Enzyme-Assisted Extraction of Oil from Wet Microalgae Scenedesmus sp. G4
Abstract
:1. Introduction
| Item | Conventional extraction | Enzyme-assisted extraction |
|---|---|---|
| Dehydration process | Needed | No |
| Organic reagent | Excess needed | Small |
| Quality of value-added biomass | Affected | Unaffected |
2. Materials and Methods
2.1. Microalgal Strain and Culture Conditions

2.2. Measurement Methods of Oil, Protein and Sugar Content
2.3. Enzyme Mixtures
2.4. Enzyme Assays
2.5. Measurement of Oil Extraction
2.6. Orthogonal Array Testing of Enzyme Mixtures Hydrolysis
| Factors | Algae concentration (g/L) | Temperature (°C) | pH | Cellulase:pectinase:hemicellulase (dry weight ratio of protein) | |
|---|---|---|---|---|---|
| Level | 1 | 2.5 | 30 | 3.5 | 1:1:1 |
| 2 | 5.0 | 40 | 4.0 | 2:1:1 | |
| 3 | 7.5 | 50 | 4.5 | 1:2:1 | |
| 4 | 10.0 | 60 | 5.0 | 1:1:2 | |
2.7. Scanning Electron Microscopy
2.8. Fourier Transform Infrared Spectroscopy Analysis
2.9. X-Ray Diffraction Analysis
3. Results and Discussion
3.1. Results of Orthogonal Array Testing on the Enzymatic Hydrolysis
| Factor | Sum of squares | Degree of freedom | F-value | Significance |
|---|---|---|---|---|
| Algae concentration (g/L) | 7907.023 | 3 | 11.336 | * |
| Temperature (°C) | 619.913 | 3 | 0.889 | - |
| pH | 2.693 | 3 | 0.004 | - |
| Cellulase:pectinase:hemicellulase (dry weight ratio of protein) | 200.723 | 3 | 0.288 | - |
| Pure error | 697.53 | 3 | - | - |
| No. | Algae concentration (g/L) | Temperature (°C) | pH | Cellulase:pectinase:hemicellulase (dry weight ratio of protein) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 2.5 | 30 | 3.5 | 1:1:1 | 86.1 |
| 2 | 2.5 | 40 | 4.0 | 2:1:1 | 61.5 |
| 3 | 2.5 | 50 | 4.5 | 1:2:1 | 86.1 |
| 4 | 2.5 | 60 | 5.0 | 1:1:2 | 69.7 |
| 5 | 5.0 | 30 | 4.0 | 1:2:1 | 41.0 |
| 6 | 5.0 | 40 | 3.5 | 1:1:2 | 30.7 |
| 7 | 5.0 | 50 | 5.0 | 1:1:1 | 51.2 |
| 8 | 5.0 | 60 | 4.5 | 2:1:1 | 34.8 |
| 9 | 7.5 | 30 | 4.5 | 1:1:2 | 23.2 |
| 10 | 7.5 | 40 | 5.0 | 1:2:1 | 20.5 |
| 11 | 7.5 | 50 | 3.5 | 2:1:1 | 27.3 |
| 12 | 7.5 | 60 | 4.0 | 1:1:1 | 28.7 |
| 13 | 10 | 30 | 5.0 | 2:1:1 | 16.4 |
| 14 | 10 | 40 | 4.5 | 1:1:1 | 12.3 |
| 15 | 10 | 50 | 4.0 | 1:1:2 | 28.7 |
| 16 | 10 | 60 | 3.5 | 1:2:1 | 16.4 |
| I | 75.850 | 41.670 | 40.125 | 44.575 | - |
| II | 39.425 | 31.250 | 39.975 | 35.000 | - |
| III | 24.925 | 48.325 | 39.100 | 41.000 | - |
| IV | 18.450 | 37.400 | 39.450 | 38.075 | - |
| R | 57.400 | 17.075 | 1.025 | 9.575 | - |
3.2. Scanning Electron Microscope Analysis of the Enzymatic Hydrolysis Products

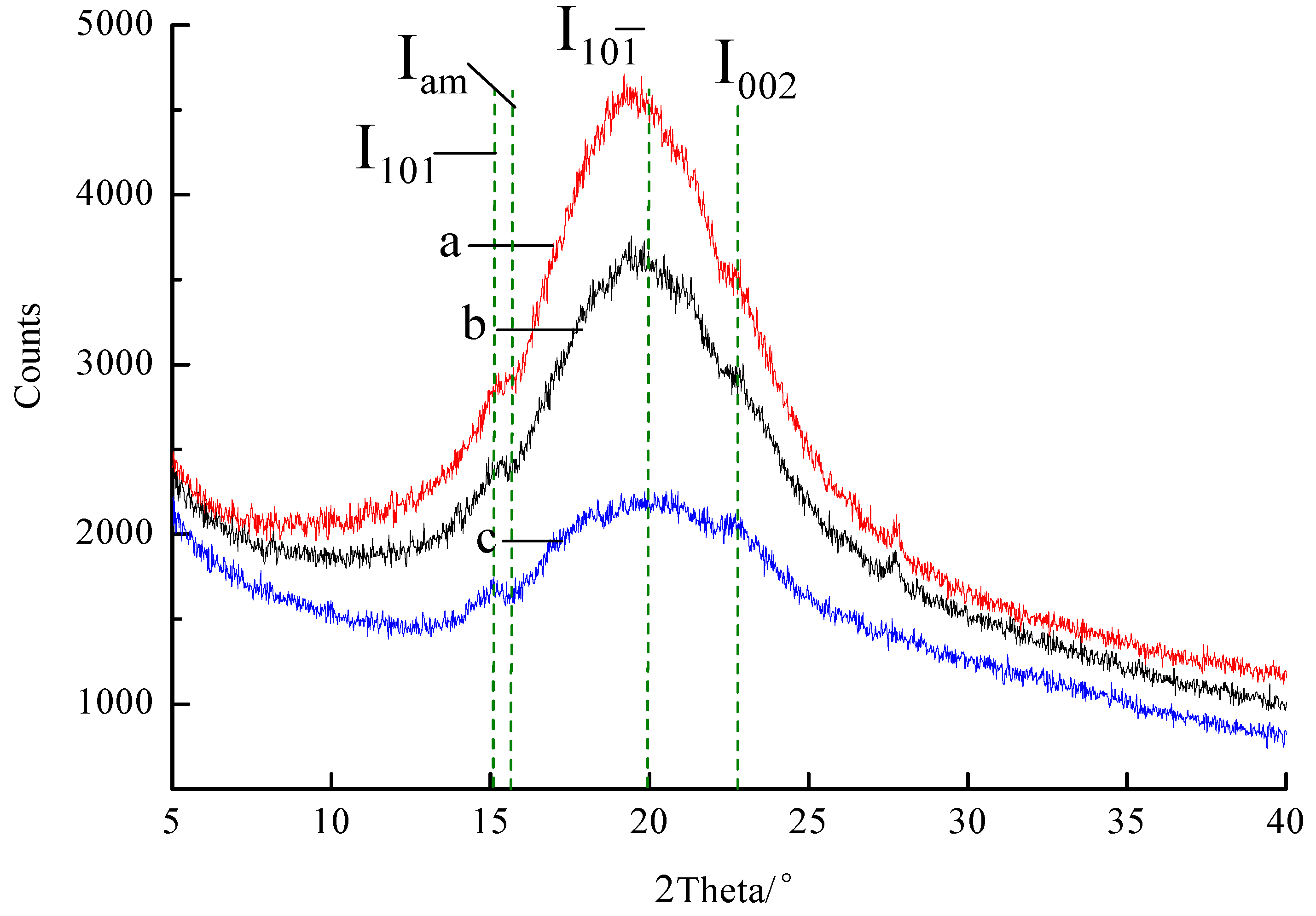
3.3. The X-ray Diffraction Analysis of the Enzymatic Hydrolysis Products
3.4. The Fourier Transform Infrared Spectroscopy Analysis of Enzymatic Hydrolysis

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel; Springer-Verlag: London, UK, 2010. [Google Scholar]
- Huo, S.; Dong, R.; Wang, Z.; Pang, C.; Yuan, Z.; Zhu, S.; Chen, L. Available resources for algal biofuel development in China. Energies 2011, 4, 1321–1335. [Google Scholar] [CrossRef]
- National Algal Biofuels Technology Roadmap; U.S. Department of Energy: Washington, DC, USA, 2010.
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tan, T. Enzyme-aided extraction of lipid from wet microalgae chlorella vulgaris. J. Bioprocess Eng. Biorefinery 2013, 4, 193–197. [Google Scholar] [CrossRef]
- Romero García, J.M.; Acién Fernández, F.G.; Fernández Sevilla, J.M. Development of a process for the production of L-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.; Herdman, M.; Stanier, R. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Chu, W.L.; Phang, S.; Goh, S.H. Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J. Appl. Phycol. 1996, 8, 389–396. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [PubMed]
- Ghose, T.K. Ghose Measurements of cellulase activities. Pure Appl. Chem. 1987, 2, 257–268. [Google Scholar]
- Meneghel, L.; Reis, G.P.; Reginatto, C.; Malvessi, E.; da Silveira, M.M. Assessment of pectinase production by Aspergillus oryzae in growth-limiting liquid medium under limited and non-limited oxygen supply. Process Biochem. 2014, 49, 1800–1807. [Google Scholar] [CrossRef]
- Diogo, J.A.; Hoffmam, Z.B.; Zanphorlin, L.M.; Cota, J.; Machado, C.B.; Wolf, L.D.; Squina, F.; Damásio, A.R.; Murakami, M.T.; Ruller, R. Development of a chimeric hemicellulase to enhance the xylose production and thermotolerance. Enzym. Microb. Technol. 2015, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, H.; Hoyer, O.; Schell, H.; Lusse, B. Reaction mechanisms involved in the influence of algogenic matter on flocculation. Wasser Abwasser Forsch. 1985, 18, 67–75. [Google Scholar]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae. Water Res. 2010, 44, 3617–3624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, N.; Sugiyama, J.; Okano, T.; Ishihara, M. Selective degradation of the cellulose Iα component in Cladophora cellulose with Trichoderma viride cellulase. Carbohydr. Res. 1997, 305, 109–116. [Google Scholar] [CrossRef]
- Atalla, R.H.; Van der Hart, D.L. Native cellulose: A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Van der Hart, D.L.; Atalla, R.H. Studies of microstructure in native celluloses using solid-state carbon-13 NMR. Macromolecules 1984, 17, 1465–1472. [Google Scholar] [CrossRef]
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Yu, J.; Zhang, X.; Tan, T. The effects of four different pretreatments on enzymatic hydrolysis of sweet sorghum bagasse. Bioresour. Technol. 2011, 102, 4585–4589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Liu, D.H. Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: A continued work. J. Chem. Technol. Biotechnol. 2008, 83, 950–956. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, S.; Wang, Z.; Cui, F.; Zou, B.; Zhao, P.; Yuan, Z. Enzyme-Assisted Extraction of Oil from Wet Microalgae Scenedesmus sp. G4. Energies 2015, 8, 8165-8174. https://doi.org/10.3390/en8088165
Huo S, Wang Z, Cui F, Zou B, Zhao P, Yuan Z. Enzyme-Assisted Extraction of Oil from Wet Microalgae Scenedesmus sp. G4. Energies. 2015; 8(8):8165-8174. https://doi.org/10.3390/en8088165
Chicago/Turabian StyleHuo, Shuhao, Zhongming Wang, Fengjie Cui, Bin Zou, Pengxiang Zhao, and Zhenhong Yuan. 2015. "Enzyme-Assisted Extraction of Oil from Wet Microalgae Scenedesmus sp. G4" Energies 8, no. 8: 8165-8174. https://doi.org/10.3390/en8088165






