Analysis of a New Liquefaction Combined with Desublimation System for CO2 Separation Based on N2/CO2 Phase Equilibrium
Abstract
:1. Introduction
2. Thermodynamic Models
2.1. Cubic Equations of State
| EOS | Function form | Coefficient |
|---|---|---|
| RK | ||
| SRK | ||
| PR | ||
2.2. Mixing Rules
| Mixing rules | a | b |
|---|---|---|
| vdW | ||
| WS | ||
| MHV2 | ||
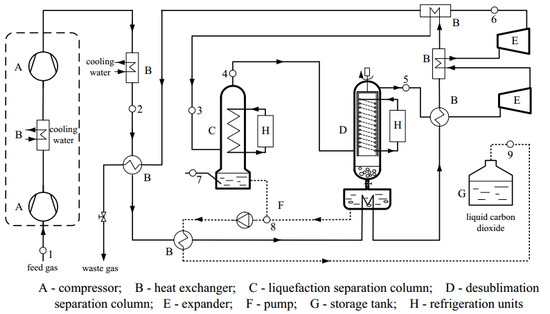
3. The New Liquefaction Combined with Desublimation System
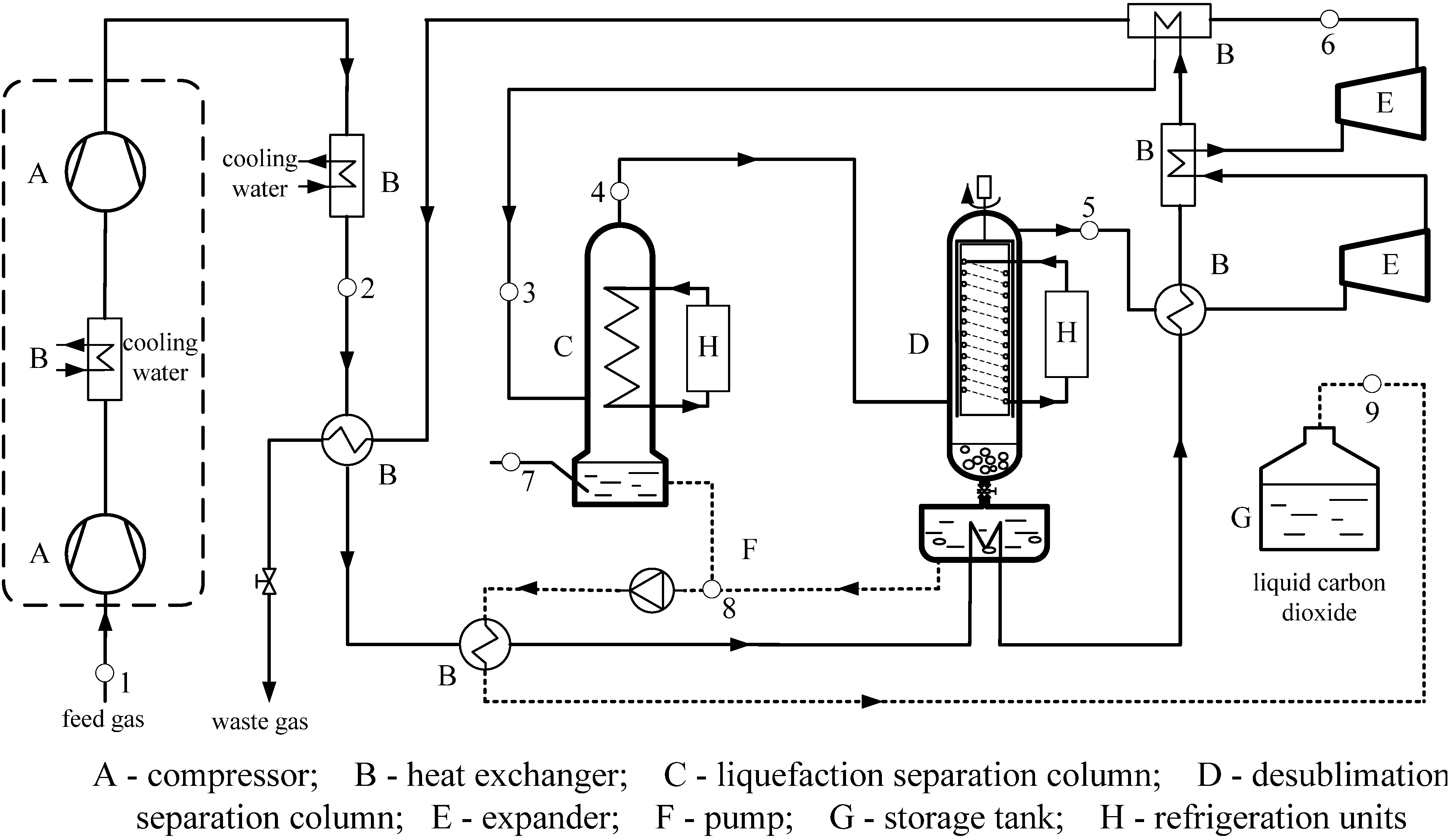
4. Method of Analysis
4.1. Thermo Model
4.2. The Separation Performance
4.3. Energy Consumption
5. Result and Discussion
5.1. Selection and Calculation of the Equations of State

5.2. Selection and Calculation of the Mixing Rules

| Models | 293.2 K | 273.2 K | 240 K | 220 K | ||||
|---|---|---|---|---|---|---|---|---|
| Ps | Vapor fraction | Ps | Vapor fraction | Ps | Vapor fraction | Ps | Vapor fraction | |
| SRK-vdW | 0.92 | 5.54 | 3.83 | 6.95 | 8.88 | 2.38 | 8.45 | 0.84 |
| SRK-MHV2 | 9.93 | 9.09 | 17.60 | 15.22 | 39.10 | 15.55 | 55.78 | 13.17 |
| SRK-WS | 9.42 | 9.07 | 15.09 | 13.30 | 33.64 | 11.22 | 47.41 | 9.52 |
| PR-vdW | 1.37 | 3.46 | 2.69 | 5.15 | 7.37 | 1.43 | 6.31 | 0.43 |
| PR-MHV2 | 7.11 | 6.49 | 12.74 | 11.33 | 32.99 | 12.76 | 50.74 | 11.51 |
| PR-WS | 8.76 | 10.28 | 14.43 | 13.88 | 33.60 | 11.31 | 48.11 | 9.76 |
5.3. Thermodynamic Properties of N2/CO2 at Low Temperature
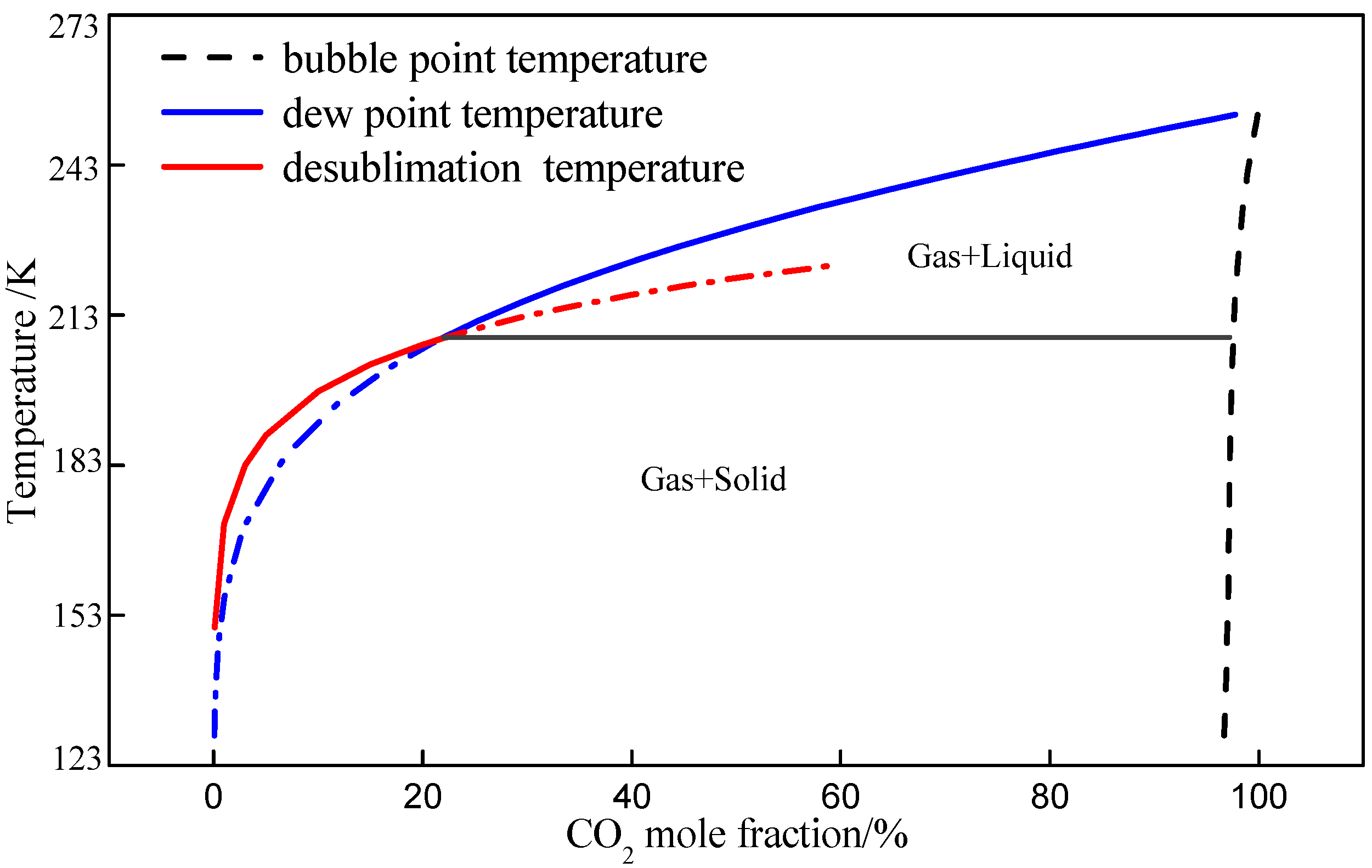

| No. | CO2 mole fraction (%) | T (K) | P (MPa) |
|---|---|---|---|
| 1 | 40 | 298.15 | 0.1 |
| 2 | 40 | 298.15 | 2 |
| 3 | 40 | 223.85 | 2 |
| 4 | 21.69 | 208.37 | 2 |
| 5 | 6.31 | 192.02 | 2 |
| 6 | 6.31 | 170.17 | 0.1 |
| 7 | 97.41 | 208.37 | 2 |
| 8 | 98.29 | 211.95 | 2 |
| 9 | 98.29 | 293.15 | 10 |
5.4. The Results of Energy Calculations
| Parameters | Value | Dimension |
|---|---|---|
| Compression work | 44,036.9 | kW |
| Expansion work | 10,715.9 | kW |
| Cold consumption by liquefaction | 16,139.01 | kW |
| Power consumption by liquefaction | 15,979.22 | kW |
| Cold consumption by desublimation | 13,841.99 | kW |
| Power consumption by desublimation | 17,814.66 | kW |
| Save power from cold energy recovery | 6171.31 | kW |
| Pump work | 604.92 | kW |
| Total work | 61,548.5 | kW |
| Unit power consumption | 0.9326 | MJ·kg−1 |
| Unit energy consumption | 3.108 | MJ·kg−1 |
| Separation methods | Pressure (MPa) | Liquefied recovery (%) | Liquid CO2 purity (%) | Solid CO2 recovery (%) | Total recovery (%) | Energy consumption (MJ·kg−1) |
|---|---|---|---|---|---|---|
| Desublimation | 0.55 | 0 | 100 | 90 | 90 | 3.416 |
| Liquefaction combined desublimation | 2 | 58.89 | 97.41 | 31.11 | 90 | 3.108 |
| Liquefaction combined desublimation | 3 | 73.04 | 95.82 | 16.96 | 90 | 3.162 |
| Liquefaction combined desublimation | 4 | 79.27 | 94.23 | 10.73 | 90 | 3.193 |
| Liquefaction separation | 6 | 85.44 | 90.44 | 0 | 85.44 | - |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Minchener, A.J.; McMullan, J.T. Sustainable clean coal power generation within a European context-the view in 2006. Fuel 2007, 86, 2124–2133. [Google Scholar] [CrossRef]
- Song, C.F.; Kitamura, Y.; Li, S.H.; Jiang, W.Z. Analysis of CO2 frost formation properties in cryogenic capture process. Int. J. Greenh. Gas Control 2013, 13, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Kelley, B.T.; Valencia, J.A.; Northrop, P.S. Controlled Freeze Zone (TM) for developing sour gas reserves. Energy Procedia 2011, 4, 824–829. [Google Scholar] [CrossRef]
- Theunissen, T.; Golombok, M.; Brouwers, J.J.H.; Bansal, G.; Benthum, R. Liquid CO2 droplet extraction from gases. Energy 2011, 36, 2961–2967. [Google Scholar] [CrossRef]
- Zanganeh, K.E.; Shafeen, A.; Salvador, C. CO2 Capture and Development of an Advanced PilotScale Cryogenic Separation and Compression Unit. Energy Procedia 2009, 1, 247–252. [Google Scholar] [CrossRef]
- Li, H.; Yan, J. Evaluating cubic equations of state for calculation of vapor-liquid equilibrium of CO2 and CO2-mixtures for CO2 capture and storage processes. Appl. Energy 2009, 86, 826–836. [Google Scholar] [CrossRef]
- Clodic, D.; Younes, M. A new method for CO2 capture: Frosting CO2 at atmospheric pressure. In Proceedings of the 6th International Conference on Greenhouse Gas Control Technologies, Kyoto, Japan, 1–4 October 2002; pp. 155–160.
- Li, H.; Jakobsen, J.P.; Wilhelmsen, Ø.; Yan, J. PVTxy properties of CO2 mixtures relevant for CO2 capture, transport and storage: Review of available experimental data and theoretical models. Appl. Energy 2011, 88, 3567–3579. [Google Scholar] [CrossRef]
- Vrabec, J.; Kedia, G.K.; Buchhauser, U.; Meyer-Pittroff, R.; Hasse, H. Thermodynamic models for vapor-liquid equilibria of nitrogen + oxygen + carbon dioxide at low temperatures. Cryogenics 2009, 49, 72–79. [Google Scholar] [CrossRef]
- Dorau, W.; Al-Wakeel, I.M.; Knapp, H. VLE data for CO2-CF2Cl2, N2-CO2, N2-CF2Cl2 and N2-CO2-CF2Cl. Cryogenics 1983, 23, 29–35. [Google Scholar] [CrossRef]
- Thiery, R.; Vidal, J.; Dubessy, J. Phase equilibria modelling applied to fluid inclusions: Liquid-vapor equilibria and calculation of the molar volume in the CO2-CH4-N2 system. Geochim. Cosmochim. Acta 1994, 58, 1073–1082. [Google Scholar] [CrossRef]
- Duan, Z.; Hu, J. A new cubic equation of state and its applications to the modeling of vapor-liquid equilibria and volumetric properties of natural fluids. Geochim. Cosmochim. Acta 2004, 68, 2997–3009. [Google Scholar] [CrossRef]
- Li, H.; Yan, J. Impacts of equations of state (EOS) and impurities on the volume calculation of CO2 mixtures in the applications of CO2 capture and storage (CCS) processes. Appl. Energy 2009, 86, 2760–2770. [Google Scholar] [CrossRef]
- Ahmad, M.; Gernert, J.; Wilbers, E. Effect of impurities in captured CO2 on liquid-vapor equilibrium. Fluid Phase Equilibria 2013, 363, 149–155. [Google Scholar] [CrossRef]
- Raabe, G.; Köhler, J. Phase equilibria in the system nitrogen-ethane and their prediction using cubic equations of state with different types of mixing rules. Fluid Phase Equilibria 2004, 222, 3–9. [Google Scholar] [CrossRef]
- Faúndez, C.A.; Valderrama, J.O. Modeling associating hydrocarbon + alcohol mixtures using the Peng-Robinson equation of state and the Wong-Sandler mixing rules. Comptes Rendus Chim. 2013, 16, 135–143. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Huron, M.J.; Vidal, J. New mixing rules in simple equations of state for representing vapour-liquid equilibria of strongly non-ideal mixtures. Fluid Phase Equilibria 1979, 3, 255–271. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, S.T. Prediction of mixture vapor-liquid equilibrium from the combined use of Peng-Robinson equation of state and COSMO-SAC activity coefficient model through the Wong-Sandler mixing rule. Fluid Phase Equilibria 2007, 254, 28–34. [Google Scholar] [CrossRef]
- Eggeman, T.; Chafin, S. Pitfalls of CO2 freezing prediction. In Proceedings of the 82nd Annual Convention of the Gas Processors Association, San Antonio, TX, USA, 10–12 March 2003.
- Yorizane, M.; Yoshimura, S.; Masuoka, H.; Miyano, Y.; Kakimoto, Y. New procedure for vapor-liquid equilibria. Nitrogen + Carbon dioxide, Methane + Freon22, and Methane + Freon12. J. Chem. Eng. Data 1985, 30, 174–176. [Google Scholar] [CrossRef]
- Al-Sahhaf, T.A.; Kidnay, A.J.; Sloan, E.D. Liquid + vapor equilibria in the N2 + CO2 + CH4 system. Ind. Eng. Chem. Fundam. 1983, 22, 372–380. [Google Scholar] [CrossRef]
- Arai, Y.; Kaminishi, G.I.; Saito, S. The experimental determination of the PVTX relations for the carbon dioxide-nitrogen and the carbon dioxide-methane systems. J. Chem. Eng. Jpn. 1971, 4, 113–122. [Google Scholar] [CrossRef]
- Xu, N.; Dong, J.; Wang, Y.; Shi, J. High pressure vapor-liquid equilibria at 293 K for systems containing nitrogen, methane and carbon dioxide. Fluid Phase Equilibria 1992, 81, 175–186. [Google Scholar] [CrossRef]
- Favre, E.; Bounanceur, R.; Roizard, D. A hybrid process combining oxygen enriched air combustion and membrane separation for post-combustion carbon dioxide capture. Sep. Purif. Technol. 2009, 68, 30–36. [Google Scholar] [CrossRef]
- Zanganeh, K.E.; Shafeen, A. A novel process integration, optimization and design approach for large-scale implementation of oxy-fired coal power plants with CO2 capture. Int. J. Greenh. Gas Control 2007, 1, 47–54. [Google Scholar] [CrossRef]
- Zanganeh, K.; Shafeen, A.; Salvador, C.; Beigzadeh, A.; Abbassi, M. CO2 processing and multi-pollutant control for oxy-fuel combustion systems using an advanced CO2 capture and compression unit (CO2CCU). Energy Procedia 2011, 4, 1018–1025. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Li, S.; Li, X.; Liang, Y.; Zhang, X. Analysis of a New Liquefaction Combined with Desublimation System for CO2 Separation Based on N2/CO2 Phase Equilibrium. Energies 2015, 8, 9495-9508. https://doi.org/10.3390/en8099495
Yang W, Li S, Li X, Liang Y, Zhang X. Analysis of a New Liquefaction Combined with Desublimation System for CO2 Separation Based on N2/CO2 Phase Equilibrium. Energies. 2015; 8(9):9495-9508. https://doi.org/10.3390/en8099495
Chicago/Turabian StyleYang, Wenchao, Shuhong Li, Xianliang Li, Yuanyuan Liang, and Xiaosong Zhang. 2015. "Analysis of a New Liquefaction Combined with Desublimation System for CO2 Separation Based on N2/CO2 Phase Equilibrium" Energies 8, no. 9: 9495-9508. https://doi.org/10.3390/en8099495
APA StyleYang, W., Li, S., Li, X., Liang, Y., & Zhang, X. (2015). Analysis of a New Liquefaction Combined with Desublimation System for CO2 Separation Based on N2/CO2 Phase Equilibrium. Energies, 8(9), 9495-9508. https://doi.org/10.3390/en8099495