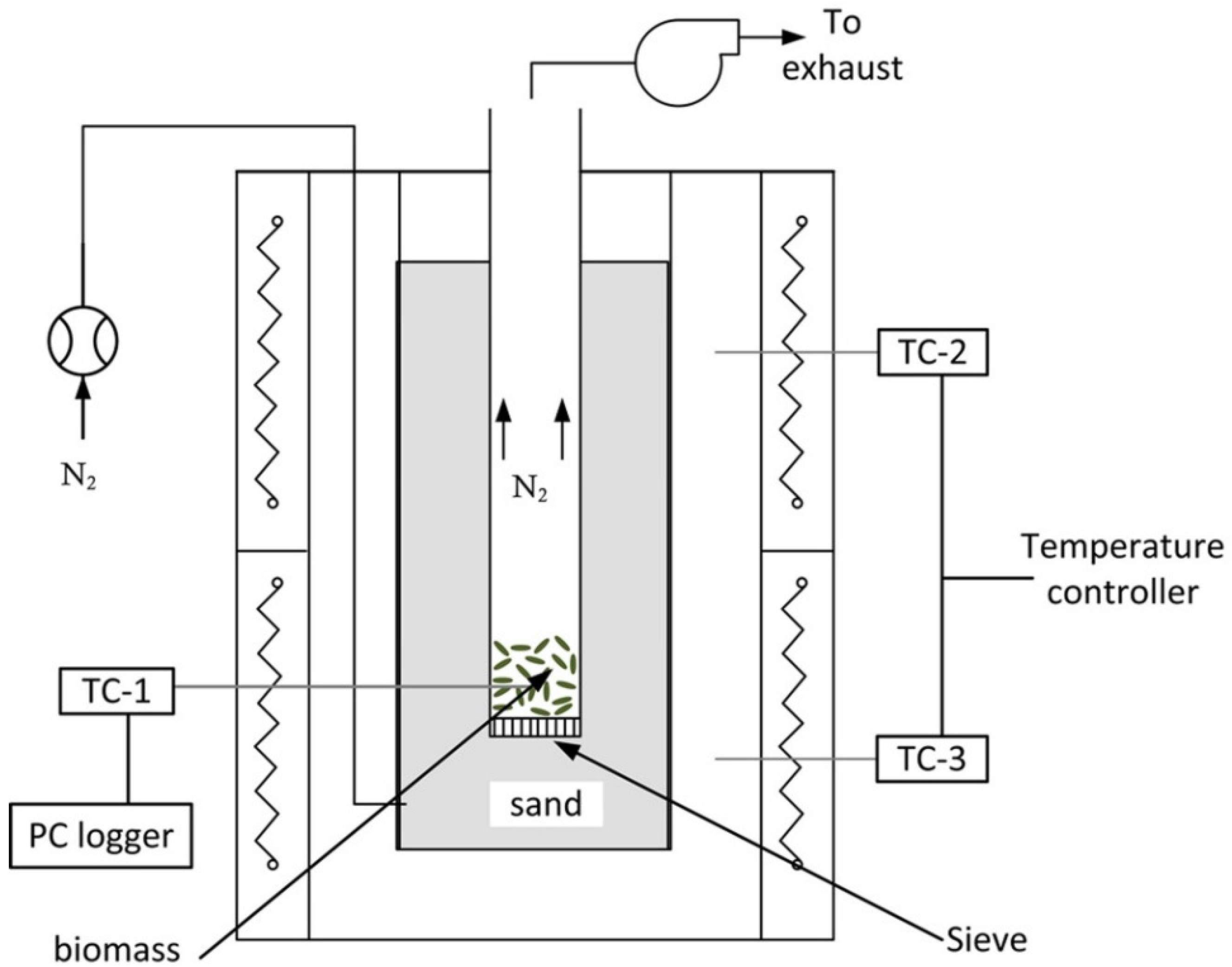
1. Introduction
Because of the environmental impacts of conventional energy sources, the world is moving to green energy options. Recently, the Dutch government set a goal to replace fossil fuels with renewable resources by 16% in 2020 and 100% in 2050. Among the renewable resources, biomass is a CO
2 neutral fuel for large-scale electricity production. However, the wide utilization of biomass is restricted due to its high moisture content, low calorific value, hygroscopic nature, low energy density and low combustion efficiency as a result of its larger particle size coupled with its high volatile matter content [
1]. Thermochemical pre-treatment of biomass via torrefaction is a method of modifying the properties of biomass to more closely resemble those of coal.
Torrefaction is a roasting of biomass in the absence of oxygen at a temperature of 220–300 °C. During the torrefaction process, the biomass partially decomposes with the release of some volatiles resulting in a uniform solid product [
2,
3]. This solid product has reduced moisture content and higher energy density compared to the originating biomass. The decomposition also destroys the fibrous structure of the biomass, which reduces the energy required for grinding. Furthermore, torrefied biomass has a more hydrophobic nature [
4] thereby reducing the costs of logistics and storage of the product. All of the above-mentioned properties make the torrefied product more coal-like and suitable for residential heating, densified biomass pellets, gasification as well as for co-firing with coal in power plants.
Torrefied biomass can be effectively utilized to increase the co-firing ratio of biomass in coal fired boilers, along with domestic heating and other commercial applications. Therefore, much research and development is currently being carried out to gain more insight into the fundamentals, technology and economics of the torrefaction process. However, more comprehensive studies are required to understand the influence of the key process parameters on the quality of the torrefied solid product specially on large scale. These kinds of studies will help to optimize the process parameters and to improve the properties of biomass for co-firing applications in coal-fired power plants.
As reported by earlier studies, the torrefaction solid product composition is influenced by many parameters, such as the biomass cell wall composition, the process parameters and particle size. Prins
et al. [
5] studied the product distribution of larch, willow and straw in standard thermogravimetric analyzer (TGA) at different torrefaction temperatures and reaction times. They reported that the cell wall composition plays a vital role in the product distribution during torrefaction next to the torrefaction process parameters. Pimehuai
et al. [
1] studied the torrefaction of sawdust, peanut husks, bagasse and water hyacinth. They reported that the temperature is the key parameter for the torrefaction process. They also noted a significant increase in energy density and hydrophobicity of these torrefied biomasses with torrefaction temperature. With respect to the physical properties of the torrefied solid product, studies by Phanphanich
et al. [
6] and Ohliger
et al. [
7] showed that grindability properties significantly depends on degree of torrefaction.
Likewise, Tumuluru
et al. [
8] focused their study solely on the influence of torrefaction on chemical compositions of
miscanthus (
giganteus) and white oak saw dust and found that a considerable loss in hydrogen content and H/C ratio occurs above 270 °C. Saddawi
et al. [
9], on the other hand, studied the possibility of combining pretreatment techniques (leaching) and torrefaction to create an improved fuel from biomass. They selected four types of biomass for their study,
miscanthus (
giganteus), short-rotation coppiced willow, eucalyptus, and wheat straw. They reported that water washing prior to torrefaction significantly reduced the ash content of all of the fuels, particularly for the herbaceous biomass,
miscanthus (
giganteus) and wheat straw. According to Hodgson
et al. [
10], miscanthus genotypes other than the current commercially cultivated
miscanthus (
giganteus) also have great potential for use in energy conversion processes. So far, no study has been published on the torrefaction behavior of
miscanthus (
sinensis).
There are few studies available that have compared woody and grassy biomass torrefaction [
2,
5,
11], but only mass/energy yields, elemental composition or individual property like grindability were discussed. In the present study, a wide range of experimental analysis techniques were used to be able to assess the quality of the torrefied beech wood and
miscanthus (
sinensis) for combustion applications. The influence of torrefaction temperature and residence time on the chemical composition, physical property (grindability) and combustion characteristics (ignition temperature) of beech wood and miscanthus are addressed
. 3. Results and Discussion
From the outset it has been made clear that the aim of the present study is to assess the influence of torrefaction process parameters on chemical, physical and combustion characteristics of torrefied biomass. Beech wood and miscanthus were studied and the results are presented and discussed in subsequent sections. For miscanthus, a residence time of 30 min was selected based on our study of beech wood torrefaction. Initially the series of beech wood torrefaction experiment was carried out for all ranges of torrefaction temperature and residence times. From the torrefied beech wood mass yield graph we observe that for 15 min residence time, the change in mass yield from temp. range 240 °C to 300 °C remained in a rather narrow range. On the other hand, for residence time of 90 min, quite significant mass loss was observed. Based on this observation, we found that 30 min can be considered the optimum residence time for miscanthus torrefaction.
Figure 3.
Photograph of beech wood and miscanthus particles and their torrefied products at different torrefaction temperatures and residence times.
Figure 3.
Photograph of beech wood and miscanthus particles and their torrefied products at different torrefaction temperatures and residence times.
3.1. Visual Observation and SEM Pictures
3.1.1. Visual Observation
The physical appearance of raw biomass and their torrefied products can be seen in
Figure 3. The first three columns show the raw and torrefied beech wood at four different torrefaction temperatures and residence times of 15 min, 30 min and 150 min. The photos for a residence time of 90 min are not shown as they are almost the same as the ones for 150 min. The last column presents the colour variation of raw and torrefied miscanthus at four torrefaction temperatures and a residence time of 30 min. It is evident that, irrespective of the biomass type, the colour of the torrefied product changes from light brown to dark brown and to black as the temperature and residence time increases. In the case of beech wood, it is observed that the colour change with increasing temperature is more prominent than with residence time, because of the exothermic nature of the reaction above 280 °C. For torrefaction temperature >280 °C, an exothermal reaction takes place results in hemicellouse decomposition of the biomass [
11]. As the process becomes exothermic, released heat increases the volatile releasing rate which includes carbon dioxide, carbon monoxide, large amount of acetic acid and other heavier products of organic molecules. The increased amount of volatiles release facilitated the removal of oxygenated and hydrogenated compounds leaving the solid torrefied product more concentrated in fixed carbon. In general, fuels with high fixed carbon tend to be more black in colour. Specifically, under severe torrefaction conditions (300 °C), the beech wood looks like charcoal. On the other hand, miscanthus shows a mild colour change with temperature, although the mass loss is higher compared to beech wood.
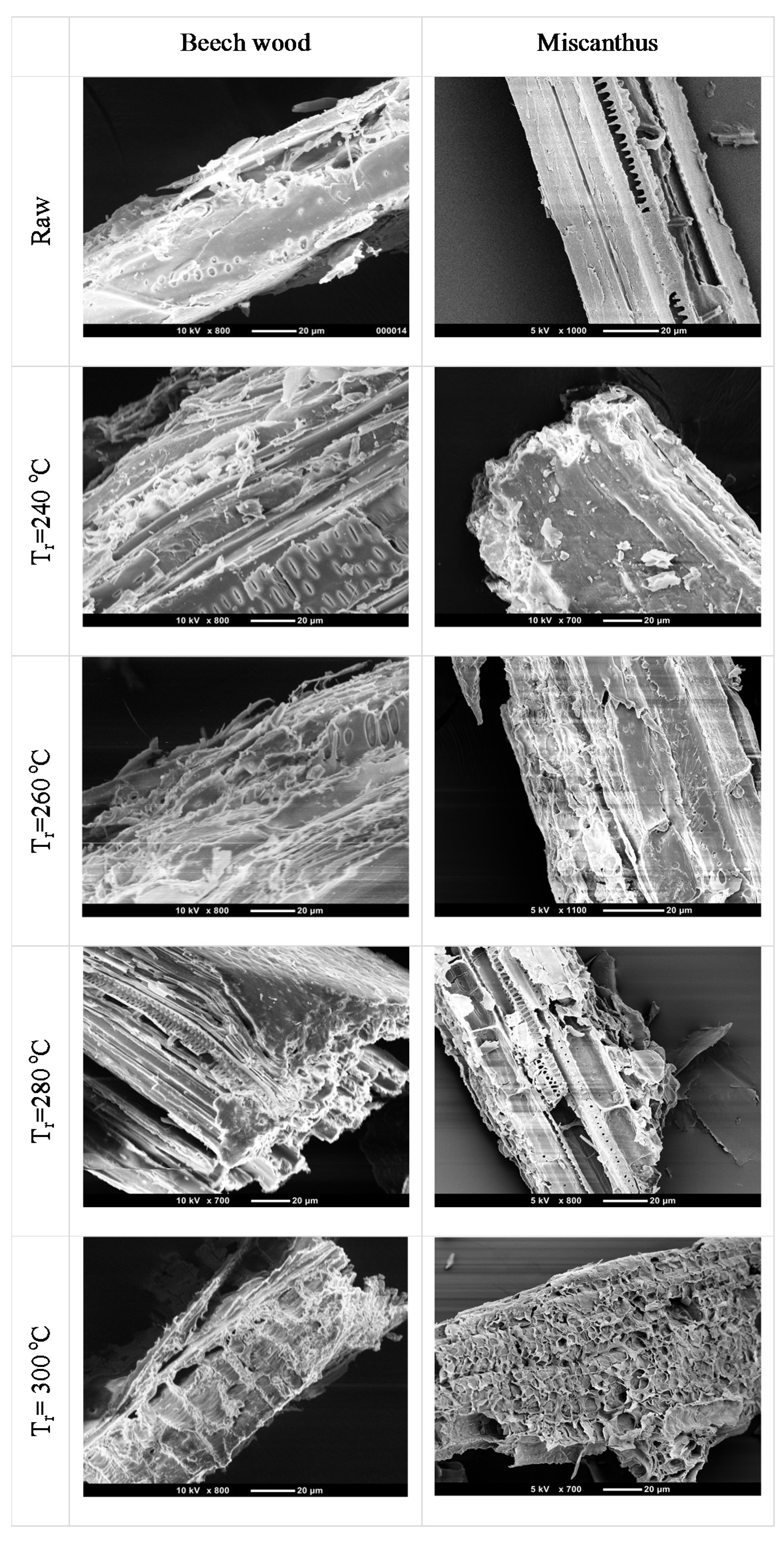
3.1.2. Scanning Electron Microscope (SEM) Pictures
Images of beech wood, miscanthus and their corresponding torrefied products at various temperatures, but at one residence time (30 min), were considered to understand the effects of torrefaction temperature.
As shown in
Figure 4, for torrefaction temperature 240 °C, the deformation of both biomass samples surfaces starts to become visible, especially for beechwood with the formation of tiny holes on the surface. On the other hand, for higher torrefaction temperatures 260 °C and 280 °C, the surface for both miscanthus and beech wood is characterized by high porosity and tubular-shape structure. The tubular-shape structure could be formed due to the decomposition of lignin material also reported by [
20,
21]. Lignin works as cementing material and considered as a ‘glue’ to bind adjacent plant cells [
22]. This might be the reason why, after torrefaction, the biomass becomes less fibrous and easily grindable with less energy consumption. Moreover, it can also be noted that, at 300 °C, the cellulose material starts to decompose and so the deformation of the cell structure can be clearly observed for both beech wood and miscanthus.
Figure 4.
SEM images of raw and torrefied beech wood (first column), raw and torrefied miscanthus (second column) at various temperatures and a residence time of 30 min.
Figure 4.
SEM images of raw and torrefied beech wood (first column), raw and torrefied miscanthus (second column) at various temperatures and a residence time of 30 min.
3.2. Proximate and Ultimate Analysis
3.2.1. Effect of Torrefaction Parameters on Proximate Analysis
Figure 5a illustrates the influence of the temperature and residence time on the volatile matter content of the torrefied solid product. Increasing the residence time from 15 min to 150 min resulted in a significant reduction in volatile content of the torrefied solid product. However, the influence of the residence time on the volatile matter of the solid product becomes more apparent for a higher torrefaction temperature (T
t > 280 °C). At around 300 °C cellulose started to decompose which resulted in significant volatile matter loss of the torrefied solid product. Moreover, for (T
t > 280 °C) and longer residence times, some slow reactive constituents of hemicellulose could get enough time to decompose, resulting in further volatile matter loss.
Figure 5.
(a) Volatile matter profile of torrefied beech wood with torrefaction temperature at various residence times VM—raw beech wood (77.9 wt%, db); (b) ash profile of torrefied beech wood with torrefaction temperature at various residence times, Ash—raw beech wood (0.7 wt%, db).
Figure 5.
(a) Volatile matter profile of torrefied beech wood with torrefaction temperature at various residence times VM—raw beech wood (77.9 wt%, db); (b) ash profile of torrefied beech wood with torrefaction temperature at various residence times, Ash—raw beech wood (0.7 wt%, db).
At a lower torrefaction temperature, increasing the residence time from 15 to 150 min reduces the volatile matter content by only 7 (wt%, dry), whereas at a higher torrefaction temperature the volatile matter content decreases by 27 (wt%, dry) for a corresponding residence time increase. Moreover, the effects of torrefaction on the ash content are presented in
Figure 5b. An increase in ash content is observed and this is due to the fact that as the temperature and residence time increase, the rate at which volatiles leave the product increases, which results in more concentrated ash in the solid product. The same trend has been seen for the fixed carbon content of the torrefied product.
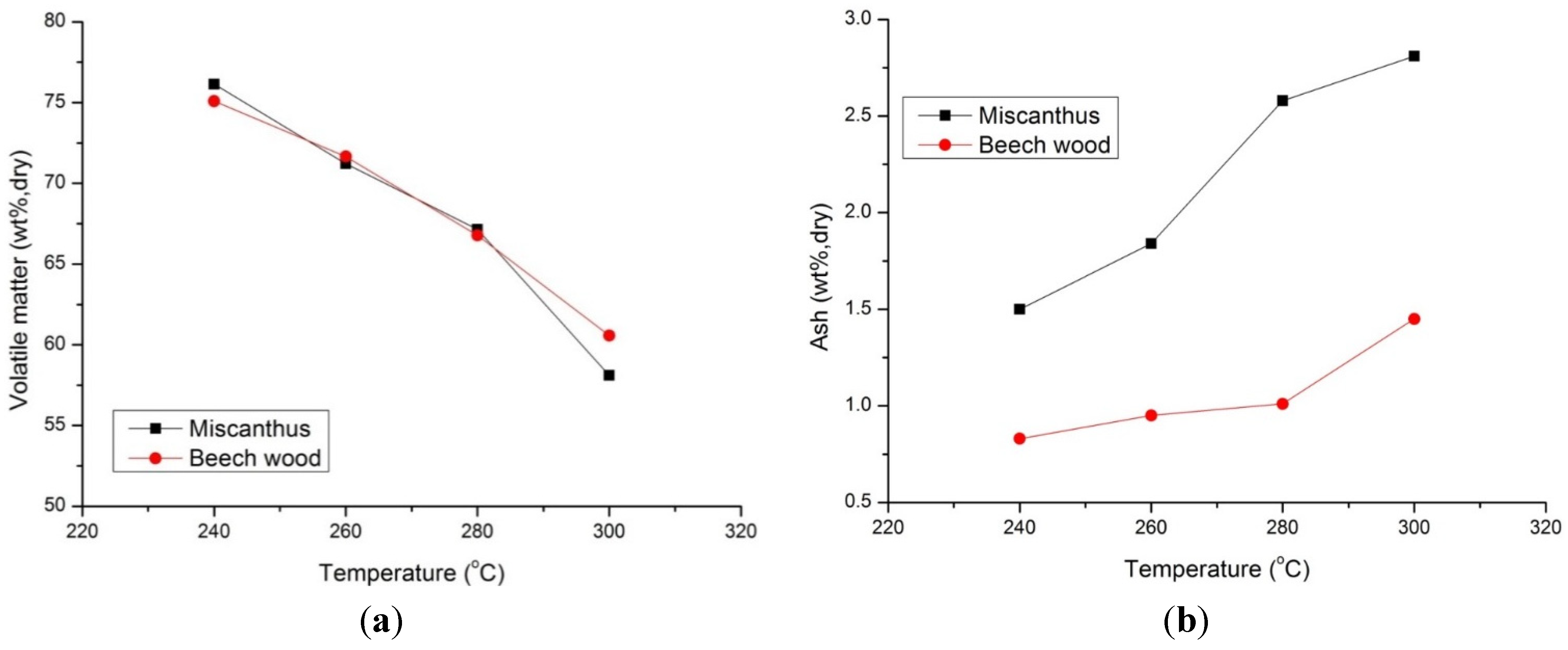
3.2.2. Effect of Biomass Type on Proximate Analysis
The influence of torrefaction temperature on the volatile matter of beech wood and miscanthus is shown in
Figure 6a. In general, within the studied ranges of process conditions, increasing torrefaction temperature has a noticeable influence to intensify the volatile matter loss. Particularly, at torrefaction temperature of 300 °C, the volatile matter present in both samples showed noticeable reduction compared to 240 °C and 260 °C. Similar observations were also made in the study of other biomass species [
6,
21]. Besides that beech wood has 37.1% hemicellulose compared to 30.4% for miscanthus, both biomass samples show the same decreasing trend in volatile matter with small differences at torrefaction temperature of 300 °C. This may be due to the same amount of volatile matter in both the biomass samples.
Figure 6.
(a) Volatile Matter (VM) profile of torrefied beech wood and miscanthus with torrefaction temperature for 30 min residence time, VM—raw beech wood (77.9 wt%, db), raw miscanthus (77.2 wt%, db); (b) Ash profile of torrefied beech wood and miscanthus with torrefaction temperature for 30 min residence time, Ash—raw beech wood (0.7 wt%, db), raw miscanthus (1.2 wt%, db).
Figure 6.
(a) Volatile Matter (VM) profile of torrefied beech wood and miscanthus with torrefaction temperature for 30 min residence time, VM—raw beech wood (77.9 wt%, db), raw miscanthus (77.2 wt%, db); (b) Ash profile of torrefied beech wood and miscanthus with torrefaction temperature for 30 min residence time, Ash—raw beech wood (0.7 wt%, db), raw miscanthus (1.2 wt%, db).
The effect of torrefaction temperature on the ash content of the torrefied miscanthus and beech wood for 30 min residence time is presented in
Figure 6b. Both biomass samples showed an increase in relative ash content with torrefaction temperature, though original miscanthus has a higher ash content compared to beech wood.
3.2.3. Ultimate Analysis and Higher Heating Value
In
Table 2, the ultimate analysis and the higher heating value of the torrefied samples are presented. Bridgeman
et al. [
2] reported an increase in carbon content and a decrease in hydrogen and oxygen after torrefaction. However, under the torrefaction conditions studied here, the hydrogen content and the nitrogen content of the sample remains nearly unchanged. The oxygen content of the torrefied sample is significantly lower compared to the originating biomass. For example, the oxygen content of the torrefied beech wood and miscanthus sample obtained under torrefaction conditions of 300 °C and 30 min residence time is 22% and 18% less than their respective originating biomass.
Another important property of biomass fuel, the higher heating value (HHV (MJ/kg, daf) is also reported in
Table 2 for all torrefied samples. From this data, it is evident that HHV of torrefied biomass has increased 2.5%, 7.6% and 15.7% against 15, 30 and 90 min for 280 °C. This increase is quite significant especially on the commercial scale. At a higher torrefaction temperature of 300 °C and a residence time of 150 min, the HHV of the torrefied beech wood, for instance, increased to 26.8 MJ/kg, daf, which is comparable to low quality coal (for example, brown coal has HHV of 27 MJ/kg, daf). In the case of miscanthus, at a higher torrefaction temperature of 300 °C and a residence time of 30 min, the heating value increased to 22.5 MJ/kg, which was comparable to beech wood under the same torrefaction conditions.
Table 2.
Heating value and ultimate analysis of torrefied beech wood and miscanthus at various temperatures and residence times.
Table 2.
Heating value and ultimate analysis of torrefied beech wood and miscanthus at various temperatures and residence times.
| Samples | Ultimate Analysis (wt%, db) | HHV (MJ/kg, db) | Energy Density (-) |
|---|
| C | H | N | O a |
|---|
| Raw Beech wood | 47.75 | 6.29 | 0.38 | 45.07 | 19.02 | 1.00 |
| TBW240_15 b | 48.38 | 5.88 | 0.15 | 44.87 | 19.16 | 1.01 |
| TBW260_15 | 48.39 | 5.74 | 0.13 | 44.89 | 19.12 | 1.00 |
| TBW280_15 | 49.95 | 4.91 | 0.17 | 44.09 | 19.50 | 1.06 |
| TBW300_15 | 50.12 | 5.74 | 0.15 | 43.11 | 19.84 | 1.07 |
| TBW240_30 | 48.22 | 5.55 | 0.14 | 45.30 | 19.01 | 1.00 |
| TBW260_30 | 49.59 | 5.62 | 0.15 | 43.69 | 19.58 | 1.03 |
| TBW280_30 | 52.48 | 4.90 | 0.23 | 41.41 | 20.47 | 1.07 |
| TBW300_30 | 51.70 | 5.51 | 0.16 | 41.50 | 20.41 | 1.14 |
| TBW240_90 | 51.07 | 5.62 | 0.15 | 42.24 | 20.19 | 1.02 |
| TBW260_90 | 51.81 | 5.45 | 0.17 | 41.55 | 20.43 | 1.07 |
| TBW280_90 | 55.86 | 5.17 | 0.19 | 37.69 | 22.00 | 1.15 |
| TBW300_90 | 55.70 | 5.35 | 0.16 | 37.25 | 22.04 | 1.34 |
| TBW240_150 | 51.44 | 5.52 | 0.14 | 41.51 | 20.31 | 1.04 |
| TBW260_150 | 55.80 | 4.84 | 0.24 | 37.65 | 21.77 | 1.08 |
| TBW280_150 | 65.35 | 4.55 | 0.25 | 27.36 | 25.71 | 1.16 |
| TBW300_150 | 65.95 | 4.61 | 0.30 | 25.10 | 26.06 | 1.39 |
| Raw Miscanthus | 47.4 | 5.7 | 0.3 | 45.3 | 18.70 | 1.00 |
| TM240_30 c | 48.01 | 5.75 | 0.34 | 44.38 | 19.00 | 1.02 |
| TM260_30 | 49.50 | 5.59 | 0.32 | 42.74 | 19.56 | 1.05 |
| TM280_30 | 50.34 | 5.42 | 0.35 | 41.29 | 19.84 | 1.08 |
| TM300_30 | 54.85 | 5.34 | 0.40 | 36.57 | 21.70 | 1.18 |
| Bituminous coal | 66.25 | 4.75 | 1.36 | 15.4 | 26.51 | - |
The elemental composition of the torrefied biomass is presented using the Van Krevelen diagram as shown in
Figure 7. This diagram clearly shows the influence that the torrefaction parameters (temperature and residence time) have on the final elemental compositions of the torrefied biomass. Due to removal of water and carbon dioxide, as one moves from the top right-hand region (lower temperature, shorter residence time) to the bottom left-hand region (higher torrefaction temperature, longer residence time) both H/C and O/C ratios decrease [
5,
22]. Similar to the findings by [
2,
22] the torrefied solid product composition is far away from charcoal or coal. However, with high torrefaction temperature and longer residence times the torrefied solid product close to the elemental composition of low quality coal can be produced. For instance, beech wood torrefied at 300 °C for a residence time of 90 min. has a comparable H/C ratio to that of bituminous coal, and its O/C ratio is slightly inferior. However, both H/C and O/C ratios are superior to that of lignite. It can also be observed that the trends are consistent for beech wood, miscanthus, wheat straw and willow [
2,
11,
22].
Figure 7.
Van-Krevelen diagram for coals, charcoal, raw and various torrefied biomasses, torrefaction temperature (Tt) and residence time (tr).
Figure 7.
Van-Krevelen diagram for coals, charcoal, raw and various torrefied biomasses, torrefaction temperature (Tt) and residence time (tr).
3.3. Mass and Energy Yields
3.3.1. Effect of Process Parameters on the Mass and Energy Yield
The mass and energy yield will be considered to evaluate the torrefaction process of biomass. The heating rate to the final temperature has an influence on the final mass yield. However, the influence is only noticeable for short residence time and temperatures above 280 °C. For torrefaction temperatures above 280 °C, the exothermic chemical reaction commences. As the process becomes exothermic, released heat increases the volatile releasing rate in turn the mass loss.
The effect is checked by removing the torrefied sample as soon as it reaches the desired temperature. As it is shown in
Table 3, it was found that the maximum mass loss during the heating time was only 30% of the total mass loss (for severe torrefaction condition, T = 300 °C and 15 min of residence time).
Table 3.
Influence of heating time on the mass yield (wt%) for different torrefaction temperature.
Table 3.
Influence of heating time on the mass yield (wt%) for different torrefaction temperature.
| Residence Time (min) | Torrefaction Temperature (°C) |
|---|
| 240 | 260 | 280 | 300 |
|---|
| 0 | 100 | 100 | 95 | 90 |
| 15 | 94 | 87 | 78 | 67 |
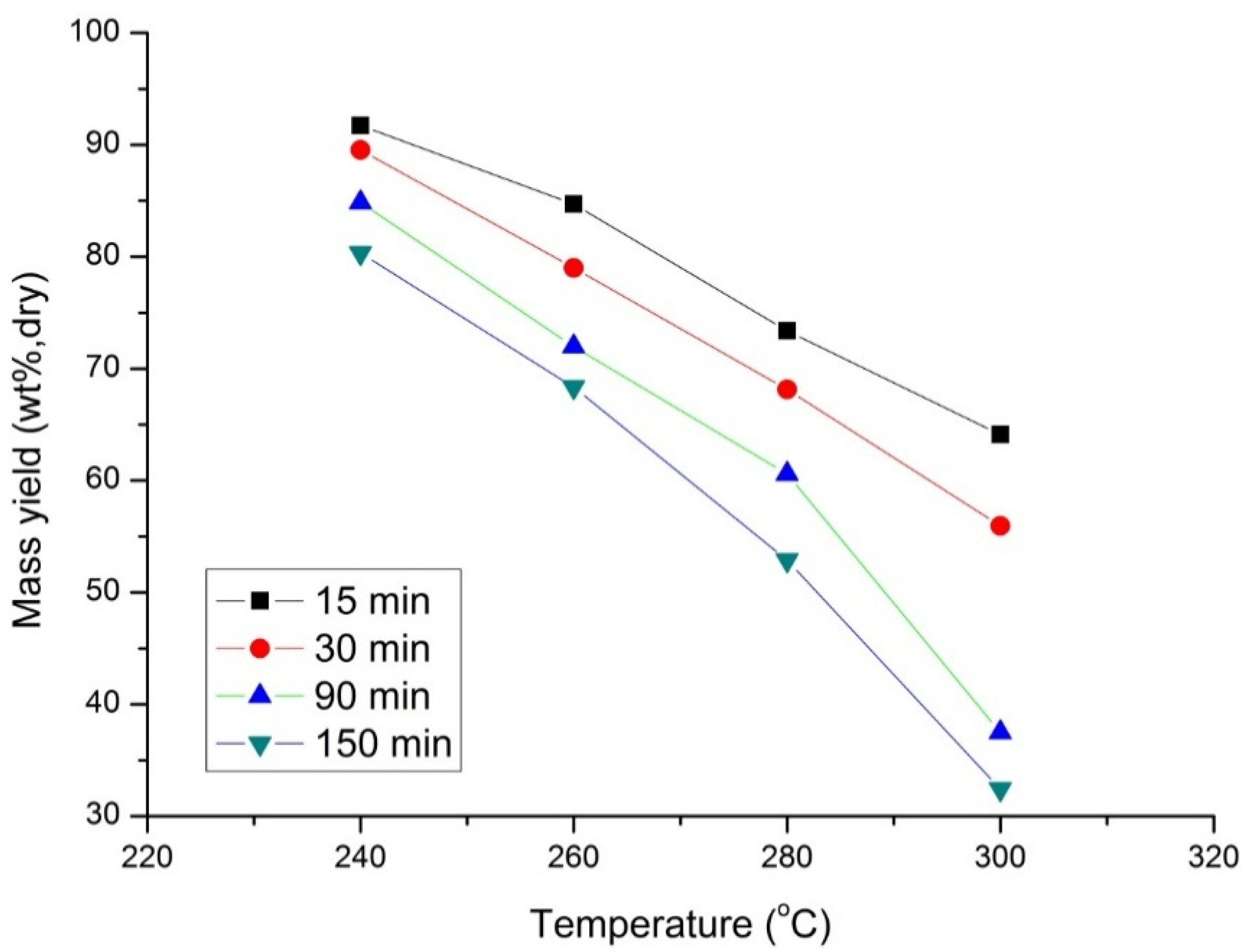
Figure 8 shows the influence of the two process parameters (temperature and residence time) on the mass yield of the torrefied product. As expected, an increase in temperature and residence time decreases the mass yield of the solid product. The decrease in mass yield is more significant for a higher torrefaction temperature (>280 °C) and residence time of 90 and 150 min. At higher temperatures cellulosic constituent of the biomass started to decompose along with the pre-decomposing constituent hemicellulose, which results in the sharp decrease of mass yield specifically at higher residence time.
Figure 8.
Mass yield of beech wood with temperature at four different torrefaction and residence times.
Figure 8.
Mass yield of beech wood with temperature at four different torrefaction and residence times.
At higher torrefaction temperatures (>280 °C) and longer residence times (>30 min), decomposition of reactive components like hemicellulose causes a large increase in mass loss. Similar results are also reported in [
3,
10]. This shows that the torrefaction residence time plays a major role in the thermal decomposition of biomass at higher temperatures (>280 °C). However, between the torrefaction process conditions, the temperature turns out be more dominant than the residence time with respect to mass reduction.
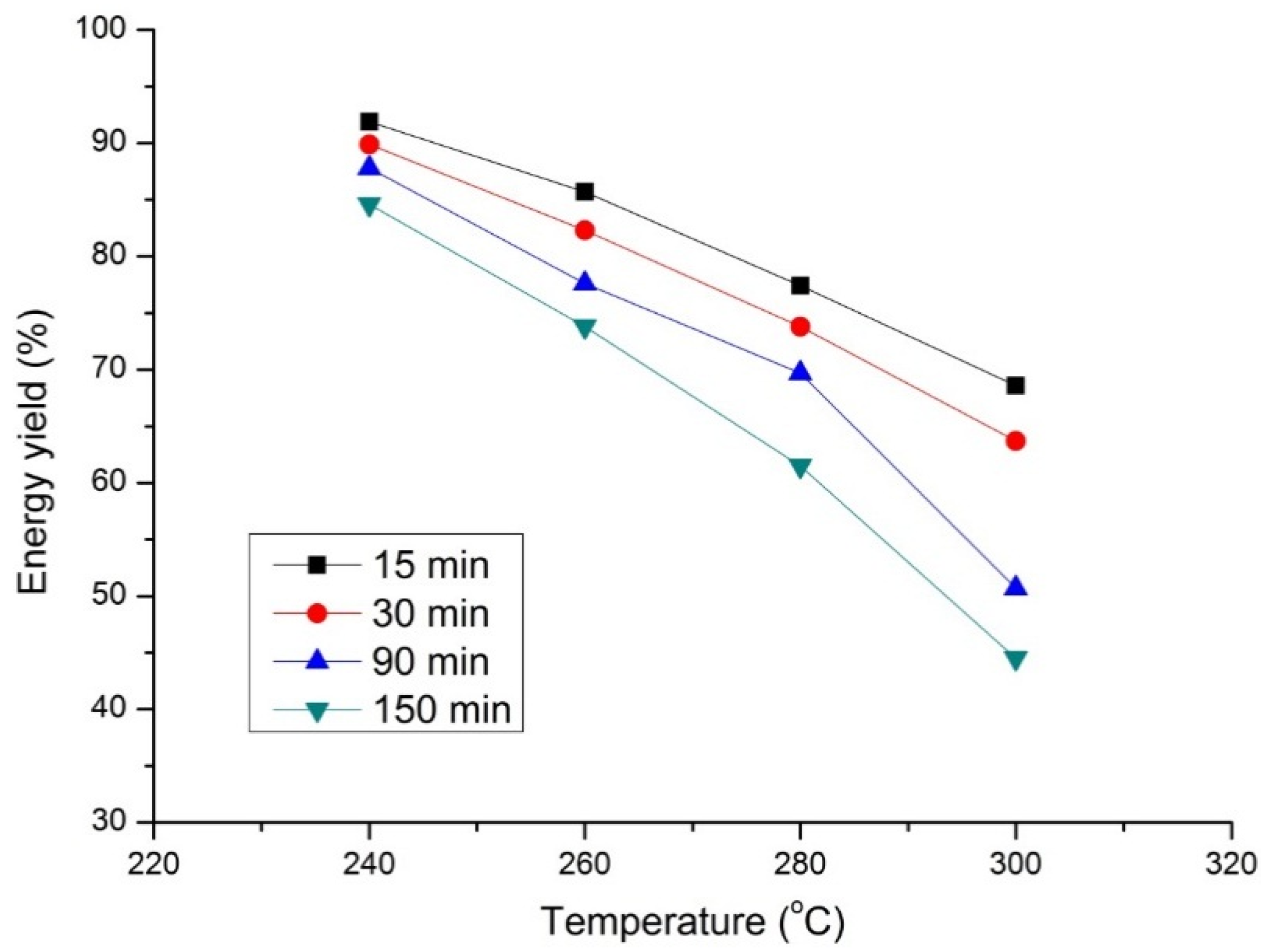
As shown in
Figure 9, the energy yield decreases with increasing temperature and residence time. Similar trends were also observed for the mass yield. Again, for a torrefaction temperature above 280 °C, the change in energy yield is more significant for residence times above 90 min. In all the cases studied, the mass reduction is greater than the energy reduction because of the loss of water and carbon dioxide, which do not contribute to the final energy content of the torrefied product.
Figure 9.
Energy yield of beech wood with temperature at four different torrefaction and residence times.
Figure 9.
Energy yield of beech wood with temperature at four different torrefaction and residence times.
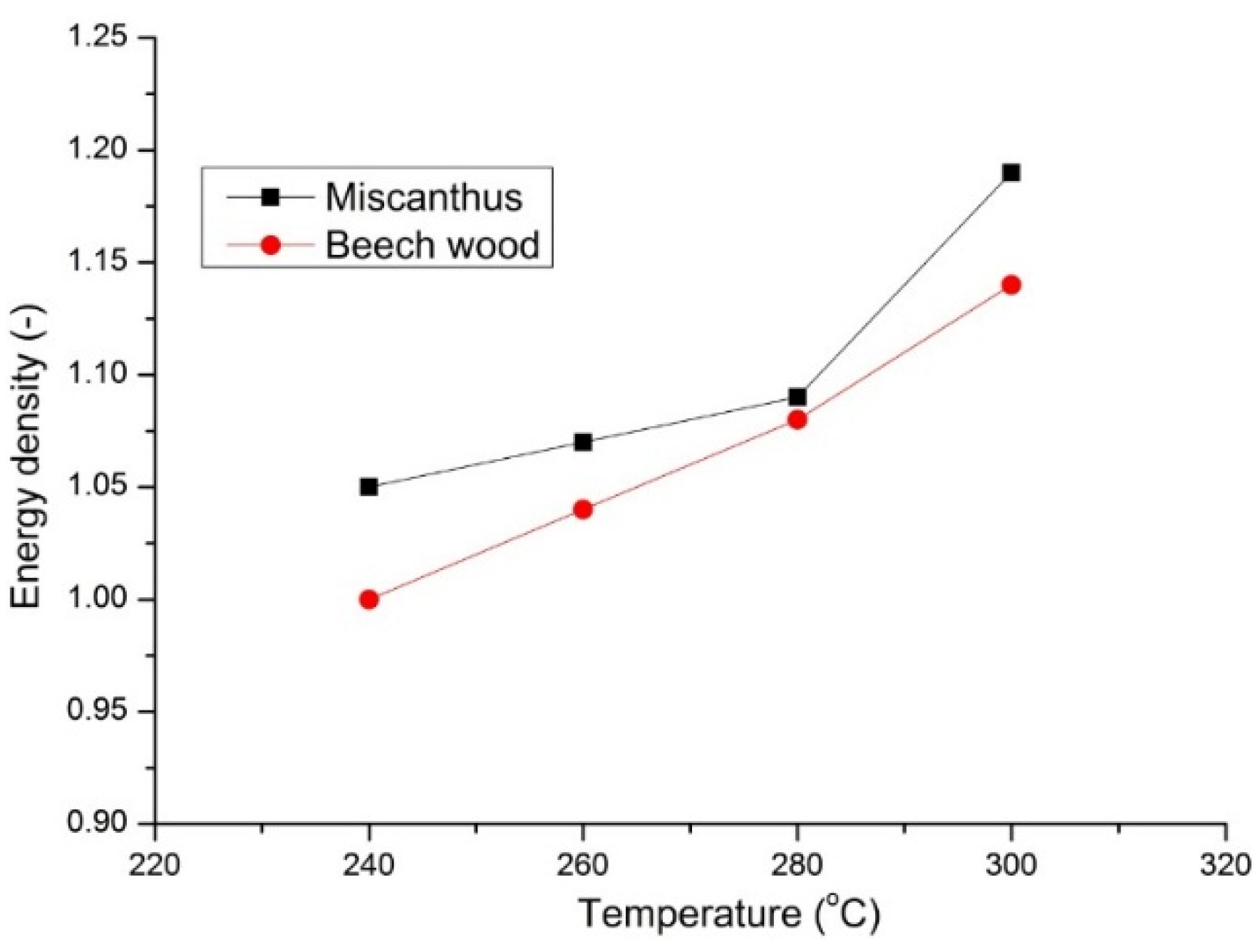
As presented in
Table 2, for a high temperature and long residence time of torrefied beech wood, the energy density increases by 40% but comes with the expense of high mass loss >60% during torrefaction. The influence of torrefaction temperature on the energy density of torrefied miscanthus and beech wood is presented in
Figure 10. Torrefaction at 300 °C and 30 min. residence time resulted in an increase in energy density of 14% for beech wood and 18% for miscanthus.
Figure 10.
Effect of torrefaction temperature on the energy density of beech wood and miscanthus for residence time of 30 min.
Figure 10.
Effect of torrefaction temperature on the energy density of beech wood and miscanthus for residence time of 30 min.
3.3.2. Effect of Biomass Type on the Mass and Energy Yield
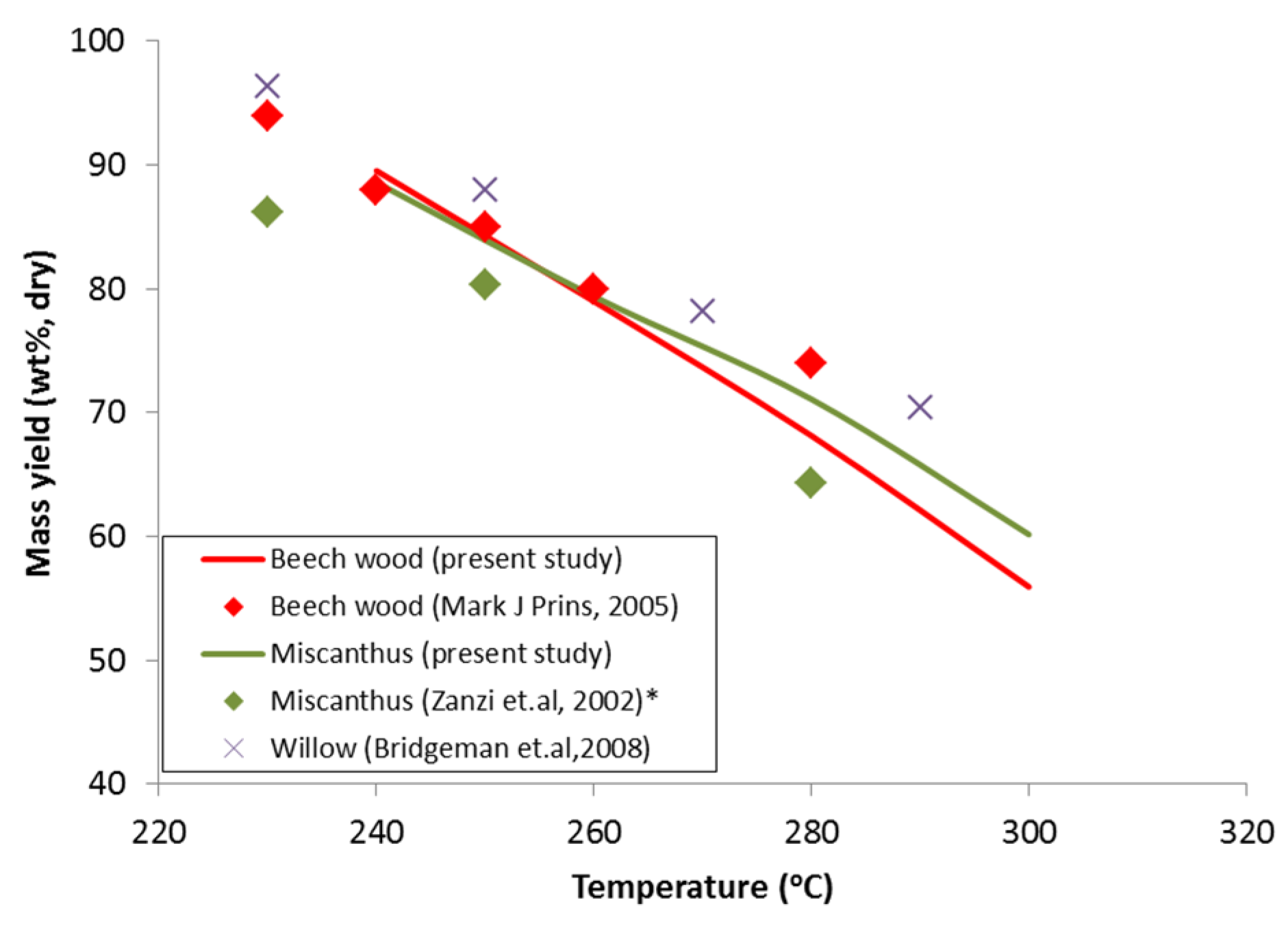
Figure 11 displays the mass yield of beech wood, miscanthus, and willow torrefied at different torrefaction temperatures. Clearly, the mass yield of the torrefied solid products decreases with torrefaction temperature. The observed mass yield of beech wood from this study was comparable to beech wood by [
5] and willow [
2], though small differences were observed for higher torrefaction temperatures. This could be due to the difference in hemicellouse fraction of the biomass samples willow (14.1, wt%) and beech wood (37.1, wt%) or different heating rates of the experimental setup used. On the other hand, the mass yield of miscanthus from this study was higher compared to the study by [
11], which could be due to the difference in torrefaction residence time.
Figure 11.
Mass yield profile
versus torrefaction temperature for a residence time of 30 min for different biomass types. * For miscanthus torrefaction by Zanzi [
11] the residence time (60 min).
Figure 11.
Mass yield profile
versus torrefaction temperature for a residence time of 30 min for different biomass types. * For miscanthus torrefaction by Zanzi [
11] the residence time (60 min).
At 300 °C, the mass yield of torrefied beech wood is 5% less than that of torrefied miscanthus. This could be due to the slightly higher hemicellouse content of beech wood (48%) compared to miscanthus (40%).
The effect of temperature on the energy yield of beech wood and miscanthus at a residence time of 30 min is presented in
Figure 12. It is clear from the figure that the energy yield of miscanthus is decreasing with the increasing temperature in the same way as beech wood. For all the torrefaction temperatures studied, miscanthus resulted in a higher energy yield than beech wood, although the difference is only 3% at the lower temperature and 8% at the higher temperature.
Figure 12.
Energy yield profile of torrefied beech wood and miscanthus vs. temperature for a residence time of 30 min.
Figure 12.
Energy yield profile of torrefied beech wood and miscanthus vs. temperature for a residence time of 30 min.
3.4. Influence of Torrefaction on the Grindability of Torrefied Beech Wood
Grindability tests were carried out in a lab scale planetary type ball-mill. Both raw and torrefied beech wood were ground and compared against bituminous coal with a hard grove index (HGI) of 52. The grindability of miscanthus was not investigated as it was not possible to grind it with the same ball-mill. In the grindability test the sieve size (dp,50), where 50% of the mass was retained, is considered the evaluating parameter for the fuel grindability. Thus fuels with a small dp,50 are easy to grind or take less energy for grinding. As known, coal by nature is a brittle fuel with good grindability property, whereas woody materials have a low grindability property due to their fibrous nature. Therefore, torrefaction could enhance the grindability property of the beech wood by reducing its fibrous nature through thermal pretreatment.
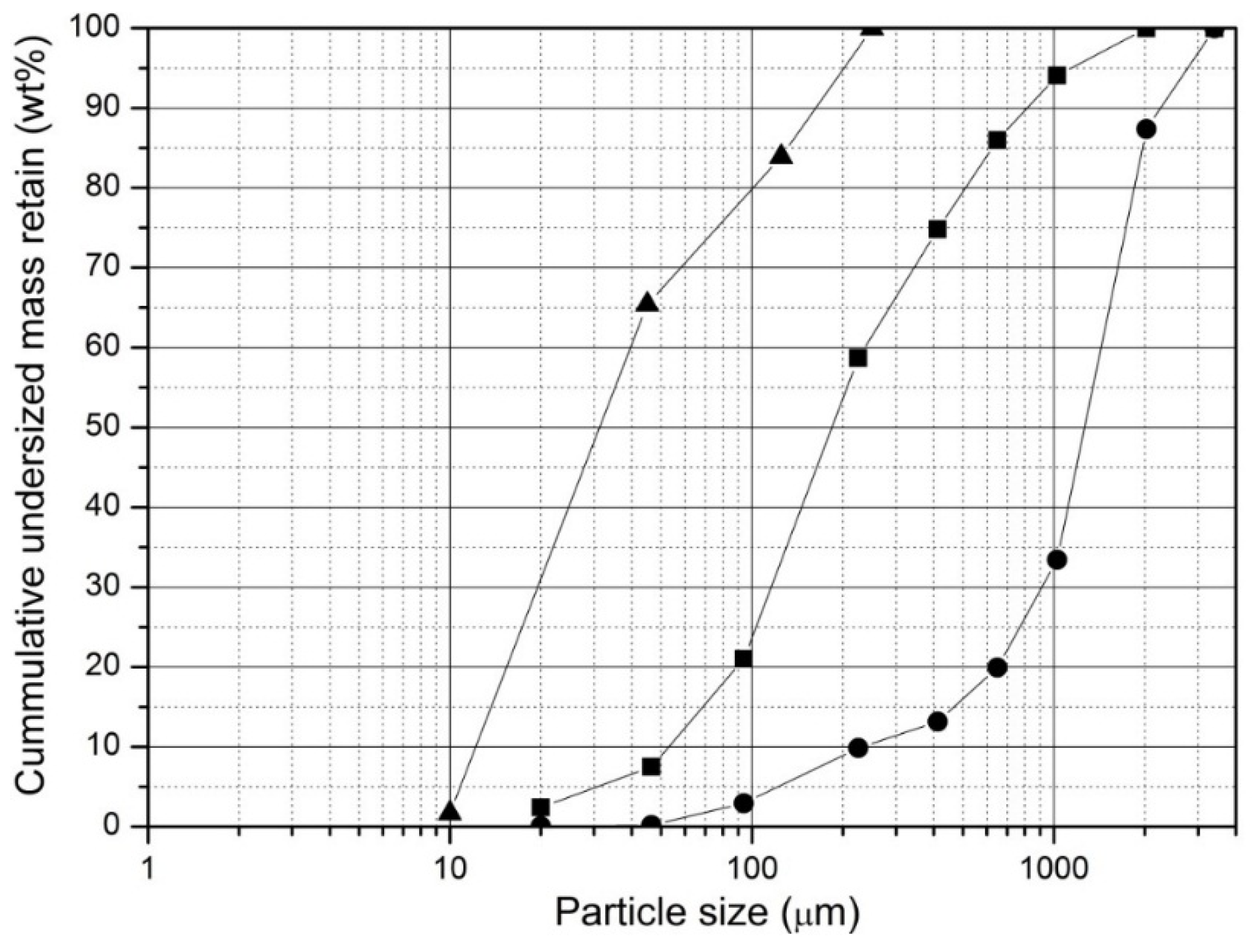
The grindability tests are summarized in
Figure 13. As can be seen from the figure, 50% of the raw beech wood passes through the 1300 µm sieve size, whereas 50% of the torrefied beech wood passes through 180 µm sieve, which is significantly less than 1300 µm while 50% of bituminous coal passes through a size of 30 µm.
These results clearly show that torrefaction of beech wood at 280 °C and a residence time of 15 min results in a solid product that is much easier to grind than the originating wood. The grinding properties of torrefied beech wood are significantly enhanced, even under the light torrefaction condition as considered here (25% weight loss during torrefaction). This might promote the increase of biomass co-firing ratio in coal-fired power plants because torrefied biomass can easily be co-milled with coal to a smaller particle size range as its fibrous nature is being reduced during the torrefaction process. Moreover the severity of the torrefaction conditions can be increased to meet the specific grinding requirements. Subsequently, the biomass char burnout could be largely enhanced.
Figure 13.
Cumulative distribution of mass retained in each sieve size for untreated and torrefied beech wood, Raw beech wood: (●), Beech wood torrefied at Tt = 280 °C and tr = 15 min: (■) and Bituminous coal with HGI = 52: (▲).
Figure 13.
Cumulative distribution of mass retained in each sieve size for untreated and torrefied beech wood, Raw beech wood: (●), Beech wood torrefied at Tt = 280 °C and tr = 15 min: (■) and Bituminous coal with HGI = 52: (▲).
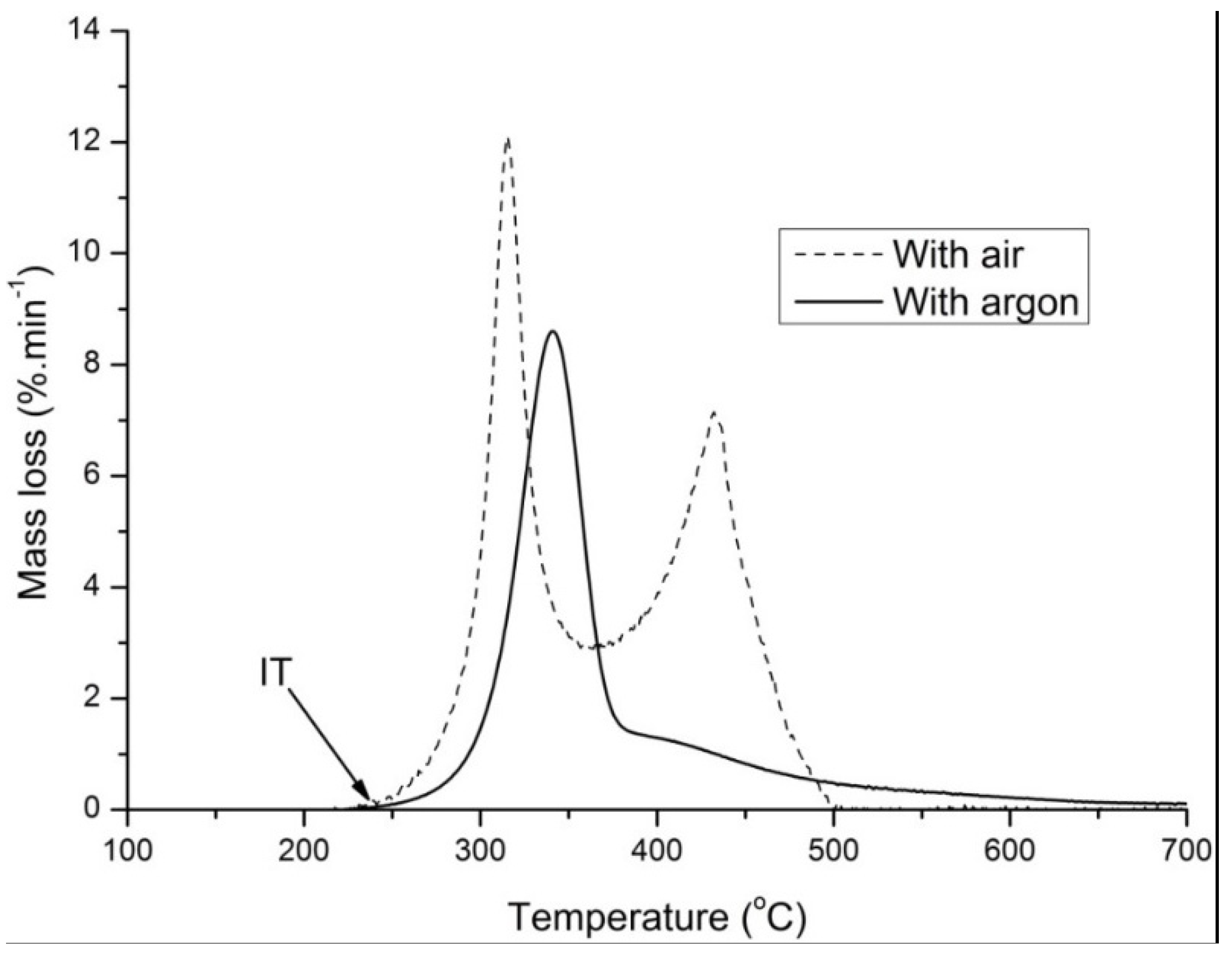
3.5. Ignition Temperature of Torrefied Beech Wood and Miscanthus (TGA)
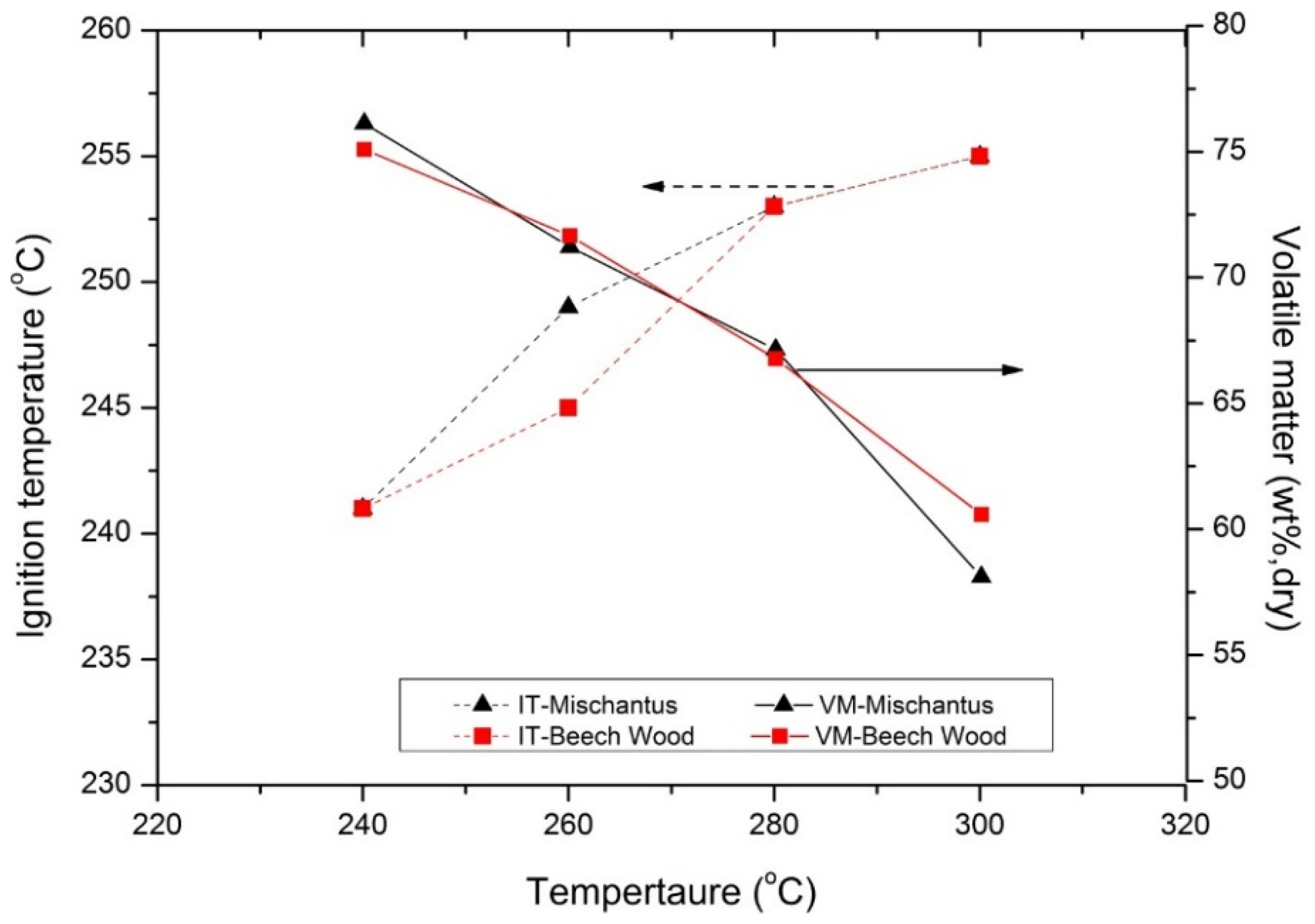
The ignition temperatures of torrefied miscanthus and beech wood are presented in
Figure 14. The ignition temperatures of raw miscanthus, beech wood and bituminous coal were determined (not shown here) to be 233 °C, 237 °C and 329 °C respectively. A fairly close ignition temperature of 235 °C was reported by [
20] for raw poplar wood at a heating rate of 5 °C/min and a sample mass of 5 mg.
Figure 14.
Ignition temperature of torrefied beech wood and miscanthus at a residence time of 30 min, IT (ignition temperature) and VM (volatile matter).
Figure 14.
Ignition temperature of torrefied beech wood and miscanthus at a residence time of 30 min, IT (ignition temperature) and VM (volatile matter).
The ignition temperature of the torrefied products increases at maximum by 20 °C compared to their original biomass as shown in
Figure 14, but it is still 75 °C lower than bituminous coal. Chen
et al. [
23] discovered that the ignition temperature of coal determined by a different experimental technique decreases with an increase in volatile matter content of the fuel. Grotkjaer
et al. [
20] has also found that the same trend can be applied to biomass as well.
As the torrefaction temperature increases, the volatile content of the solid torrefied product decreases (also discussed in preceding sections) and the structure becomes more porous (highly reactive), due to the depolymerization of lignin that binds the material, as discussed in
Section 3.1. The lower the contents of volatile matter retained in the torrefied product, the higher the ignition temperature will be, while increased porosity of the torrefied product can lower the ignition temperature due to increased diffusion rates of oxygen
etc. These two properties of the torrefied product might compete with each other in determining the final fuel ignition temperature.
Overall a slight increase in the ignition temperature of the torrefied biomass was observed as the torrefaction temperature increases. However, the ignition temperatures of the torrefied products are still far from bituminous coal.
4. Conclusions
In this study, the torrefaction of beech wood and miscanthus has been studied with a batch reactor for temperatures between 240–300 °C and residence times of 15–150 min. Various properties of torrefied biomass, which are of paramount importance for co-fired power plants and fuel handling systems, have been investigated and the results have been presented. The mass/energy yield of beech wood and miscanthus was found to decrease steadily with the increase of torrefaction temperature and residence time, whereas the type of experimental setup employed was found to have little or no effect on the mass yield of the torrefaction process. Furthermore, observation of the mass and energy yield curve also revealed that the torrefaction temperature is the most vital process parameter in influencing the mass/energy yield for the whole range of torrefaction study. On the other hand, the residence time becomes important only for torrefaction temperatures >280 °C. Based on the ultimate analyses, the torrefaction process intensifies the carbon and reduces the oxygen content of the torrefied sample as the process conditions get more severe (high temperature and longer residence time). Of the two biomass samples studied, miscanthus has shown higher energy densification together with higher energy yield as compared to beech wood at a residence time of 30 min. These differences could be attributed to the difference in the initial particle size used for analysis and the composition of the biomass. Grindability of beech wood is improved notably by the process of torrefaction. Even for lightly torrefied beech wood (280 °C and 15 min of residence time) with (~25, wt%) mass loss, a remarkable improvement in grindability has been found. This was demonstrated by the reduction of the (dp,50) parameter from 1300 µm for raw beech wood to 180 µm for torrefied beech wood, which is fairly close to 30 µm for bituminous coal. The results of the combustion study showed that the overall ignition temperature change for both beech wood and miscanthus due to torrefaction is insignificant (237–255 °C) compared to bituminous coal with 329 °C. As a whole, torrefaction temperature determines the characteristics of the torrefied fuel compared to other parameters like residence time and geometry of the reactor.