Expression of Heat Shock Proteins in Human Fibroblast Cells under Magnetic Resonant Coupling Wireless Power Transfer
Abstract
:1. Introduction
2. Results and Discussion
Expression of Heat Shock Proteins
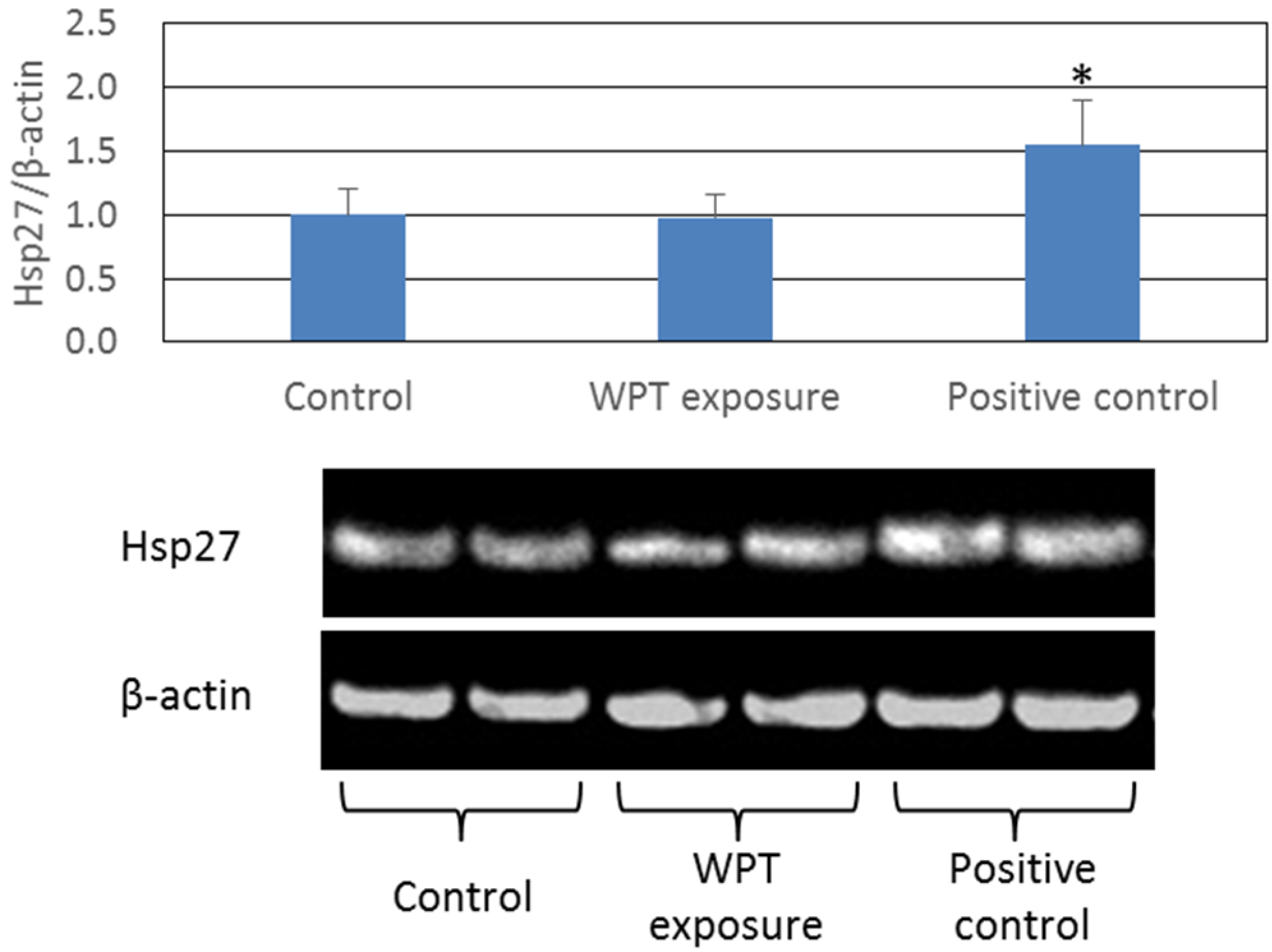


3. Experimental Section
3.1. Exposure System
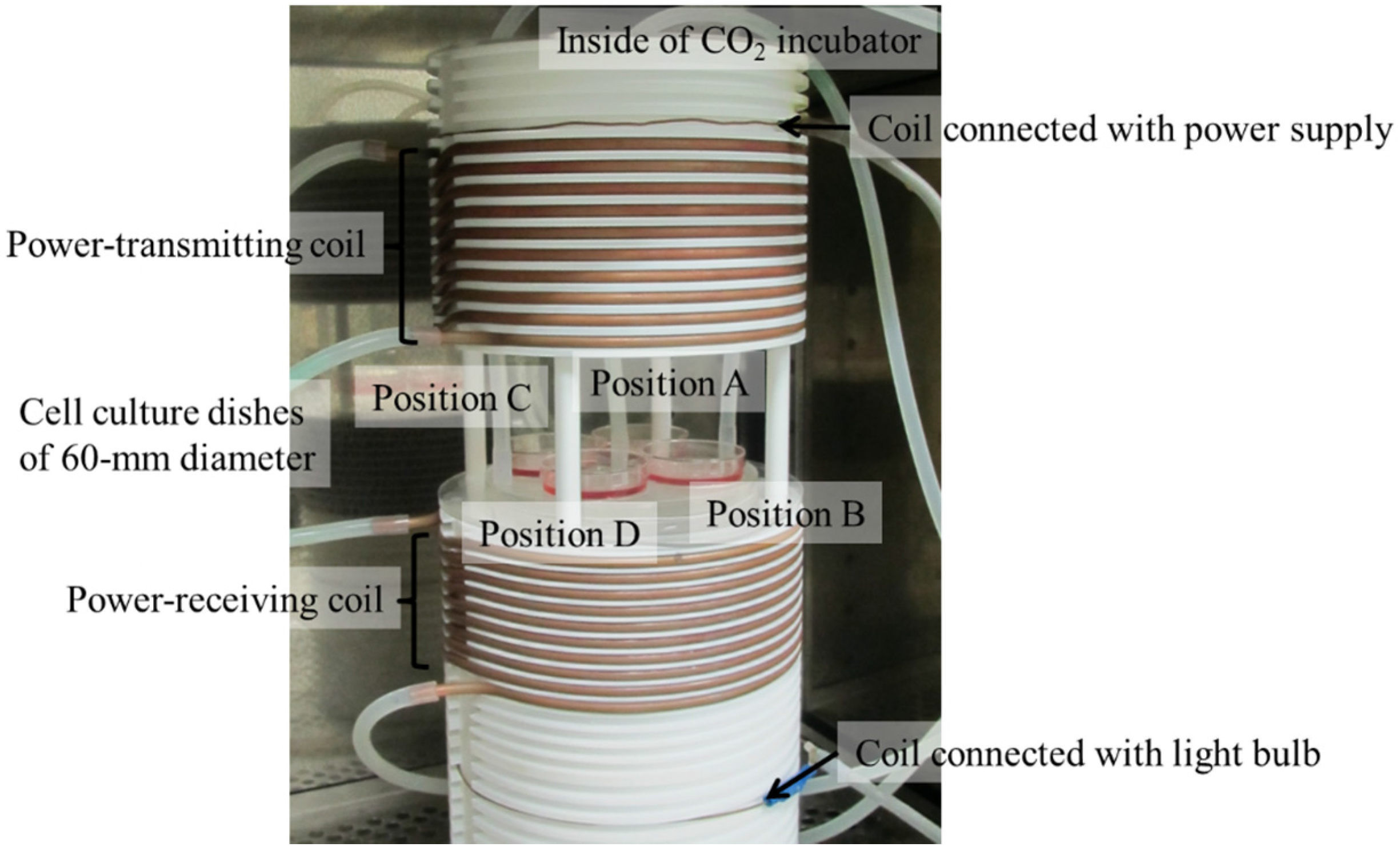
| Type | Position A | Position B | Position C | Position D |
|---|---|---|---|---|
| Magnetic Field | 169.2 ± 2.5 A/m (±1.5%) | 170.7 ± 2.2 A/m (±1.3%) | 167.6 ± 1.7 A/m (±1.0%) | 171.5 ± 2.2 A/m (±1.3%) |
| SAR | 21.8 ± 9.5 W/kg | 21.3 ± 10.0 W/kg | 21.6 ± 12.1 W/kg | 20.7 ± 9.7 W/kg |
3.2. Cells and Culture Conditions
3.3. Western Blotting
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shinohara, N. Power without wire. IEEE Microw. Mag. 2011, 12, S64–S73. [Google Scholar] [CrossRef]
- Kurs, A.; Karalis, A.; Moffatt, R.; Joannpoilos, J.D.; Fisher, P.; Soljacic, M. Wireless power transfer via strongly coupled magnetic resonances. Sci. Mag. 2007, 317, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, Y.; Taniya, A.; Horiuchi, M.; Kobayashi, S. Development of kW Class Wireless Power Transmission System for EV Using Magnetic Resonant Method. In Proceedings of the 1st International Electric Vehicle Technology Conference, Yokohama, Japan, 17–19 May 2011.
- Chun, Y.; Park, S.; Kim, J.; Kim, J.; Kim, H.; Kim, J.; Kim, N.; Ahn, S. Electromagnetic compatibility of resonance coupling wireless power transfer in on-line electric vehicle system. IEICE Trans. Commun. 2014, 97, 416–423. [Google Scholar] [CrossRef]
- Sony develops highly efficient wireless power transfer system based on magnetic resonance. Available online: http://www.sony.net/SonyInfo/News/Press/200910/09-119E/ (accessed on 18 September 2015).
- Miyakoshi, J.; Horiuchi, E.; Nakahara, T.; Sakurai, T. Magnetic fields generated by an induction heating (IH) cook top do not cause genotoxicity in vitro. Bioelectromagnetics 2007, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Nakasono, S.; Ikehata, M.; Dateki, M.; Yoshie, S.; Shigemitsu, T.; Negishi, T. Intermediate frequency magnetic fields do not have mutagenic, co-mutagenic or gene conversion potentials in microbial genotoxicity tests. Mutat. Res. 2008, 649, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Taki, M. Measurement of magnetic field from an induction heating hob and estimation of induced current density in human body. IEEJ Trans. Fundam. Mater. 2005, 125, 427–433. [Google Scholar] [CrossRef]
- Lin, J.C. Dosimetric comparison between different quantities for limiting exposure in the RF band: Rationale and implications for guidelines. Health Phys. 2007, 92, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Verschaeve, L.; Juutilainen, J.; Lagroye, I.; Miyakoshi, J.; Saunders, R.; de Seze, R.; Tenforde, T.; van Rongen, E.; Veyret, B.; Xu, Z. In vitro and in vivo genotoxicity of radiofrequency fields. Mutat. Res. 2010, 705, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmia; Cao, Y.; Scarfic, M.R. Adaptive response in mammalian cells exposed to non-ionizing radiofrequency fields: A review and gaps in knowledge. Mutat. Res. 2014, 760, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Hirata, A. Evaluation of induced electric field and compliance procedure for wireless power transfer system in an electrical vehicle. Phys. Med. Biol. 2013, 58, 7583–7593. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Wake, K.; Watanabe, S. Incident electric field effect and numerical dosimetry for a wireless power transfer system using magnetically coupled resonances. IEEE Trans. Microw. Theory Tech. 2013, 61, 3461–3469. [Google Scholar] [CrossRef]
- Christ, A.; Douglas, M.G.; Cooper, E.B.; Sample, A.P.; Waters, B.H.; Smith, J.R.; Kuster, N. Evaluation of wireless resonant power transfer systems with human electromagnetic exposure limits. IEEE Trans. Electromagn. Compat. 2013, 55, 265–274. [Google Scholar] [CrossRef]
- Sunohara, T.; Laakso, I.; Hirata, A.; Onishi, T. Analysis of in-situ electric field and specific absorption rate in human models for wireless power transfer with induction coupling. Phys. Med. Biol. 2014, 59, 3721–3735. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhu, C.; Lu, R.; Mao, S.; Qi, Y. Intermediate frequency magnetic field generated by a wireless power transmission device does not cause genotoxicity in vitro. Bioelectromagnetics 2014, 35, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Shinohara, N.; Miyakoshi, J. In Vitro Evaluation of Genotoxic Effects under Magnetic Resonant Coupling Wireless Power Transfer. Int. J. Environ. Res. Public Health 2015, 12, 3853–3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Extremely Low Frequency Fields, Environmental Health Criteria Monograph No. 238; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- Miyakoshi, J.; Mori, Y.; Yaguchi, H.; Ding, G.; Fujimori, A. Suppression of heat-induced HSP-70 by simultaneous exposure to 50 mT magnetic field. Life Sci. 2000, 66, 1187–1196. [Google Scholar] [CrossRef]
- Sakurai, T.; Kiyokawa, T.; Kikuchi, K.; Miyakoshi, J. Intermediate frequency magnetic fields generated by an induction heating (IH) cooktop do not affect genotoxicities and expression of heat shock proteins. Int. J. Radiat. Biol. 2009, 85, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koyama, S.; Komatsubara, Y.; Suzuki, Y.; Taki, M.; Miyakoshi, J. Effects of a 2450 MHz high-frequency electromagnetic field with a wide range of SARs on the induction of heat-shock proteins in A172 cells. Bioelectromagnetics 2006, 27, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Fujimichi, Y.; Hamada, N. Ionizing irradiation not only inactivates clonogenic potential in primary normal human diploid lens epithelial cells but also stimulates cell proliferation in a subset of this population. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Maeda, M.; Maezawa, H.; Usami, N.; Kobayashi, K. Bystander cell killing in normal human fibroblasts is induced by synchrotron X-ray microbeams. Radiat. Res. 2010, 173, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Straface, E.; Vona, R.; Ascione, B.; Matarrese, P.; Strudthoff, T.; Franconi, F.; Malorni, W. Single exposure of human fibroblasts (WI-38) to a sub-cytotoxic dose of UVB induces premature senescence. FEBS Lett. 2007, 581, 4342–4348. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Kang, G.Y.; Lee, J.S.; Choi, J.I.; Lee, J.W.; Hong, S.C.; Myung, S.H.; Lee, Y.S. Effects on micronuclei formation of 60-Hz electromagnetic field exposure with ionizing radiation, hydrogen peroxide, or c-Myc overexpression. Int. J. Radiat. Biol. 2012, 88, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Torsello, A.; Tedesco, B.; Fasanella, S.; Boninsegna, A.; D’Ascenzo, M.; Grassi, C.; Azzena, G.B.; Cittadini, A. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: Possible involvement of a redox mechanism. Biochim. Biophys. Acta 2005, 1743, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.A.; Alfieri, R.R.; Petronini, P.G.; Brigotti, M.; Campanini, C.; Borghetti, A.F. Attenuated expression of 70-kDa heat shock protein in WI-38 human fibroblasts during aging in vitro. Exp. Cell Res. 1999, 252, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.A.; Alfieri, R.R.; Desenzani, S.; Petronini, P.G.; Borghetti, A.F. Proteasome inhibition increases HuR level, restores heat-inducible HSP72 expression and thermotolerance in WI-38 senescent human fibroblasts. Exp. Gerontol. 2004, 39, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, M.; Zhou, J.; Zhang, X. HSPs 27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). Int. J. Oncol. 2014, 45, 18–30. [Google Scholar] [PubMed]
- Mizuno, K.; Miyakoshi, J.; Shinohara, N. In vitro exposure system using magnetic resonant coupling wireless power transfer. Wirel. Power Transf. 2014, 1, 97–107. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz–100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar]
- Ahlbom, A.; Bergqvist, U.; Bernhardt, J.H.; Cesarini, J.; Court, L.A.; Grandolfo, M.; Hietanen, M.; Mckinlay, A.F.; Repacholi, M.H.; Sliney, D.H.; et al. Guidelines for Limiting Exposure to Time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, K.; Shinohara, N.; Miyakoshi, J. Expression of Heat Shock Proteins in Human Fibroblast Cells under Magnetic Resonant Coupling Wireless Power Transfer. Energies 2015, 8, 12020-12028. https://doi.org/10.3390/en81012020
Mizuno K, Shinohara N, Miyakoshi J. Expression of Heat Shock Proteins in Human Fibroblast Cells under Magnetic Resonant Coupling Wireless Power Transfer. Energies. 2015; 8(10):12020-12028. https://doi.org/10.3390/en81012020
Chicago/Turabian StyleMizuno, Kohei, Naoki Shinohara, and Junji Miyakoshi. 2015. "Expression of Heat Shock Proteins in Human Fibroblast Cells under Magnetic Resonant Coupling Wireless Power Transfer" Energies 8, no. 10: 12020-12028. https://doi.org/10.3390/en81012020
APA StyleMizuno, K., Shinohara, N., & Miyakoshi, J. (2015). Expression of Heat Shock Proteins in Human Fibroblast Cells under Magnetic Resonant Coupling Wireless Power Transfer. Energies, 8(10), 12020-12028. https://doi.org/10.3390/en81012020





