A Complementary Biodiesel Blend from Soapnut Oil and Free Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Fatty acid | High-oleic FFAs [13] | SNME and FFA-based biodiesel blend |
|---|---|---|
| Palmitic acid (C16:0) | 1.39 | 3.0 |
| Stearic acid (C18:0) | 4.30 | 2.6 |
| Oleic acid (C18:1) | 81.7 | 65.2 |
| Linoleic acid (C18:2) | 11.2 | 9.4 |
| Linolenic acid (C18:3) | 0.19 | 1.6 |
| Arachidic acid (C20:0) | 0.8 | 3.5 |
| Eicosenoic acid (C20:1) | 0.3 | 14.5 |
| Lignoceric acid (C24:0) | 0.1 | 0.0 |
| Saturated fatty acids | 6.59 | 9.1 |
| Unsaturated fatty acids | 93.39 | 90.7 |
2.2. Experimental Procedures
2.3. Analysis of Fuel Properties
2.4. Reaction Rate Kinetics for Consumption of Antioxidant
3. Results and Discussion
3.1. Properties of SNME and FFA-based Biodiesel Blends
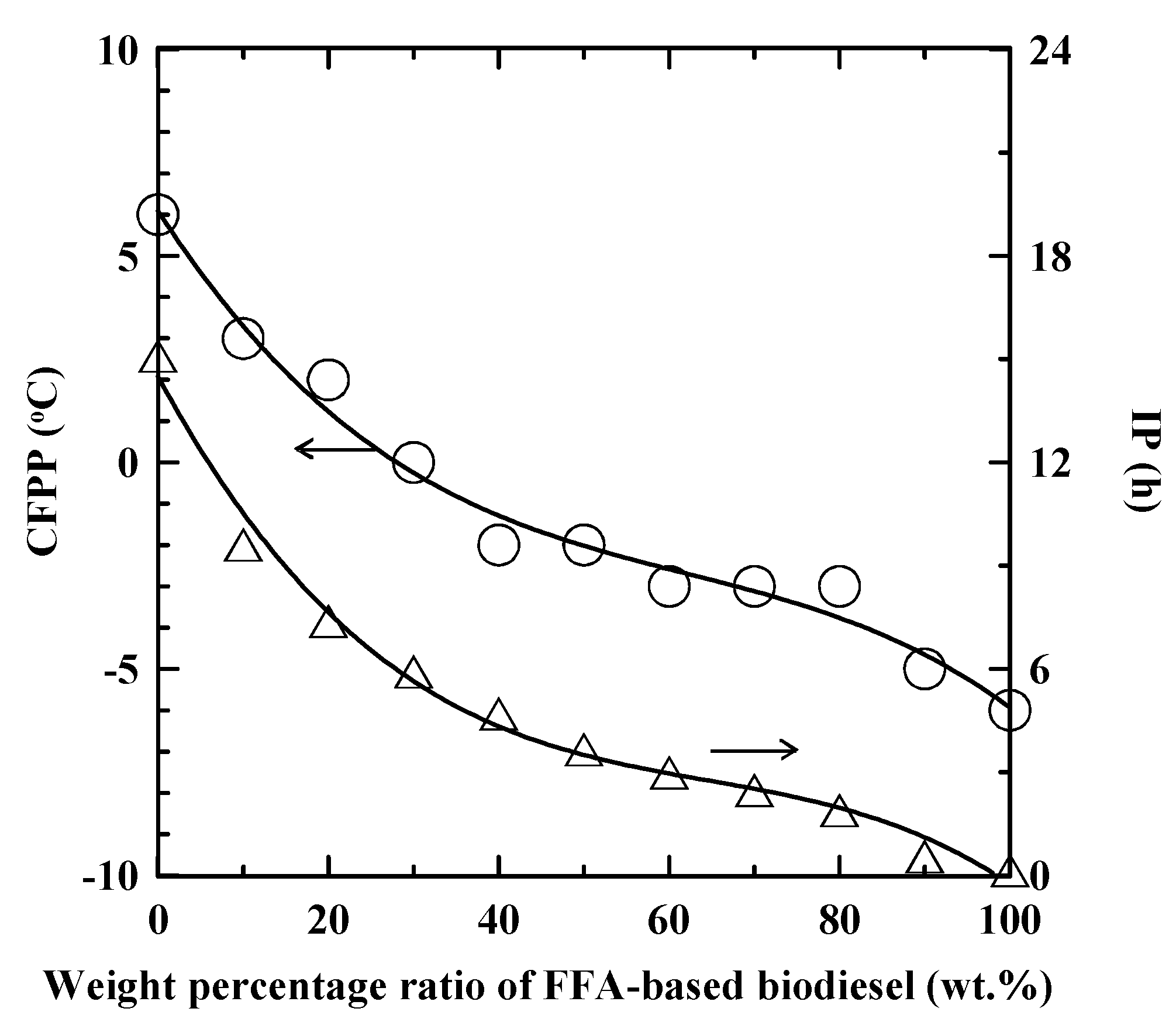
| Property | Unit | SNME and FFA-based biodiesel blend | ASTM D6751 | CNS 15072 | EN 14214 |
|---|---|---|---|---|---|
| Acid value | mg KOH/g | 0.18 | 0.5 max | 0.5 max | 0.5 max |
| Cetane number | - | 58 | 47 min | 51 min | 51 min |
| CFPP | °C | −2 | a | 0 max | a |
| Density at 15 °C | kg/m3 | 878 | b | 860–900 | 860–900 |
| Ester content | % (m/m) | 98.6 | b | 96.5 min | 96.5 min |
| Flash point | °C | 169 | |||
| Iodine value | g I2/100 g | 95.7 | b | 120 max | 120 max |
| Kinematic viscosity at 40 °C | mm2/s | 4.82 | 1.9–6.0 | 3.50–5.0 | 3.50–5.0 |
| Oxidation stability, 110 °C | h | 4.7 | 3.0 min | 6 min | 6 min |
3.2. Temperature Dependence of IP and Enhanced with Addition of PDA
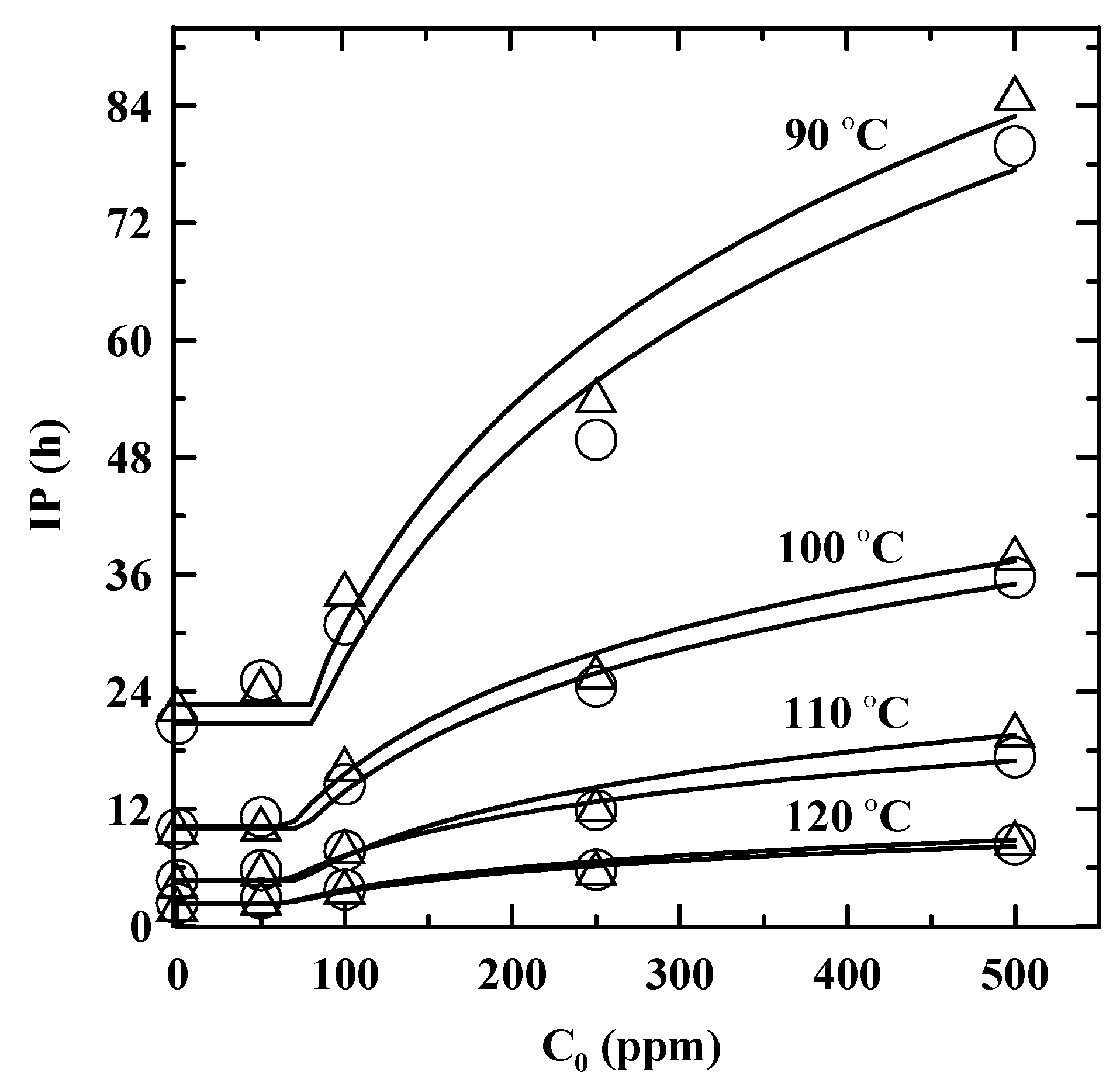
| T(°C ) | Method | IPORIG (h) | Stabilization factor a | kf (h−1) | Ccr (ppm) | R2 |
|---|---|---|---|---|---|---|
| 90 | EN 14,112 | 20.70 | 3.86 | 0.0320 | 81.4 | 0.977 |
| EN 15,751 | 22.74 | 3.75 | 0.0309 | 77.8 | 0.958 | |
| 100 | EN 14,112 | 9.94 | 3.59 | 0.0827 | 63.8 | 0.976 |
| EN 15,751 | 10.25 | 3.73 | 0.0768 | 62.6 | 0.944 | |
| 110 | EN 14,112 | 4.70 | 3.60 | 0.166 | 66.9 | 0.970 |
| EN 15,751 | 4.70 | 4.20 | 0.126 | 74.1 | 0.967 | |
| 120 | EN 14,112 | 2.33 | 3.58 | 0.346 | 66.3 | 0.977 |
| EN 15,751 | 2.30 | 3.93 | 0.316 | 63.8 | 0.958 |
3.3. Reaction Rate Kinetics for Consumption of PDA
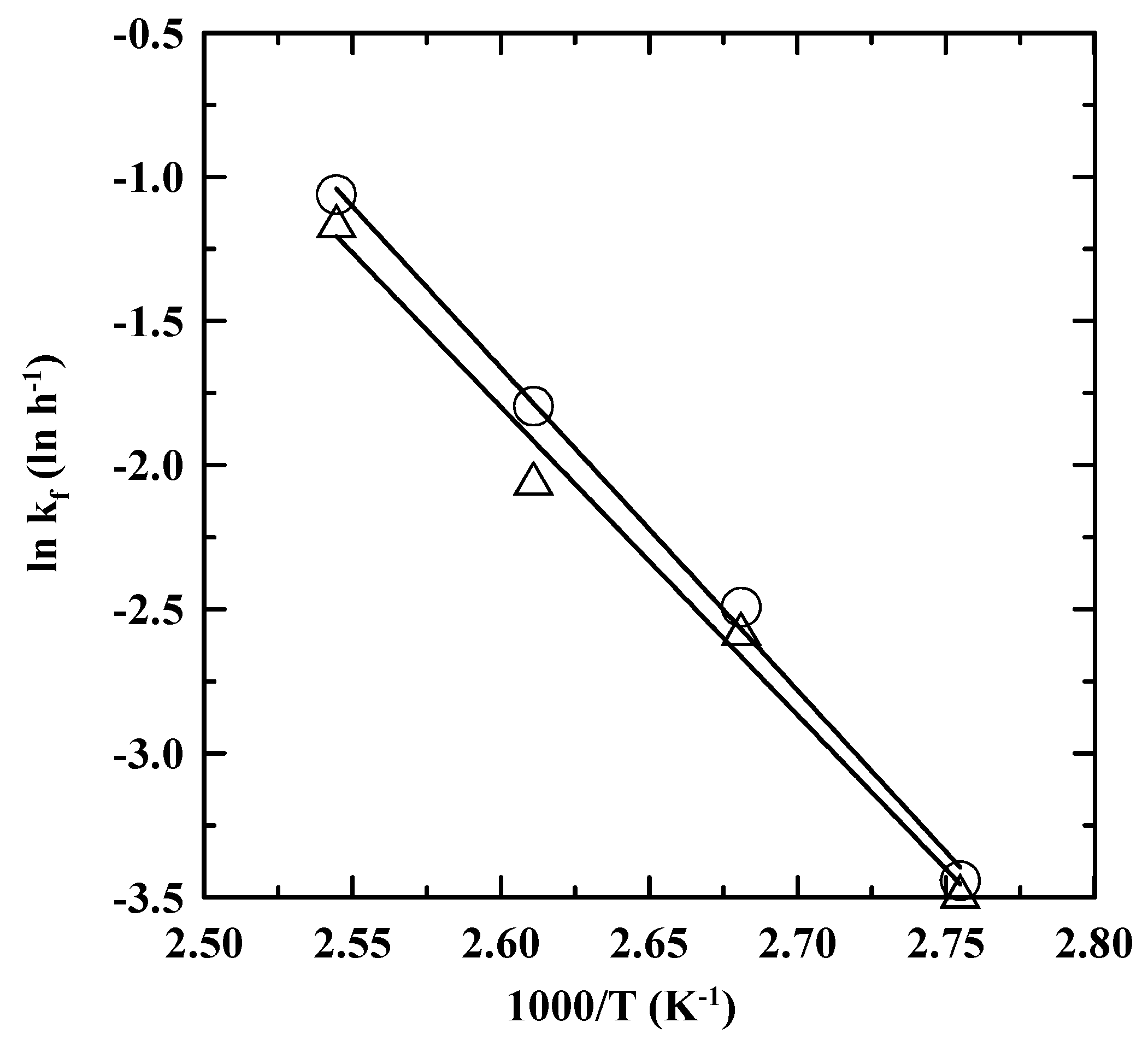
3.4. IP at Ambient Temperature as Obtained by an Extrapolation Method

| C0 (ppm) | EN 14,112 | EN 15,751 |
|---|---|---|
| 0 | ln IP = −0.0728 T + 9.58, R2 = 0.999 | ln IP = −0.0763 T + 9.98, R2 = 1 |
| ln IP = 10391.35/T − 25.568, R2 = 0.999 | ln IP = 10896.89/T − 26.9, R2 = 1 | |
| 100 | ln IP = −0.0708 T + 9.84, R2 = 0.999 | ln IP = −0.0717 T + 10.0, R2 = 0.999 |
| ln IP = 10139/T − 24.5, R2 = 0.998 | ln IP = 10381/T − 25.1, R2 = 0.995 | |
| 250 | ln IP = −0.072 T + 10.4, R2 = 1 | ln IP= −0.0734 T + 10.6, R2 = 1 |
| ln IP = 10270/T − 24.3, R2 = 0.999 | ln IP = 10477/T − 24.8, R2 = 0.999 | |
| 500 | ln IP = −0.075 T + 11.1, R2 = 0.999 | ln IP = −0.0737 T + 11.1, R2 = 0.998 |
| ln IP = 10706/T − 25.1, R2 = 0.999 | ln IP = 10520/T − 24.5, R2 = 0.998 |
 : EN 14,112 and EN 15,751, respectively, as predicted by the reaction rate kinetics.
: EN 14,112 and EN 15,751, respectively, as predicted by the reaction rate kinetics.
 : EN 14,112 and EN 15,751, respectively, as predicted by the reaction rate kinetics.
: EN 14,112 and EN 15,751, respectively, as predicted by the reaction rate kinetics.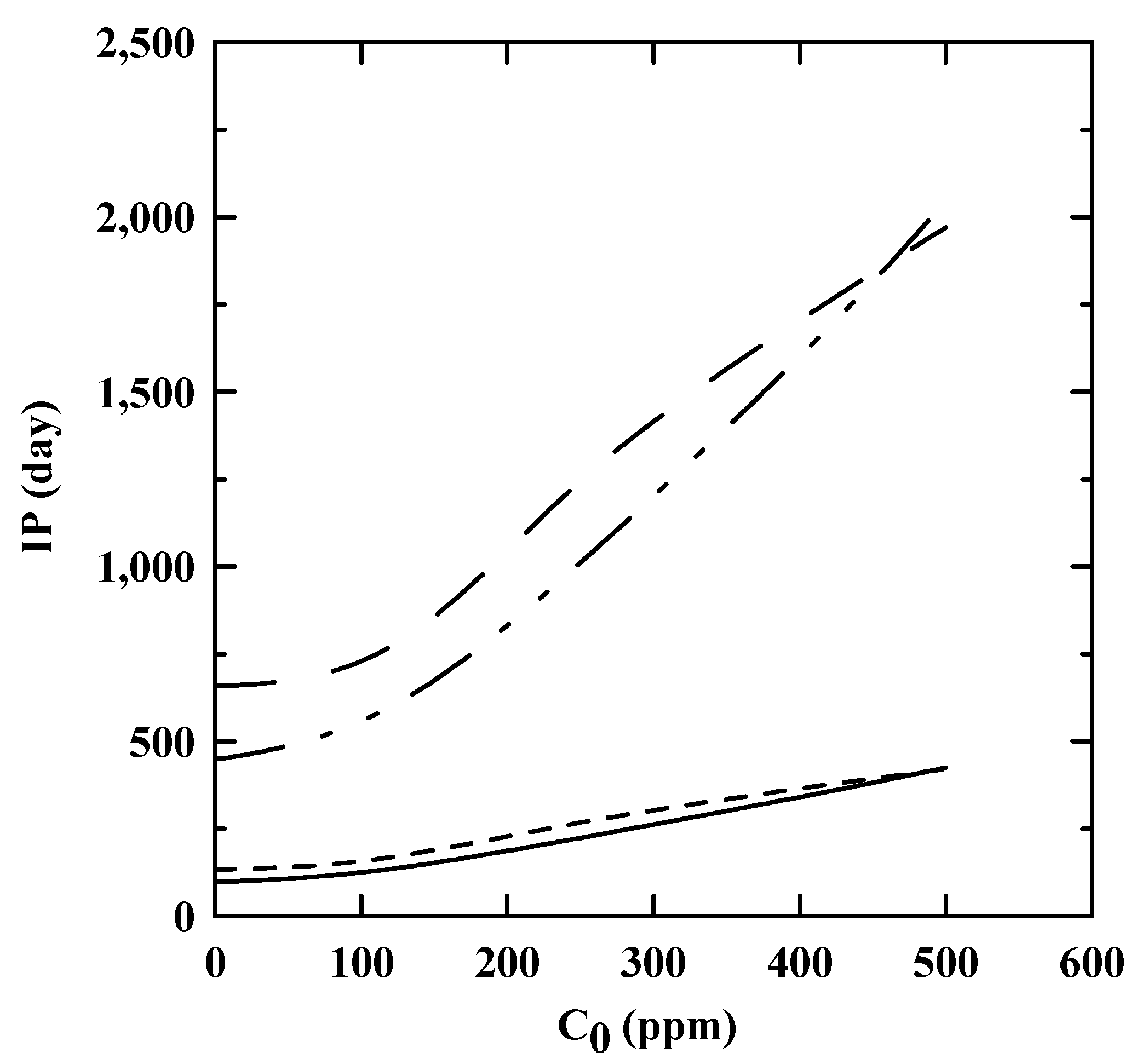
4. Conclusions
Acknowledgement
References
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Moser, B.R.; Knothe, G.; Cermak, S.C. Biodiesel from meadowfoam (Limnanthes alba L.) seed oil: Oxidative stability and unusual fatty acid composition. Energy Environ. Sci. 2010, 3, 318–327. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.P.; Park, S.C.; Kim, Y.J.; Lee, J.S. Blending effects of biodiesels on oxidation stability and low temperature flow properties. Bioresour. Technol. 2008, 99, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Schober, S.; Mittellbach, M. The impact of antioxidants on biodiesel oxidation stability. Eur. J. Lipid Sci. Technol. 2004, 106, 382–389. [Google Scholar] [CrossRef]
- Haryana-online.com. Ritha. 2007. Available online: http://www.haryana-online.com/Flora/ritha.htm (accessed on 17 August 2012).
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, J.; Du, M.; Zhang, J.; Jiang, J. Fatty acid composition analysis of Sapindus mukorossi Gaerth seed oil (in Chinese). China Oils Fats 2009, 34, 74–76. [Google Scholar]
- Misra, R.D.; Murthy, M.S. Performance, emission and combustion evaluation of soapnut oil-diesel blends in a compression ignition engine. Fuel 2011, 90, 2514–2518. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiang, T.H.; Chen, J.H. An optimum biodiesel combination: Jatropha and soapnut oil biodiesel blends. Fuel 2011, 92, 377–380. [Google Scholar] [CrossRef]
- Ng, W.K.; Lim, P.K.; Boey, P.L. Dietary lipid and palm oil source affects growth, fatty acid composition and muscle α-tocopherol concentration of African catfish, Clarias gariepinus. Aquaculture 2003, 215, 229–243. [Google Scholar] [CrossRef]
- Aranda, D.A.G.; Santos, R.T.P.; Tapanes, N.C.O.; Ramos, A.L.D.; Antunes, O.A.C. Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catal. Lett. 2008, 122, 20–25. [Google Scholar] [CrossRef]
- Ooi, Y.S.; Zakaria, R.; Mohamed, A.R.; Bhatia, S. Catalytic conversion of palm oil-based fatty acid mixture to liquid fuel. Biomass Bioenergy 2004, 27, 477–484. [Google Scholar] [CrossRef]
- Chen, Y.H.; Luo, Y.M. Oxidation stability of biodiesel derived from free fatty acids associated with kinetics of antioxidants. Fuel Process. Technol. 2011, 92, 1387–1393. [Google Scholar] [CrossRef]
- Waynick, J.A. Characterization of Biodiesel Oxidation and Oxidation Products; National Renewable Energy Laboratory: Lakewood, CO, USA, 2005; pp. 3–5. [Google Scholar]
- Karavalakis, G.; Stournas, S.; Karonis, D. Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 2010, 89, 2483–2489. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of antioxidants on the oxidation stability of methyl soyate (biodiesel). Fuel Process. Technol. 2005, 86, 1071–1085. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energy Fuels 2008, 22, 4301–4306. [Google Scholar] [CrossRef]
- Araújo, S.V.; Luna, F.M.T.; Rola, E.M., Jr.; Azevedo, D.C.S.; Cavalcante, C.L., Jr. A rapid method for evaluation of the oxidation stability of castor oil FAME: Influence of antioxidant type and concentration. Fuel Process. Technol. 2009, 90, 1272–1277. [Google Scholar] [CrossRef]
- Denisov, E.T.; Khudyakov, I.V. Mechanisms of action and reactivities of the free radicals of inhibitors. Chem. Rev. 1987, 87, 1313–1357. [Google Scholar] [CrossRef]
- Domingos, A.K.; Saad, E.B.; Vechiatto, W.W.D.; Wilhelm, H.M.; Ramos, L.P. The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J. Braz. Chem. Soc. 2007, 18, 416–423. [Google Scholar] [CrossRef]
- Guzman, R.D.; Tang, H.; Salley, S.; Ng, K.Y.S. Synergistic effects of antioxidants on the oxidation stability of soybean oil- and poultry fat-based biodiesel. J. Am. Oil Chem. Soc. 2009, 86, 459–467. [Google Scholar] [CrossRef]
- Xin, J.; Imahara, H.; Saka, S. Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel 2009, 88, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Chen, J.H.; Luo, Y.M.; Shang, N.C.; Chang, C.H.; Chang, C.Y.; Chiang, P.C.; Shie, J.L. Property modification of jatropha oil biodiesel by blending with other biodiesels or adding antioxidants. Energy 2011, 36, 4415–4421. [Google Scholar] [CrossRef]
- Tang, H.; Guzman, R.D.; Salley, S.O.; Ng, K.Y.S. The oxidation stability of biodiesel: Effects of FAME composition and antioxidant. Lipid Technol. 2008, 20, 249–252. [Google Scholar] [CrossRef]
- Bondioli, P.; Gasparoli, A.; Bella, L.; Tagliabue, S.; Toso, G. Biodiesel stability under commercial storage conditions over one year. Eur. J. Lipid Sci. Technol. 2003, 105, 735–741. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-H.; Tang, T.-C.; Chiang, T.-H.; Huang, B.-Y.; Chang, C.-Y.; Chiang, P.-C.; Shie, J.-L.; Franzreb, M.; Chen, L.-Y. A Complementary Biodiesel Blend from Soapnut Oil and Free Fatty Acids. Energies 2012, 5, 3137-3148. https://doi.org/10.3390/en5083137
Chen Y-H, Tang T-C, Chiang T-H, Huang B-Y, Chang C-Y, Chiang P-C, Shie J-L, Franzreb M, Chen L-Y. A Complementary Biodiesel Blend from Soapnut Oil and Free Fatty Acids. Energies. 2012; 5(8):3137-3148. https://doi.org/10.3390/en5083137
Chicago/Turabian StyleChen, Yi-Hung, Ting-Cheng Tang, Tsung-Han Chiang, Bo-Yu Huang, Ching-Yuan Chang, Pen-Chi Chiang, Je-Lueng Shie, Matthias Franzreb, and Lu-Yen Chen. 2012. "A Complementary Biodiesel Blend from Soapnut Oil and Free Fatty Acids" Energies 5, no. 8: 3137-3148. https://doi.org/10.3390/en5083137
APA StyleChen, Y.-H., Tang, T.-C., Chiang, T.-H., Huang, B.-Y., Chang, C.-Y., Chiang, P.-C., Shie, J.-L., Franzreb, M., & Chen, L.-Y. (2012). A Complementary Biodiesel Blend from Soapnut Oil and Free Fatty Acids. Energies, 5(8), 3137-3148. https://doi.org/10.3390/en5083137






