Scholar] [CrossRef]
- Hu, B.; Xia, C. Factors influencing the measured surface reaction kinetics parameters. Asia-Pac. J. Chem. Eng. 2016, 11, 327–337. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, J.; Li, M.; Chen, Y.; Yan, M.; Xia, C. Plasma Glow Discharge as a Tool for Surface Modification of Catalytic Solid Oxides: A Case Study of La0.6Sr0.4Co0.2Fe0.8O3−δ Perovskite. Energies 2016, 9, 786. https://doi.org/10.3390/en9100786
Zhang Y, Ma J, Li M, Chen Y, Yan M, Xia C. Plasma Glow Discharge as a Tool for Surface Modification of Catalytic Solid Oxides: A Case Study of La0.6Sr0.4Co0.2Fe0.8O3−δ Perovskite. Energies. 2016; 9(10):786. https://doi.org/10.3390/en9100786
Chicago/Turabian StyleZhang, Yanxiang, Jingbo Ma, Mei Li, Yu Chen, Mufu Yan, and Changrong Xia. 2016. "Plasma Glow Discharge as a Tool for Surface Modification of Catalytic Solid Oxides: A Case Study of La0.6Sr0.4Co0.2Fe0.8O3−δ Perovskite" Energies 9, no. 10: 786. https://doi.org/10.3390/en9100786




