1. Introduction
The condition of power transformer insulation is paramount since its reliability is important to society and power utilities along with the techno-economic growth of any country. For power transformers to operate normally it is important to isolate live parts from each other and the ground, current not leaking from one path to another through materials not intended to be a conducting path. Insulation includes all three forms of matter, sometimes with a simple shape but often involved a combination of forms, such as the solid/liquid or solid/gas. Heat dissipation can be achieved by circulating certain fluids (liquids/gases), which also ensure electrical insulation of energized conductors. The insulating-fluids market is therefore likely to be dominated by liquids [
1]. Petroleum based oils, the so-called mineral oils, are used as the sole insulation material only in regions where, by design, electrical stresses are relatively low. The functions of oil are to strengthen the dielectric properties of solid insulation, to electrically insulate active parts from grounded ones, to dissipate heat from the core and coils to the radiators, and to quench eventual arcs [
2].
The insulating oils used in large power transformers may suffer from thermal, electrical and/or environmental stresses, even under service conditions [
1]. This will, in turn, cause and accelerate the deterioration of the insulation, posing a potential threat to the equipment safety. Aging is a phenomenon resulting in slow and irreversible changes in material properties [
3,
4,
5,
6]. The process implies the decomposition of vulnerable hydrocarbons resulting in a modification of the macromolecular structure of the liquid and solid materials. In general, transformer oil loses its stability gradually and becomes decomposed and oxidized [
3,
4,
5,
6,
7,
8,
9].
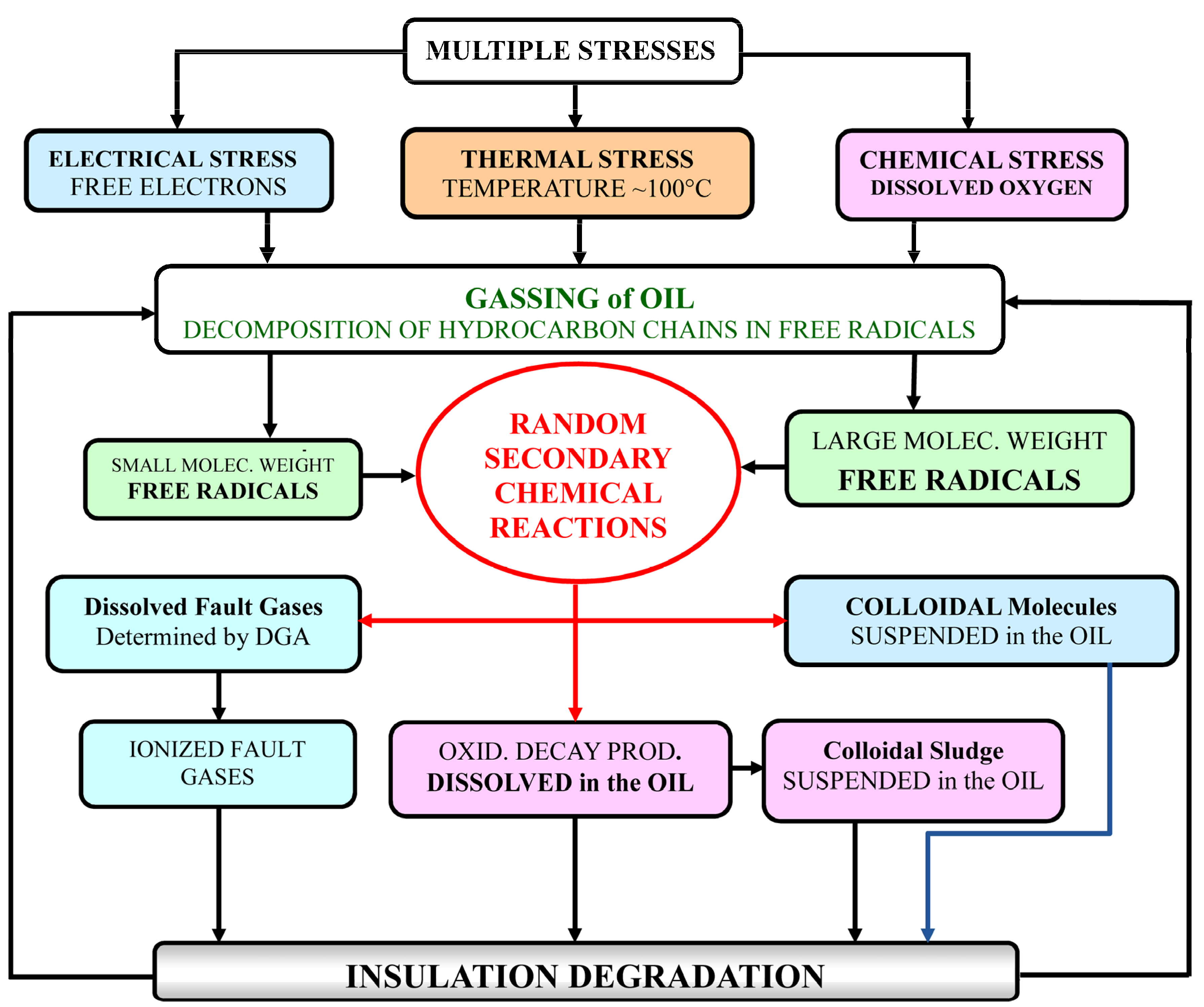
Electrical stress together with heat and moisture, in the presence of oxygen, oxidises the oil producing free radicals, acids, and sludge that are deleterious to the transformer [
6,
10,
11]. Free radicals may be generated by thermal decomposition, electrical discharge, electrolysis at an electrode, mechanical damage, chemical reactions, and high energy radiation and are thus implicated in the ageing process [
12,
13]. John at The Nineteen Symposium on Electrical Insulation underscored the paramount importance of free radicals in the physical organic chemistry of mineral insulating oils [
14]. These by-products are deleterious to the transformer and catalyze further oxidation of the oil. The electric stress accelerates ageing, possibly by increasing the precipitation of acid products of oil degradation onto paper surfaces. Chemically speaking, the acid build-up will worsen the insulating paper tensile strength. Aggressive decay products being absorbed by the solid insulation attack the cellulose fibers too. Sludge produced may stick onto the large surface of transformer boards stopping heat being dissipated which results in overheating of insulation and accelerates degradation processes. The cracking process of cellulose (depolymerisation by a succession of chemical reactions) causes chain scissions, the release of gases and water into the surrounding oil and some large molecules such as furfurals [
15]. In composite oil-impregnated dielectrics for high voltage insulation, oil is usually the weaker component of the system, both in dielectric strength and in reaction to environmental stress. Knowledge of the stability of insulating oils under electrical stress is of upmost importance to both electrical-equipment designers and operating engineers. Free radicals are very reactive and can adversely affect the chemical, physical, and dielectric properties of the insulating liquid [
12,
13]. Therefore, the detection of free radicals in oil may provide useful information on the inception of oil/paper system degradation. The method proposed allows the condition of insulating oil to be assessed through investigation of free radicals.
An attempt to develop on-line diagnostic tools and field monitoring techniques by Electron spin resonance (ESR) was explored previously [
12]. From the obtained results, the authors concluded that the ESR technique was not suitable for on-line monitoring of the condition of oil/paper insulation of high voltage equipment. In this manuscript, a sensitive spectrophotometric method is used as a quality control tool for the determination of free radical scavenging activity.
3. Experimental Procedures
3.1. Ionization Procedure
Free electrons are the primary source of energy for the breakdown of vulnerable covalent bonds (approximately 4 eV ≈ 386 kJ·mol
−1). Pioneering work by Forster [
22] describes the mechanism by which high-voltage fields interact with insulating oils. His extensive research in this field reveals that electrons escape from the conduction band of the metal conductor and are emitted from its surface, especially during very short but frequent commutation voltage surges [
23]. Lesser quantities of energy below this threshold simply will not do the job. The free electrons injected into the liquid insulation are accelerated by the electric field. The collision of a fast electron with a hydrocarbon molecule M may be either elastic or inelastic, leading to very different results:
(ii) Inelastic collision:
or
Whereas stable molecules reaching their singlet excitation level usually release the absorbed energy as a quantum of harmless fluorescent light:
Vulnerable molecules decompose and generate a pair of free radicals:
As the population of free radicals increases, some gaseous or liquid fractions may capture a free electron and form an ion:
The accumulation of such ionized molecules increases the dissipation factor of oil-paper insulation. Alternatively, large free radicals may combine, leading to the formation of an insoluble colloidal suspension.
A Merell-based test cell type, defined in the ASTM Test Method D 6180 [
24], was used (
Figure 2). The free electrons process are generated by a cylindrical copper electrode 15 mm (0.6 in) in diameter and 10 mm (0.4 in) long sealed in a 500 mL round bottomed glass flask. The electrode is placed in the center of the discharge cell and suspended above the oil. An oil volume of 50 mL was used to perform the tests.
Figure 2.
Discharge cell according to ASTM Designation D6180 [
23].
Figure 2.
Discharge cell according to ASTM Designation D6180 [
23].
3.2. Free Radical Assessment Technique
Test specimen sampled from a transformer generally exhibits little change in 24 h (chemical reactions between free radicals is possible only by contact). A test specimen which has undergone accelerated aging contains a large amount of free radicals. As a result, the probability of a reaction between them is higher than insitu conditions. Therefore the free radical measurement must be performed within a short period of time. Both types of specimens should be sealed or nitrogen blanketed to prevent reaction with oxygen. Therefore, test specimens taken from transformers shall be tested 24 h following sampling while test specimen that has undergone any accelerated aging process is to be tested within 24 h.
The T60U UV-VIS spectrophotometer (by P.G. Instruments Ltd., Lutterworth, UK), having a wavelength accuracy ±1 nm was used to assess the relative concentration of free radicals in electrical insulating oils. Microsoft-based software is provided to process the data. One pathway by which a liquid dielectric degrades results from the homolytic cleavage of some vulnerable hydrocarbon bonds under the effect of a high voltage.
The reactive free radical reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), is added to a solution whose free radical concentration is to be determined. The rate that the DPPH disappears is directly proportional to the relative free radical content at a particular instant of time. The DPPH solution has an intense blue color, which changes to yellow as unpaired electrons are coupled. This reaction is monitored spectrophotometrically. The DPPH solution has a maximum absorbance at 520 nm. The disappearance of DPPH can be determined by plotting the absorbance against time at 520 nm. The presence of free radicals in solution will increase the rate at which DPPH disappears from the background solution; the higher the free radical concentration in the test specimen, the faster DPPH disappears. The relative free radical concentration of an insulating oil test specimen is determined as follows: initially, the absorbance of the background solution of known concentration is recorded. Subsequently the decreasing absorbance of the oil specimen added to the background solution is plotted. Finally the subtraction of the oil specimen absorbance from the background solution results in a display curve of the reaction. The absorbance at 240 s/4 min from the beginning of the reaction is reported [
13].
There is a direct correlation between the disappearance of the DPPH tracer and the concentration of radicals at a certain time. The concentration of DPPH of 10
−5 mole, even at this low level, is blue-violet coloured. The formation of a new species due to DPPH reacting with the free electron of another free radical changes the colour from blue-violet to yellow, with a corresponding decrease in absorbance of the light at blue-violet wavelengths and the increase in absorbance at yellow wavelengths [
25]. The larger the quantities of radicals present in the oil, the faster the reduction of light at blue-violet wavelengths and the increase in absorbance at yellow wavelengths. The scavenging activity of DPPH can be represented by the following chemical reactions, where AH symbolizes an anti-oxidant and R a free radical:
Using the UV-VIS (ultraviolet visible) spectrophotometer to sweep from 480 to 580 nm every 60 s, the measurement of the amplitude was achieved by confirming the validity in amplitude and wavelength by using a control solution. The DPPH reference solution at a 0.01% concentration normally provides a constant absorbance of 0.500 (±0.005) at 520 nm. The absorption information can be presented also by percent transmittance as function of wavelength or wavenumber. The inverse relationship between absorbance (Abs) and transmittance (
T) is not linear, but logarithmic [
26].
3.2.1. Materials Needed
Any automated Visible Spectrophotometer capable of handling matched liquid samples using glass cuvettes of 1 cm sample path length, along with spectrograde toluene and spectrograde stable DPPH are needed. The testing procedure is done at ambient temperature 25 °C (±5 °C). Any differences due to the properties of the glass cuvettes used in the tests are compensated for using the reference solution.
The DPPH decolouration in presence of free radicals is best rationalised as resulting from an abstraction reaction between DPPH and the solvent (tri-chlorobenzene, toluene or benzene) [
27]. A 0.01% DPPH standard solution in toluene from an intermediate 0.1% DPPH stock solution is prepared. 0.1 grams of DPPH is dissolved in a 100 mL volumetric flask and filled to the mark with toluene for 0.1% DPPH stock solution. 5 mL of 0.1% DPPH solution is put into a 50 mL volumetric flask and fill to the mark with toluene for 0.01% DPPH solution.
3.2.2. Preparation of the Reference Solution
2 mL of the test specimen is added to a 10 mL volumetric flask and filled to the mark with toluene. The solvent diminishes the viscosity of oil to accelerate free radicals collision to couple their unpaired electrons.
3.2.3. Measurement of the Reference Solution
The scan region is set from 480 nm to 580 nm. A glass cuvette is filled with the reference solution and the instrument adjusted to read zero absorbance.
3.2.4. Preparation of the Background Solution
2 mL of DPPH standard solution (0.01%) is added to a 10 mL volumetric flask and filled to the mark with toluene. This allows having the same concentration as the oil solution.
3.2.5. Measurement of the Background Solution
The background solution is scanned to determine the maximum absorbance and its corresponding wavelength which should be 0.5 ± 0.005 at 520 nm. If it is not, the DPPH standard solution should be adjusted by either adding more stock solution or toluene and the method standard run again.
Using the time scan mode (480 nm to 580 nm), the absorbance of background solution is recorded at 520 nm for 10 min at one minute intervals. The values should be 0.500 (±0.005). This data is saved.
3.2.6. Preparation of the Test Specimen Solution
2 mL of DPPH (eventually adjusted) and 2 mL of the oil sample are added to a 10 mL volumetric flask.
3.2.7. Measurement of the Test Specimen
The following steps allow collection and analysis of data:
- -
Begin timing for 2.0 min when the first drop of oil contacts the DPPH solution. Fill to the mark with toluene and transfer the test specimen solution into a glass cuvette.
- -
After two minutes has elapsed from the beginning of the reaction, record and scan the spectrum at one minute intervals for at least 10 min.
- -
Subtract the test specimen spectrum from the background solution to obtain the display curve.
- -
The magnitude at 520 nm is then recorded for each measurement.
4. Results of the Investigations
The investigations were carried out on virgin/new Voltesso 35 oil, and aged/used oil collected from a power transformer that has been in service for 20 years. The measurements were done before and after exposure to electrical discharge activity in the oil for 5 h. The observed reduction in absorbance reflects the kinetic of reaction between the free radicals in the DPPH solution and in the oil test specimen. The subtraction of test specimen absorbance from background solution results in the increasing display curves shown in
Figure 3 and
Figure 4, the change in absorbance at 520 nm representing the total consumption of free radicals with time. The slope of the curve at a particular point also gives an indication of the rate of the reaction.
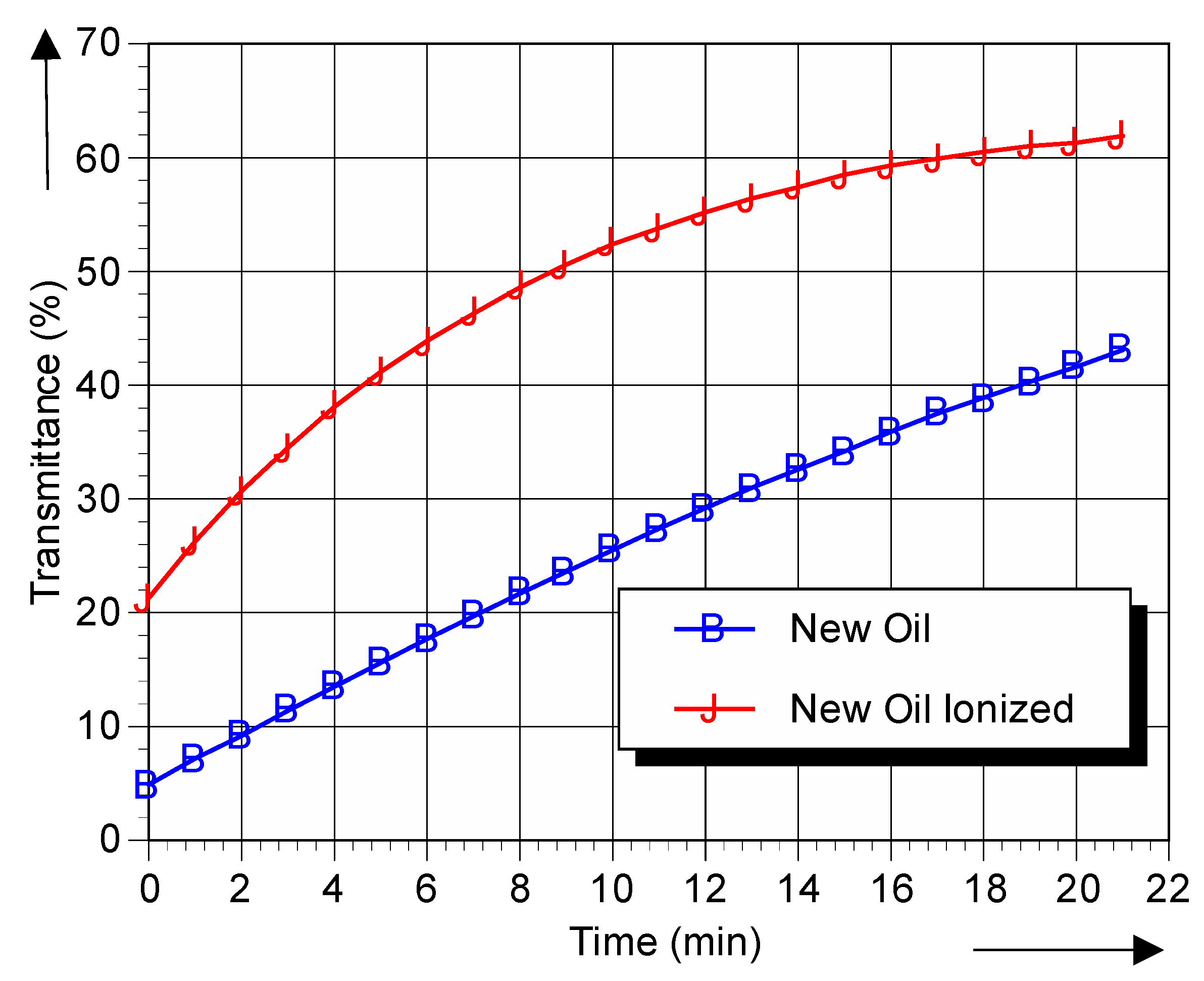
Figure 3.
Transmittance with DPPH of new oil and new oil ionized as a function of time.
Figure 3.
Transmittance with DPPH of new oil and new oil ionized as a function of time.
A comparison of the free radical reactions in new and aged oil, with no ionization and after ionization, was carried out to determine their stability under electrical discharge. The ionization procedure was performed according to ASTM D6180 [
24]. The results of the investigations are presented in
Figure 3 and
Figure 4.
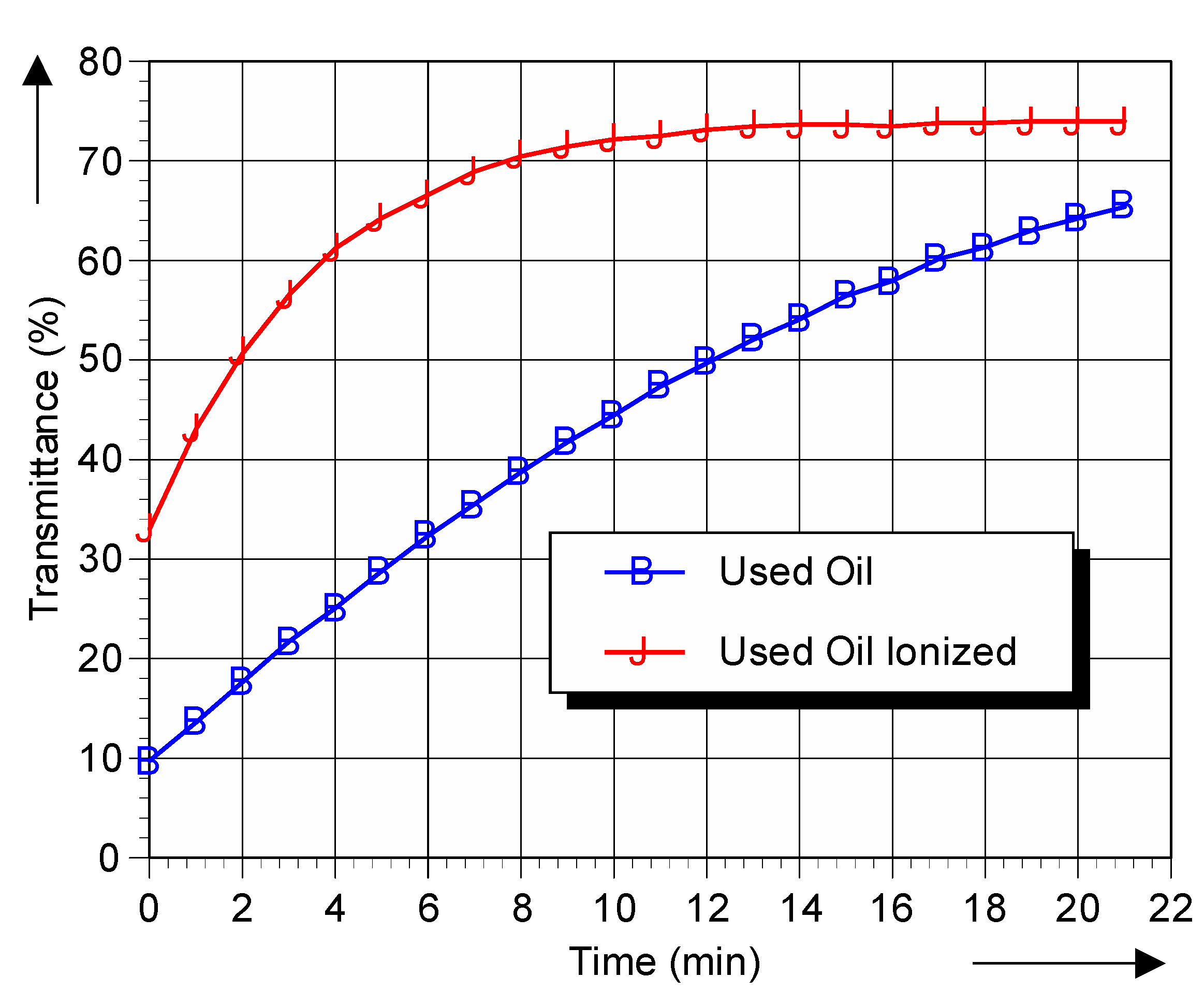
Figure 4.
Transmittance with DPPH of used oil and used oil ionized as a function of time.
Figure 4.
Transmittance with DPPH of used oil and used oil ionized as a function of time.
It can be seen that, over time, more radicals react with the DPPH. Comparison of the initial value for new and used oils demonstrates that the presence of free radicals is increased in aged oils, i.e., within 2 min more DPPH has changed from blue-violet to yellow.
The DPPH demonstrates the presence of free radicals, and can therefore serve as free radicals chemical marker in insulating fluids. The results clearly show the increase in transmittance if the oil is aged, after 10 min the transmittance has become 40% for new oil yet has become 65% for aged oil. In addition, the rate of change of the DPPH concentration is much faster in used ionized oil than in new ionized oil. These results demonstrate the applicability of the test method in determining the relative Free Radical Content of Insulating Oils of Petroleum Origin.
The quantitative relationship between the free radicals content in the test specimen and absorbance is given by reporting its value at 2 min [
13]. The measurement of the relative free radical content of the four samples, with each sample determined six times, is reported in
Table 1.
Table 1.
Data used to develop repeatability estimates.
Table 1.
Data used to develop repeatability estimates.
| Oil Condition | Percent Transmittance Values at Two Minutes after Mixing |
|---|
| New oil | 9.2 ± 0.3 |
| New oil ionized | 30.7 ± 0.4 |
| Used oil | 17.66 ± 0.4 |
| Used oil ionized | 50.65 ± 0.6 |
Repeatability
The repeatability or test-retest reliability has been evaluated based on the analysis with the same method, using the same spectrophotometer and the same oil sample (new naphthenic-based mineral oil) repeated on different days. Note that two measurements have been performed on days 8, 9, and 15 at 6 h intervals. This data is listed in
Table 2. These results suggest that the repeatability is 0.31 Absorbance units.
Table 2.
Data used to develop repeatability estimates.
Table 2.
Data used to develop repeatability estimates.
| Day Tested | Two Minutes after Mixing |
|---|
| Absorbance | Transmittance (%) |
|---|
| Day 1 | 1.40 | 3.9811 |
| Day 8 | 1.46 | 3.4674 |
| Day 8 | 1.42 | 3.8019 |
| Day 9 | 1.44 | 3.6308 |
| Day 9 | 1.38 | 4.1687 |
| Day 15 | 1.47 | 3.3884 |
| Day 15 | 1.43 | 3.7154 |
| Day 28 | 1.47 | 3.3884 |
| Day 29 | 1.47 | 3.3884 |
| Day 33 | 1.51 | 3.0903 |
5. Conclusions
In this manuscript, a laboratory testing technique is proposed to assess free radical concentration in insulating mineral oil. A reactive free radical reagent: 2,2-diphenyl-1-picrylhydrazyl (DPPH), was added to a solution whose free radical concentration is to be determined using a UV-VIS spectrophotometric method. The quantitative relationship between a standard electrical stress during a given period of time and the amount of vulnerable hydrocarbons in mineral insulating oils was addressed. All generated free radicals, large and small, are paramagnetic. In a real life conditions, these are attracted by the windings contributing to the partial discharges. Hence, these unstable molecules can be particularly harmful during a transient over voltage, when the generated gases and ionized decay products can initiate incipient electrical failure.
In addition, the theoretical premises that, incipient electrical discharges contribute to free radical generation, is experimentally confirmed under laboratory conditions.
This testing method which is applicable to new oils as well as naturally or artificially oxidized oil, may be adopted for Measurement of the relative Free Radical Content of Insulating Oils of Petroleum Origin. With this test method, the role played by free radicals in the oil decaying and gassing can be investigated.
Acknowledgments
The authors are much indebted to the anonymous reviewers for their valuable comments/suggestions which allowed improving the English expressions and technical quality of the manuscript.
Author Contributions
John Sabau designed this research and gave the whole guidance; Issouf Fofana and Amidou Betie collected all the data, carried out calculations, result display and analysis and wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deng, S.; Sakurai, A. Crude oil spot price forecasting based on multiple crude oil markets and timeframes. Energies 2014, 7, 2761–2779. [Google Scholar] [CrossRef]
- Fofana, I. 50 years in the development of insulating liquids. IEEE Electr. Insul. Mag. 2013, 29, 13–25. [Google Scholar] [CrossRef]
- Singha, S.; Asano, R.; Frimpong, G.; Claiborne, C.C.; Cherry, D. Comparative aging characteristics between a high oleic natural ester dielectric liquid and mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 149–158. [Google Scholar] [CrossRef]
- Ruijin, L.; Chao, G.; Ke, W.; Lijun, Y.; Grzybowski, S.; Huigang, S. Investigation on thermal aging characteristics of vegetable oil-paper insulation with flowing dry air. Insul. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1649–1658. [Google Scholar]
- Zmarzły, D.; Dobry, D. Analysis of properties of aged mineral oil doped with C60 fullerenes. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1119–1126. [Google Scholar] [CrossRef]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Hadjadj, Y.; Meghnefi, F.; Fofana, I.; Ezzaidi, H. On the feasibility of using poles computed from frequency domain spectroscopy to assess oil impregnated paper insulation conditions. Energies 2013, 6, 2204–2220. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, S.; Grzybowski, S. Reliability evaluation method for oil-paper insulation in power transformers. Energies 2011, 4, 1362–1375. [Google Scholar] [CrossRef]
- Asano, R.; Page, S.A. Reducing environmental impact and improving safety and performance of power transformers with natural ester dielectric insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 50, 134–141. [Google Scholar] [CrossRef]
- Ruijin, L.; Yuandi, L.; Pei, G.; Haibin, L.; Huanhuan, X. Thermal aging effects on the moisture equilibrium curves of mineral and mixed oil-paper insulation systems. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 842–850. [Google Scholar]
- Wada, J.; Ueta, G.; Okabe, S.; Amimoto, T. Techniques to inhibit transformer insulating oil degradation—Effectiveness evaluation of the removal of degradation products using adsorbents. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2307–2316. [Google Scholar] [CrossRef]
- Abdolall, K. Feasibility of free radical detection for condition assessment of oil/paper insulation of transformers. In Proceedings of the Conference Record of the 2008 IEEE International Symposium on Electrical Insulation (ISEI), Vancouver, BC, Canada, 9–12 June 2008; pp. 182–186.
- Pryor, W.A. Introduction to Free Radical Chemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1966. [Google Scholar]
- Tanaka, J. Free radicals in electrical insulation. In Proceedings of the 19th Symposium of Electrical Insulating Materials, Osaka, Japan, 13–15 June 1986.
- Lundgaard, L.E. Ageing of Cellulose in mineral-oil insulated transformers. CIGRE 323, 45–63.
- Fofana, I.; Sabau, J. Application of petroleum-based oil in power transformers. In Natural Gas Research Progress; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2008; Chapter 6. [Google Scholar]
- Fofana, I.; Borsi, H.; Gockenbach, E.; Farzaneh, M. Aging of transformer insulating materials under selective conditions. Eur. Trans. Electr. Power 2007, 17, 450–470. [Google Scholar] [CrossRef]
- Ferguson, R.; Lobeiras, A.; Sabau, J. Suspended particles in the liquid insulation of aging power transformers. Electr. Insul. Mag. 2002, 18, 17–23. [Google Scholar] [CrossRef]
- Wang, W.; Yue, C.; Gu, J.; Du, J.; Li, F.; Yang, K. Status assessment of polymeric materials in mineral oil under electro-thermal aging by frequency-domain dielectric spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 831–841. [Google Scholar] [CrossRef]
- Mineral Insulating Oils—Oxidation Stability Test Method Based on Differential Scanning Calorimetry (DSC); PD IEC/TR 62036; British Standards Institution (BSI): London, UK, 2007.
- Sabau, J.; Fofana, I.; Bouaicha, A.; Hadjadj, Y.; Farzaneh, M. An environmentally friendly dissolved oxygen and moisture removal system for freely breathing transformers. IEEE Electr. Insul. Mag. 2010, 26, 30–38. [Google Scholar] [CrossRef]
- Forster, E.O. Progress in the field of electric properties of dielectric liquids. IEEE Trans. Electr. Insul. 1990, 25, 45–53. [Google Scholar] [CrossRef]
- Armanini, D.; Bosotti, O.; Centenari, A.; Ghirelli, A.; Romani, A.; Vallini, F. Aging and deterioration of HV current transformer insulation by very fast transient over voltages. L’Energ. Electr. 1989, 5, 251–256. [Google Scholar]
- Standard Test Method for Stability of Insulating Oils of Petroleum Origin under Electrical Discharge; ASTM D6180-05; ASTM International: West Conshohocken, PA, USA, 2005.
- Sabau, J.; Stokhuyzen, R. The electrochemical stability of mineral insulating oils. In Electrical Insulating Materials: International Issues; ASTM STP 1376; Hirschler, M.M., Ed.; American Society of Testing and Materials: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Settle, F.A. Handbook of Instrumental Techniques for Analytical Chemistry; Prentice Hall: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Lorimer, J.P.; Kershawt, D.; Mason, T.J. Investigation of the consumption of diphenylpicrylhydrazyl in solution in the absence and presence of ultrasound. J. Chem. Soc. Faraday Trans. 1995, 91, 1067–1074. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).