The improved Hydrogen Storage Performances of the Multi-Component Composite: 2Mg(NH2)2–3LiH–LiBH4
Abstract
:1. Introduction
2. Results and Discussion
2.1. The hydrogen Desorption Properties of 2Mg(NH2)2–3LiH–LiBH4 Composite
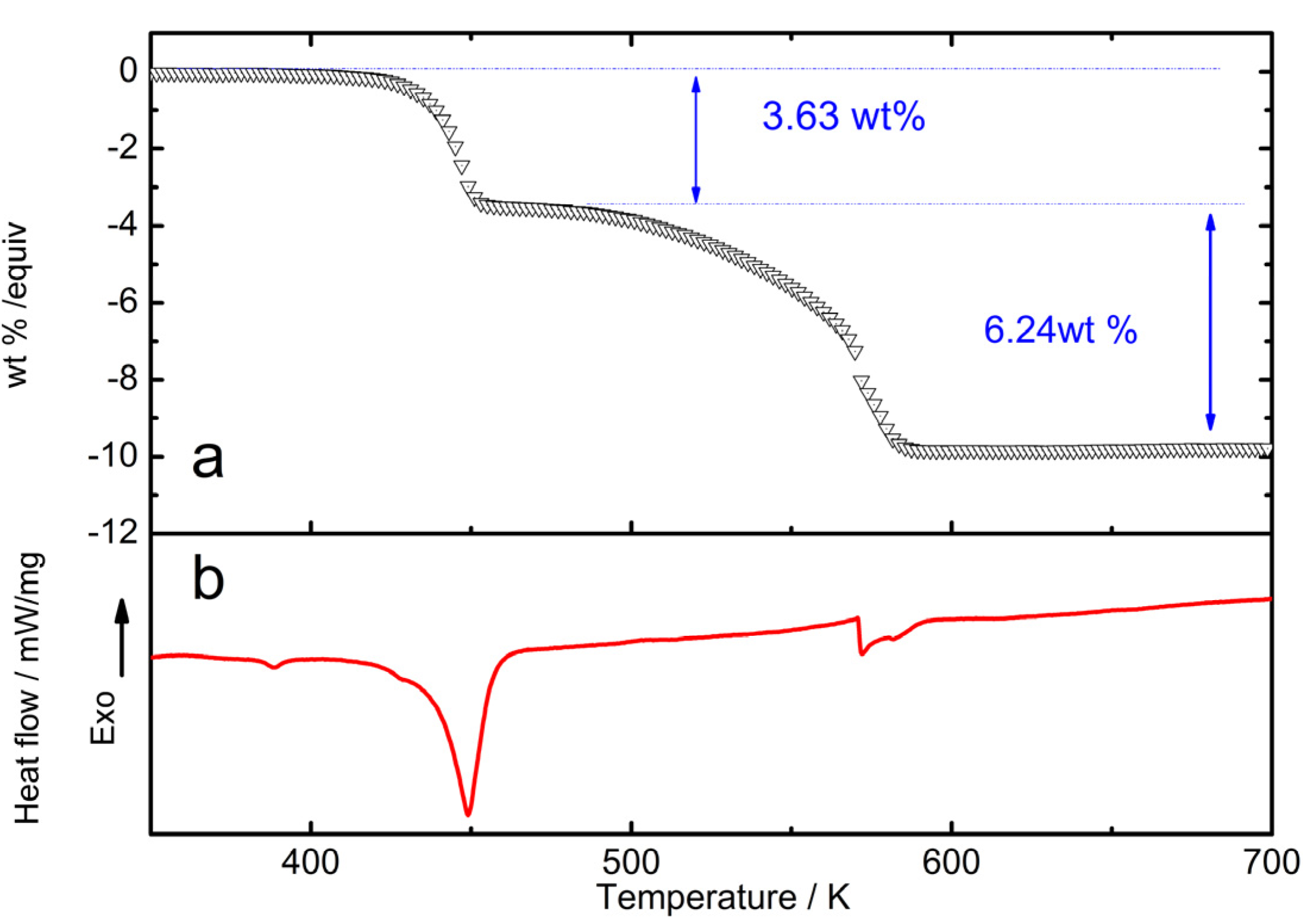
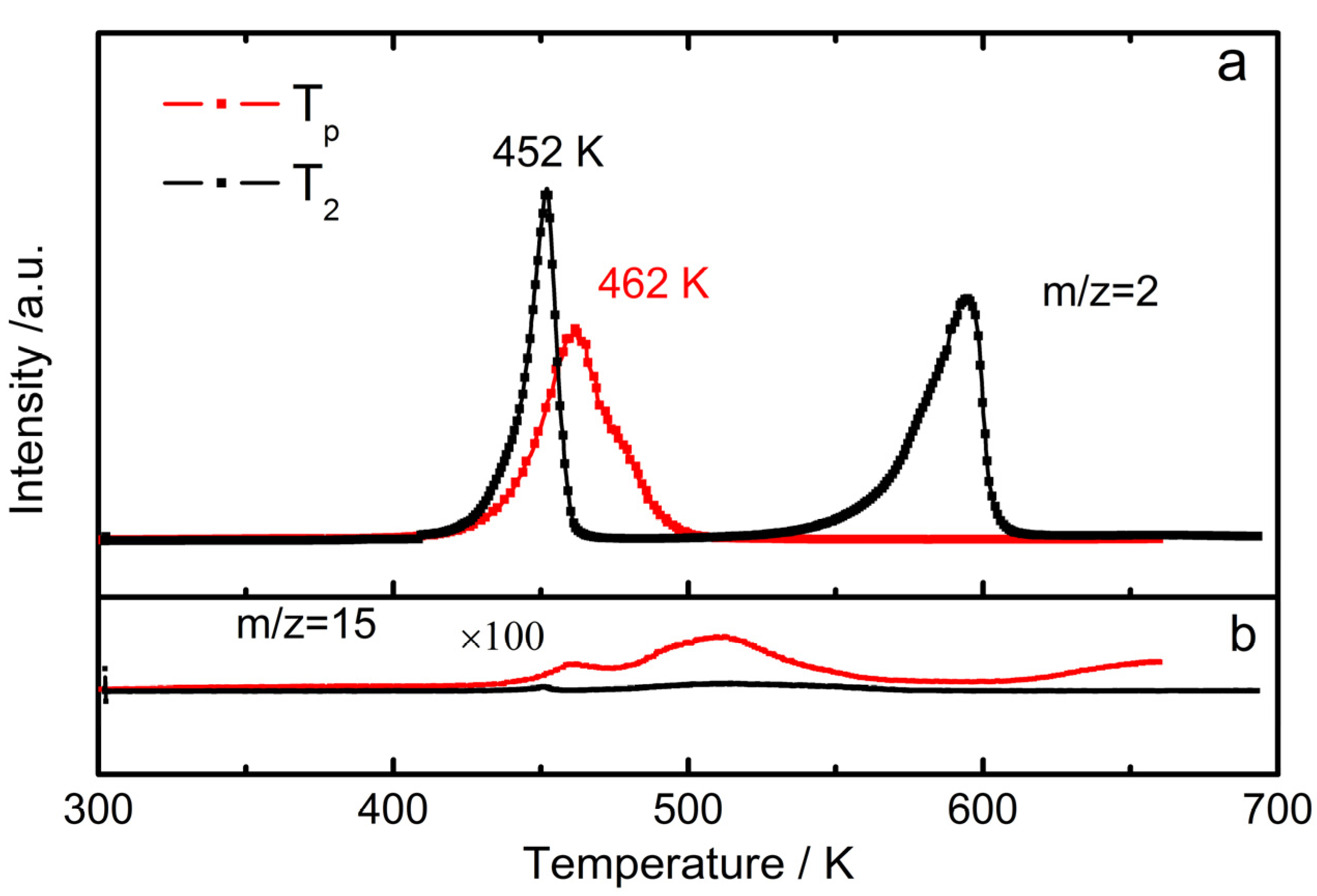
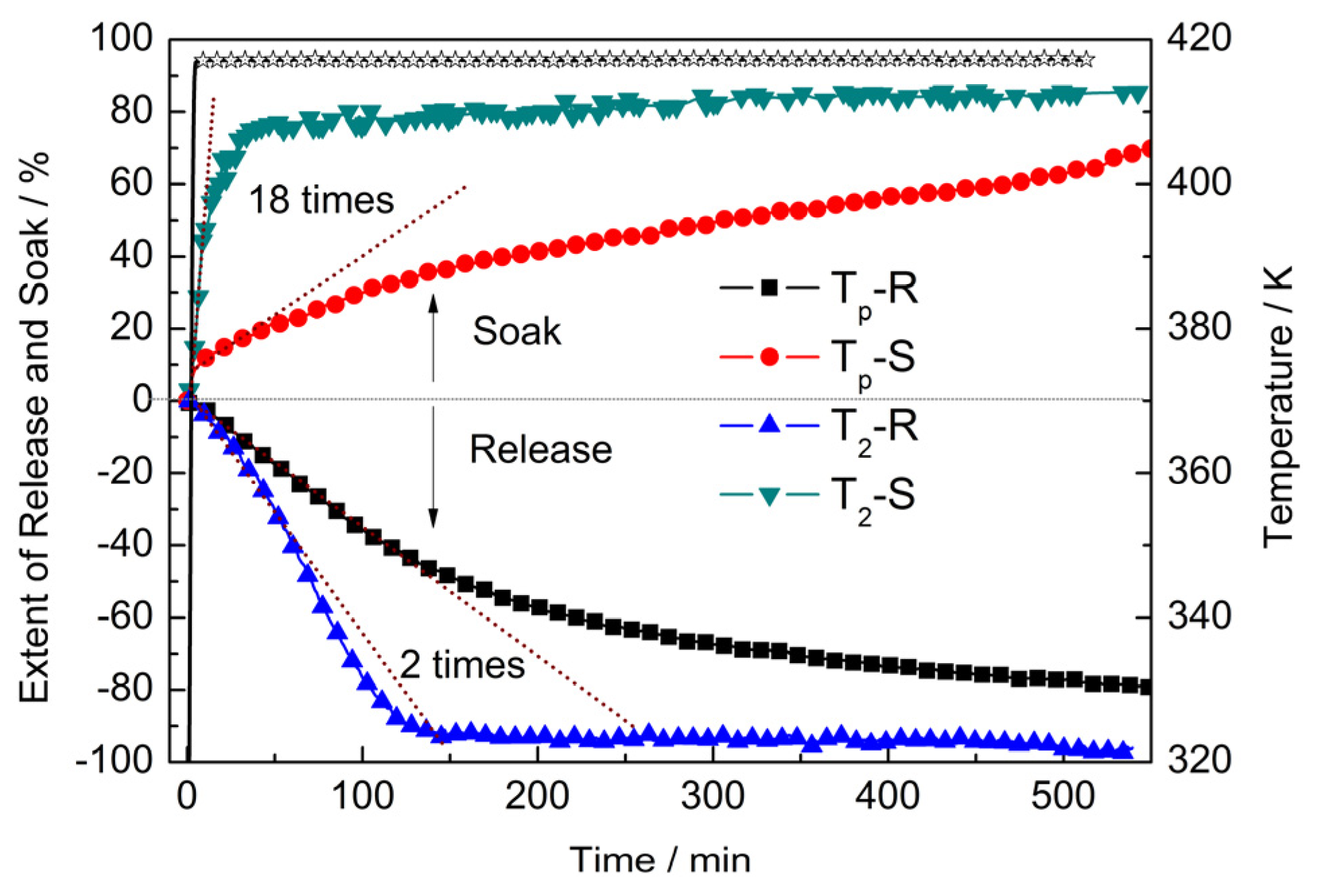

2.2. Pressure–Composition–Isotherm (PCI) Dehydrogenation at High Pressure
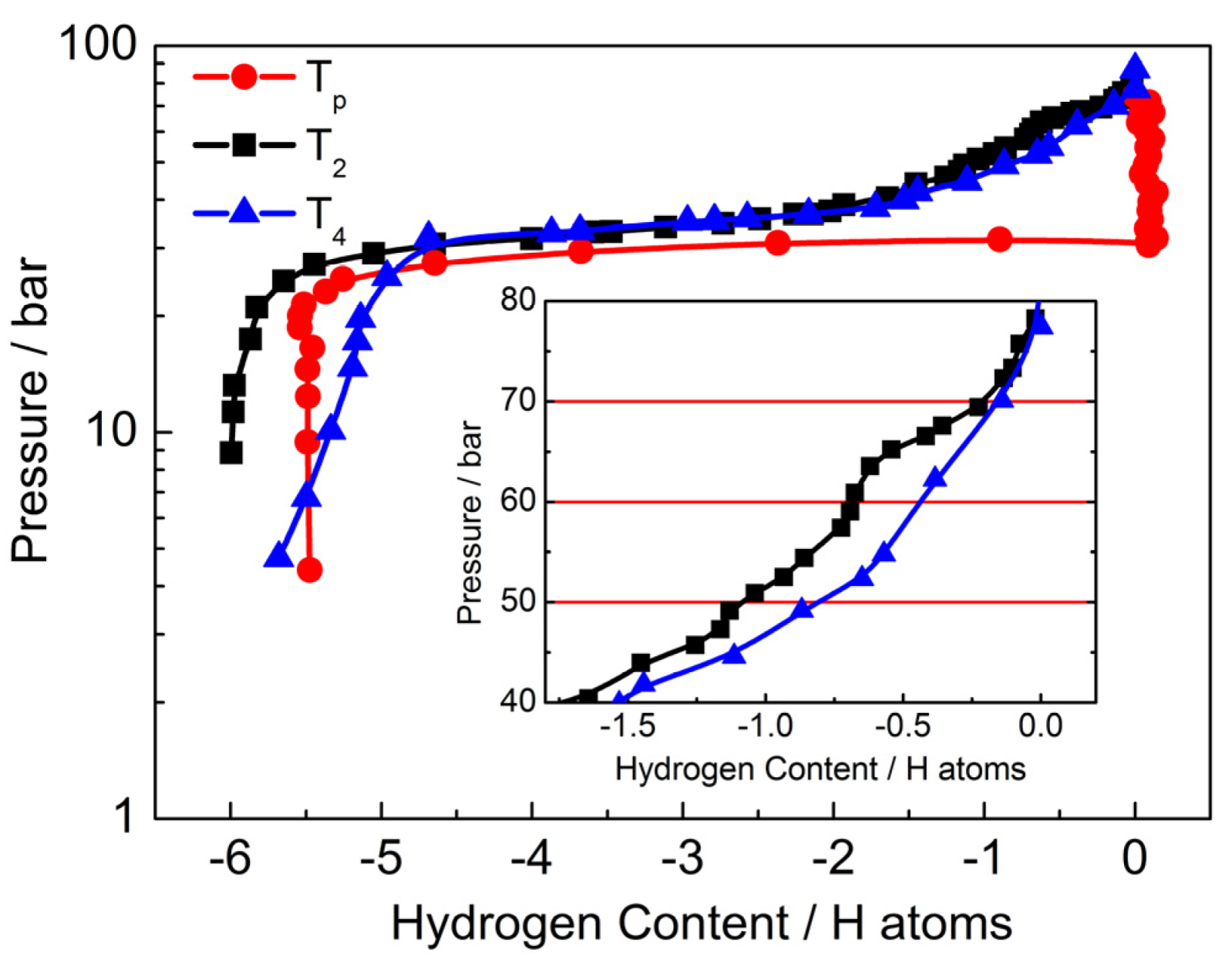

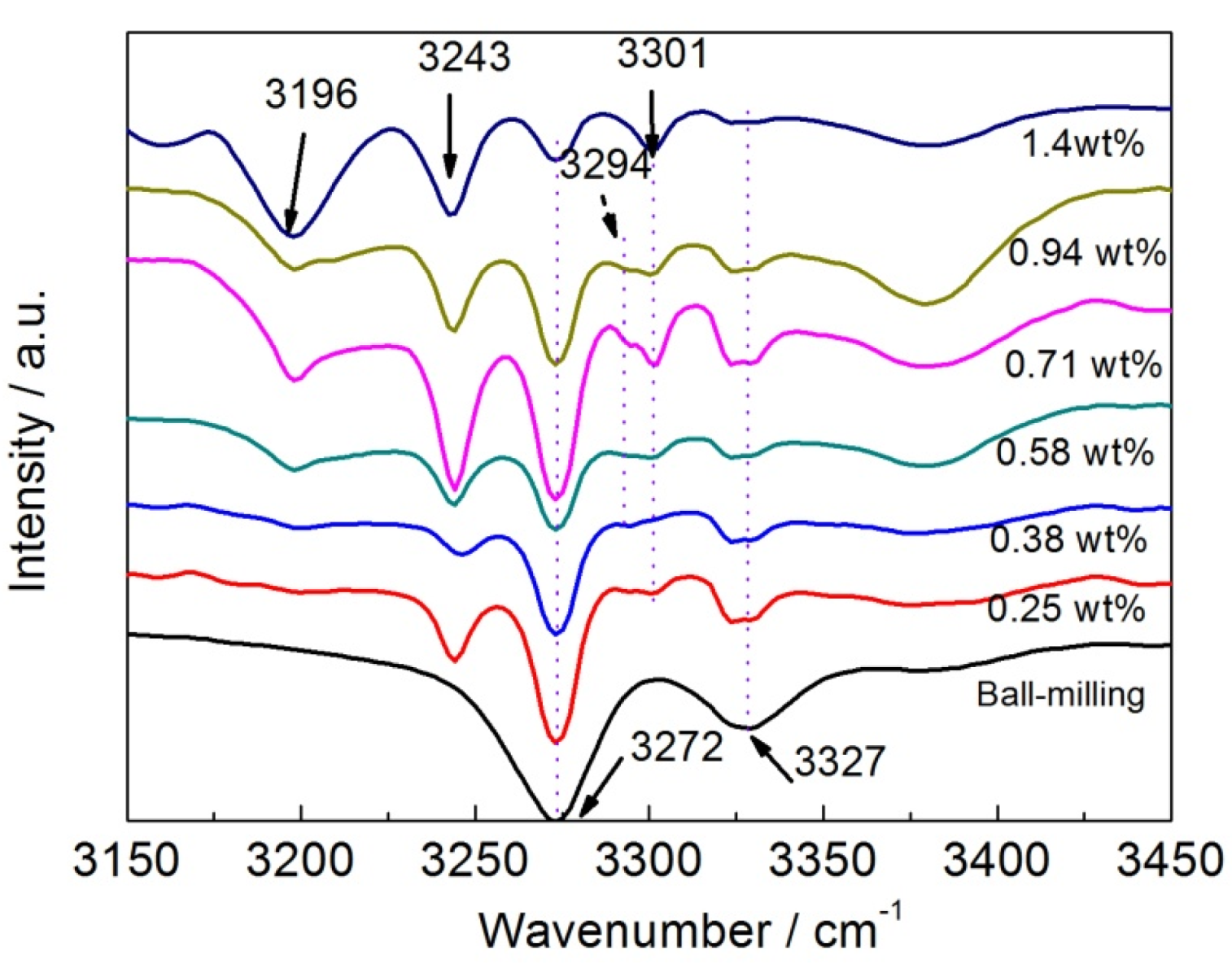
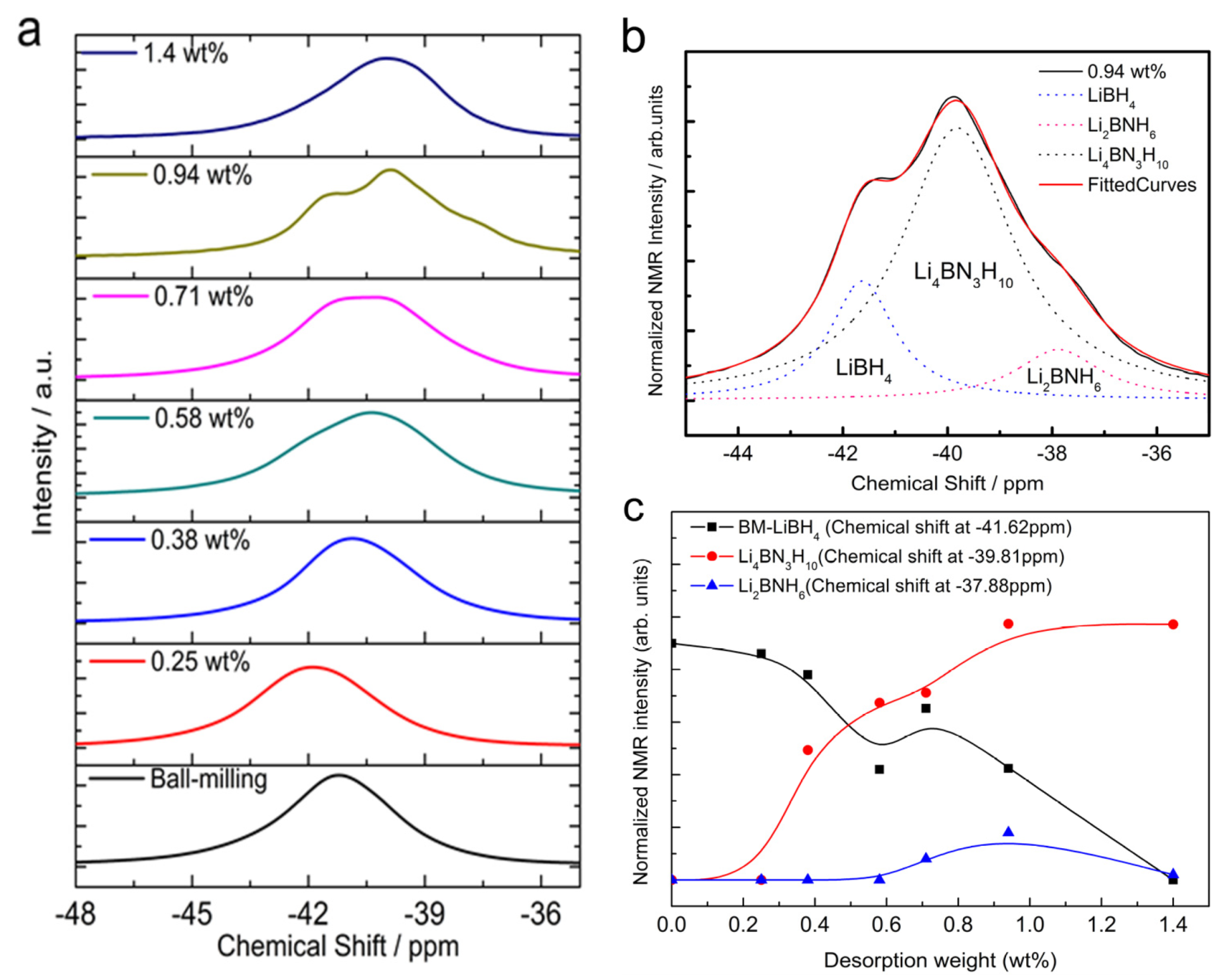
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhu, M. Recent progress in hydrogen storage. Mater. Today 2008, 11, 36–43. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 1997, 253, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible hydrogen storage via titanium-catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. B 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O. Magnesium alanate—A material for reversible hydrogen storage. J. Alloy. Compd. 2003, 356, 418–422. [Google Scholar] [CrossRef]
- Chlopek, K.; Frommen, C.; Leon, A.; Zabara, O.; Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.; Zuttel, A.; Orimo, S. Thermodynamical stability of calcium borohydride Ca(BH4)2. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Zuttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloy. Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z. Metal–N–H systems for the hydrogen storage. Scr. Mater. 2007, 56, 817–822. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.T.; Luo, J.Z.; Lin, J.Y.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Luo, W. (LiNH2–MgH2): A viable hydrogen storage system. J. Alloy. Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Orimo, S. Synthesis and dehydriding studies of Mg–N–H systems. J. Power Sources 2004, 138, 309–312. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.J.; Chen, P. Ternary imides for hydrogen storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Wu, H. Structure of ternary imide Li2Ca(NH)2 and hydrogen storage mechanisms in amide–hydride system. J. Am. Chem. Soc. 2008, 130, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wu, G.; Hu, J.; Liu, Y.; Chen, P.; Luo, W.; Wang, J. Reversible hydrogen storage by a Li–Al–N–H complex. Adv. Funct. Mater. 2007, 17, 1137–1142. [Google Scholar] [CrossRef]
- Hino, S.; Ichikawa, T.; Leng, H.Y.; Fujii, H. Hydrogen desorption properties of the Ca–N–H system. J. Alloy. Compd. 2005, 398, 62–66. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Wu, G.; Xiong, Z.; Chen, P. Structural and compositional changes during hydrogenation/dehydrogenation of the Li–Mg–N–H system. J. Phys. Chem. C 2007, 111, 18439–18443. [Google Scholar] [CrossRef]
- Leng, H.; Ichikawa, T.; Fujii, H. Hydrogen storage properties of Li–Mg–N–H systems with different ratios of LiH/Mg(NH2)2. J. Phys. Chem. B 2006, 110, 12964–12968. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, J.; Stewart, K.; Clift, M.; Gross, K. Li–Mg–N–H: Recent investigations and development. J. Alloy. Compd. 2007, 446, 336–341. [Google Scholar] [CrossRef]
- Xiong, Z.T.; Wu, G.T.; Hu, J.J.; Chen, P.; Luo, W.F.; Wang, J. Investigations on hydrogen storage over Li–Mg–N–H complex—The effect of compositional changes. J. Alloy. Compd. 2006, 417, 190–194. [Google Scholar] [CrossRef]
- Chen, X.Y.; Guo, Y.H.; Yu, X.B. Enhanced dehydrogenation properties of modified Mg(NH2)2–LiBH4 composites. J. Phys. Chem. C 2010, 114, 17947–17953. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Towata, S.; Ninomiya, A.; Nakamori, Y.; Orimo, S. Crystal structure analysis of novel complex hydrides formed by the combination of LiBH4 and LiNH2. Appl. Phys. A 2006, 83, 277–279. [Google Scholar] [CrossRef]
- Weidner, E.; Dolci, F.; Hu, J.J.; Lohstroh, W.; Hansen, T.; Bull, D.J.; Fichtner, M. Hydrogenation reaction pathway in Li2Mg(NH)2. J. Phys. Chem. C 2009, 113, 15772–15777. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.F.; Gu, Y.J.; Gao, M.X.; Pan, H.G. Improved hydrogen-storage thermodynamics and kinetics for an RbF-doped Mg(NH2)2–2LiH system. Chem.Asian J. 2013, 8, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, Y.F.; Gao, M.X.; Pan, H.G. Understanding the role of K in the significantly improved hydrogen storage properties of a KOH-doped Li–Mg–N–H system. J. Mater. Chem. A 2013, 1, 5031–5036. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, C.; Li, B.; Gao, M.X.; Pan, H.G. Metathesis reaction-induced significant improvement in hydrogen storage properties of the KF-added Mg(NH2)2–2LiH system. J. Phys. Chem. C 2013, 117, 866–875. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, T.; Wu, G.T.; Li, W.; Liu, Y.F.; Araujo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z.T.; et al. Potassium-modified Mg(NH2)2/2 LiH system for hydrogen storage. Angew. Chem. Int. Ed. 2009, 48, 5828–5832. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Fichtner, M.; Chen, P. Investigation on the properties of the mixture consisting of Mg(NH2)2, LiH, and LiBH4 as a hydrogen storage material. Chem. Mater. 2008, 20, 7089–7094. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, Y.F.; Wu, G.T.; Xiong, Z.T.; Chua, Y.S.; Chen, P. Improvement of hydrogen storage properties of the Li–Mg–N–H system by addition of LiBH4. Chem. Mater. 2008, 20, 4398–4402. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Rubidium hydride: An exceptional dehydrogenation catalyst for the lithium amide/magnesium hydride system. J. Phys. Chem. C 2013, 117, 6554–6560. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Siegel, D.J.; Halliday, D.; Drews, A.; Carter, R.O., 3rd; Wolverton, C.; Lewis, G.J.; Sachtler, J.W.; Low, J.J.; et al. A self-catalyzing hydrogen-storage material. Angew. Chem. Int. Ed. 2008, 47, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.F.; Gu, J.; Gao, M.X.; Pan, H.G. Synergetic effects of in situ formed CaH2 and LiBH4 on hydrogen storage properties of the Li–Mg–N–H system. Chem. Asian J. 2013, 8, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.F.; Gu, J.; Gu, Y.J.; Gao, M.X.; Pan, H.G. Mechanistic investigations on significantly improved hydrogen storage performance of the Ca(BH4)2-added 2LiNH2/MgH2 system. Int. J. Hydrog. Energy 2013, 38, 5030–5038. [Google Scholar] [CrossRef]
- Pan, H.G.; Shi, S.B.; Liu, Y.F.; Li, B.; Yang, Y.J.; Gao, M.X. Improved hydrogen storage kinetics of the Li–Mg–N–H system by addition of Mg(BH4)2. Dalton Trans. 2013, 42, 3802–3811. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.F.; Li, C.; Gao, M.X.; Pan, H.G. In situ formation of lithium fast-ion conductors and improved hydrogen desorption properties of the LiNH2–MgH2 system with the addition of lithium halides. J. Mater. Chem. A 2014, 2, 3155–3162. [Google Scholar] [CrossRef]
- Cao, H.J.; Wu, G.T.; Zhang, Y.; Xiong, Z.T.; Qiu, J.S.; Chen, P. Effective thermodynamic alteration to Mg(NH2)2–LiH system: Achieving near ambient-temperature hydrogen storage. J. Mater. Chem. A 2014, 2, 15816–15822. [Google Scholar] [CrossRef]
- Chater, P.A.; David, W.I.F.; Anderson, P.A. Synthesis and structure of the new complex hydride Li2BH4NH2. Chem. Commun. 2007, 45, 4770–4772. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, W.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Structures and crystal chemistry of Li2BNH6 and Li4BN3H10. Chem. Mater. 2008, 20, 1245–1247. [Google Scholar] [CrossRef]
- Borgschulte, A.; Jones, M.O.; Callini, E.; Probst, B.; Kato, S.; Zuttel, A.; David, W.I.F.; Orimo, S. Surface and bulk reactions in borohydrides and amides. Energy Environ. Sci. 2012, 5, 6823–6832. [Google Scholar] [CrossRef]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H.; et al. Complex hydrides with (BH4)− and (NH2)− anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cao, H.; Wu, G.; He, T.; Chen, P. The improved Hydrogen Storage Performances of the Multi-Component Composite: 2Mg(NH2)2–3LiH–LiBH4. Energies 2015, 8, 6898-6909. https://doi.org/10.3390/en8076898
Wang H, Cao H, Wu G, He T, Chen P. The improved Hydrogen Storage Performances of the Multi-Component Composite: 2Mg(NH2)2–3LiH–LiBH4. Energies. 2015; 8(7):6898-6909. https://doi.org/10.3390/en8076898
Chicago/Turabian StyleWang, Han, Hujun Cao, Guotao Wu, Teng He, and Ping Chen. 2015. "The improved Hydrogen Storage Performances of the Multi-Component Composite: 2Mg(NH2)2–3LiH–LiBH4" Energies 8, no. 7: 6898-6909. https://doi.org/10.3390/en8076898




