Intermolecular Hydrogen Transfer in Isobutane Hydrate
Abstract
:1. Introduction
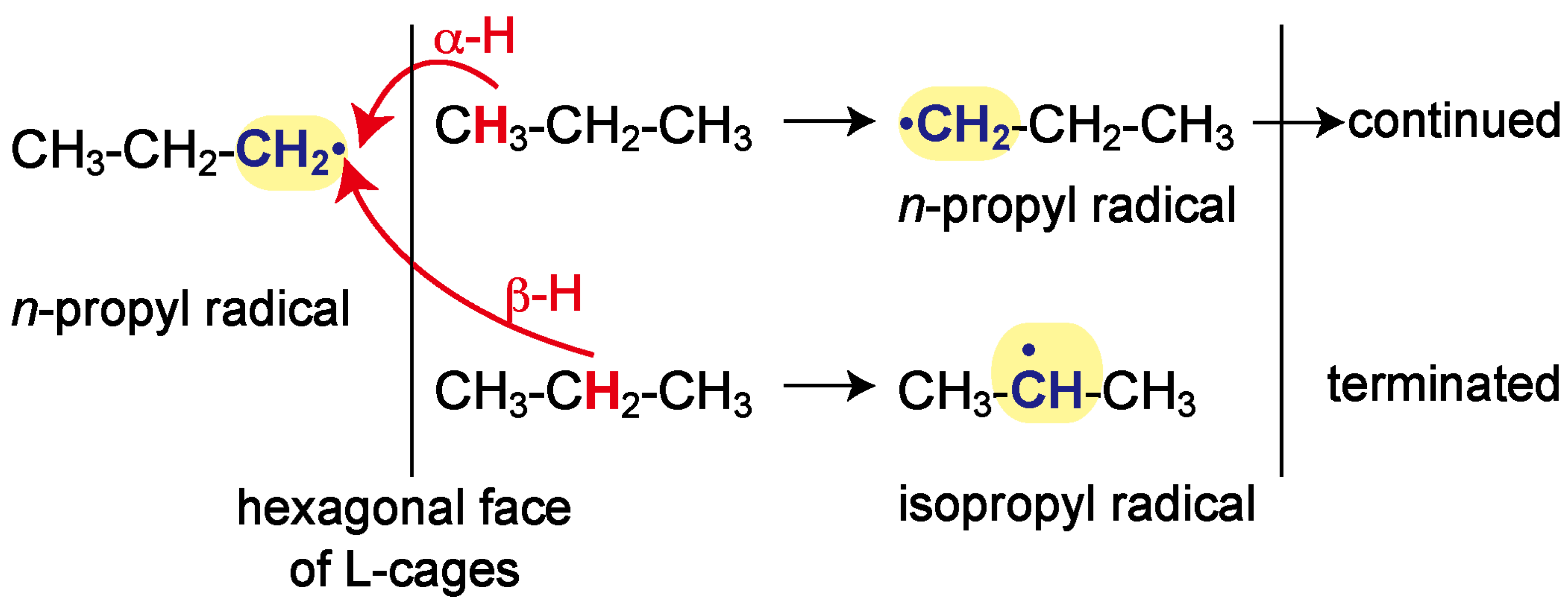
2. Results and Discussion
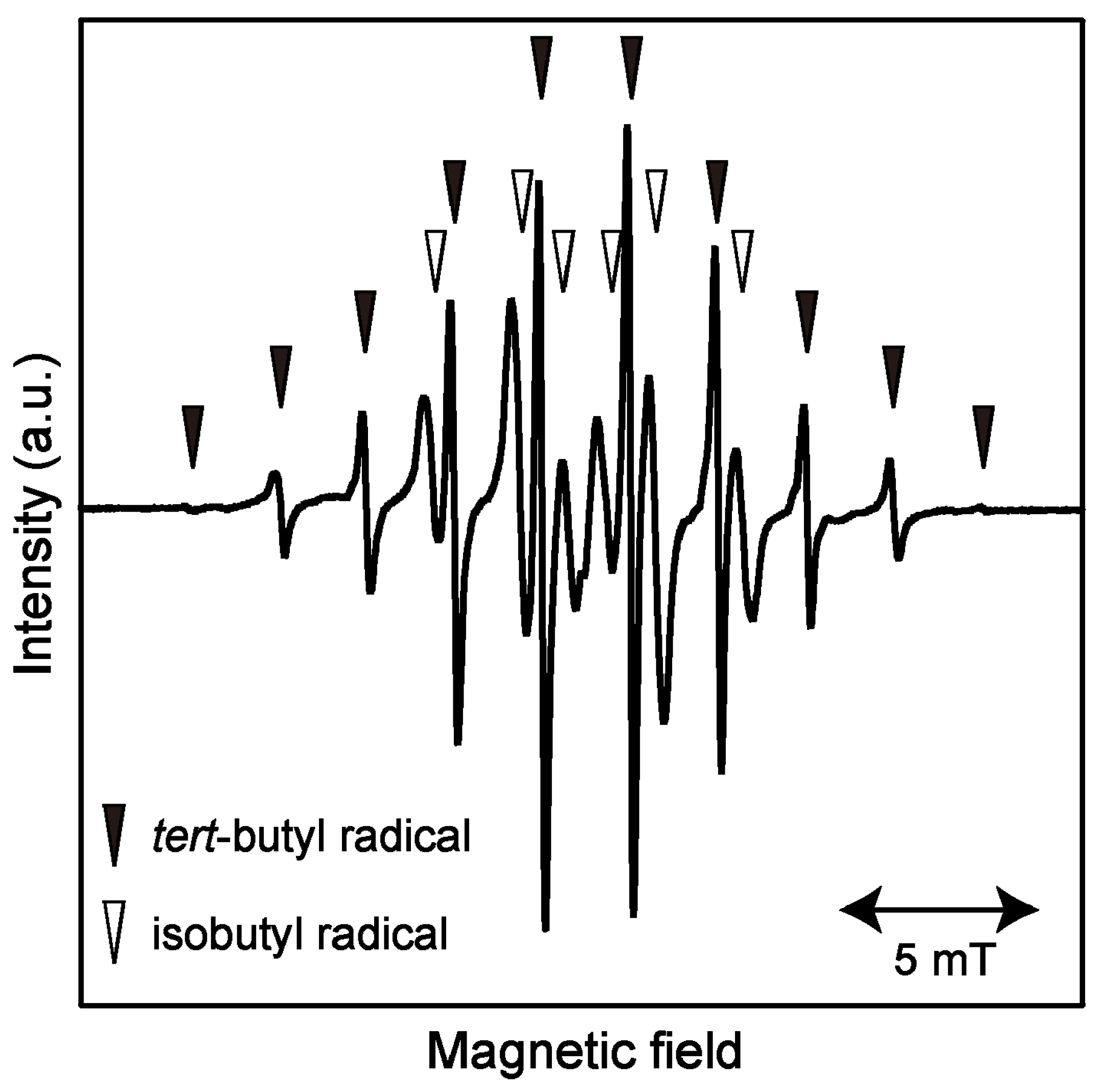
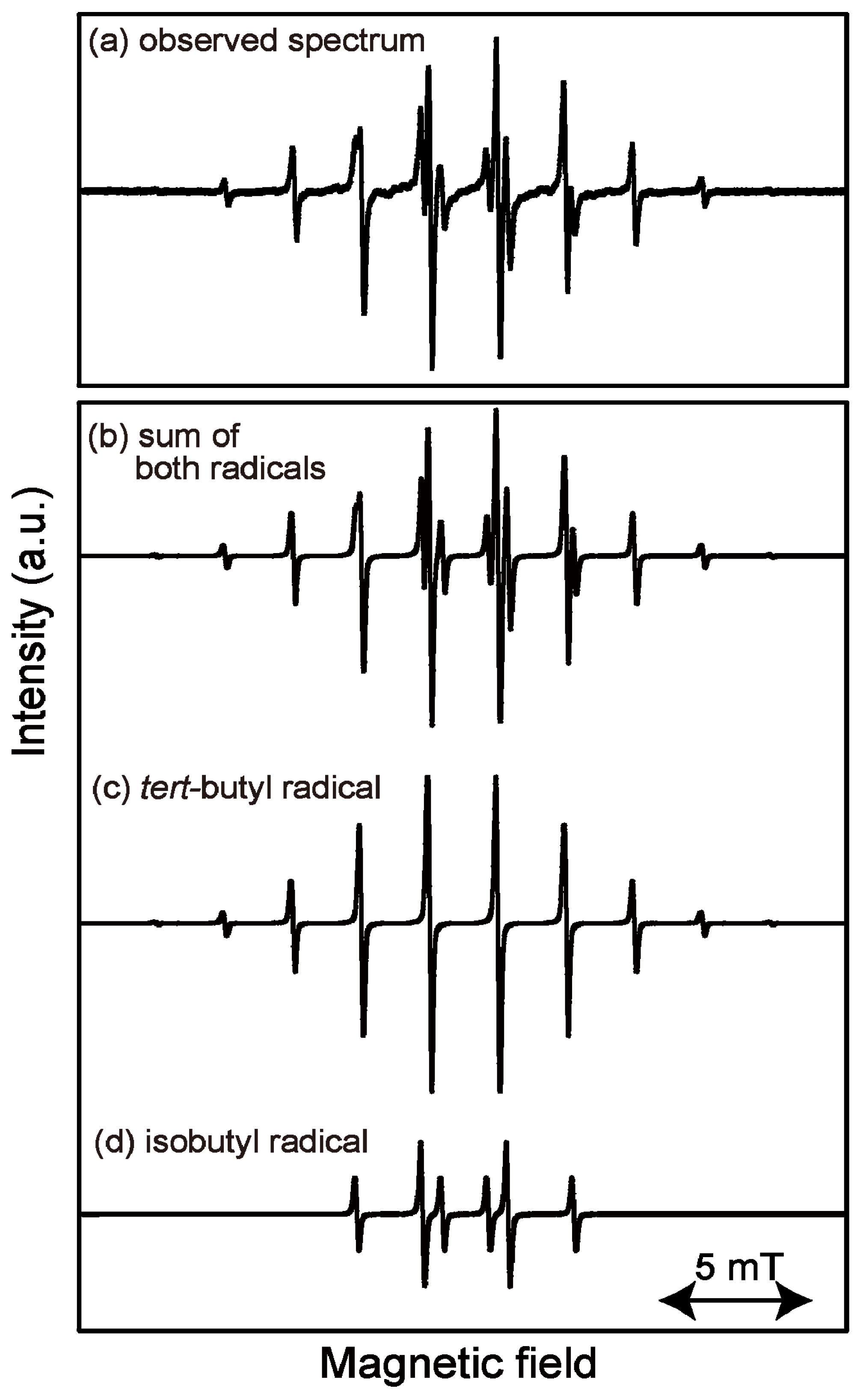
2.1. ESR Spectrum of Induced Radicals
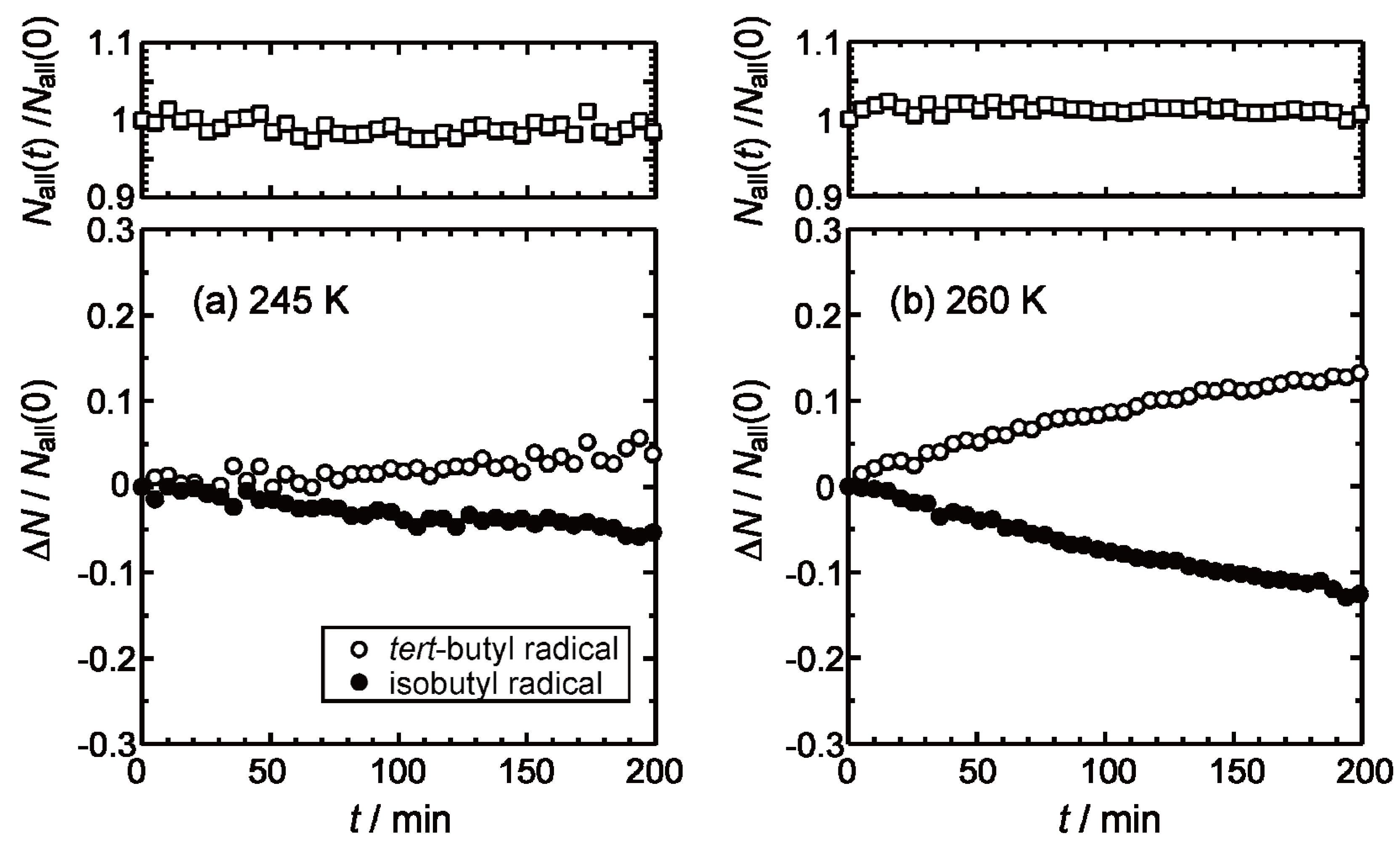
2.2. Isothermal Annealing Measurements



3. Experimental Section
4. Conclusions
Acknowledgments
References
- Takeya, K.; Tani, A.; Yada, T.; Ikeya, M.; Ohgaki, K. Electron spin resonance study on γ-ray-induced methyl radicals in methane hydrates. Jpn. J. Appl. Phys. 2004, 43, 353–357. [Google Scholar] [CrossRef]
- Takeya, K.; Nango, K.; Sugahara, T.; Ohgaki, K.; Tani, A. Activation energy of methyl radical decay in methane hydrate. J. Phys. Chem. B 2005, 109, 21086–21088. [Google Scholar] [CrossRef] [PubMed]
- Takeya, K.; Nango, K.; Sugahara, T.; Ohgaki, K.; Tani, A.; Ito, H.; Okada, M.; Kasai, T. Electron spin resonance study on gamma-ray-induced ethyl radical in ethane hydrate. Jpn. J. Appl. Phys. 2007, 46, 3066–3070. [Google Scholar] [CrossRef]
- Ohgaki, K.; Nakatsuji, K.; Takeya, K.; Tani, A.; Sugahara, T. Hydrogen transfer from guest molecule to radical in adjacent hydrate-cages. Phys. Chem. Chem. Phys. 2008, 10, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, P. Free radicals and reactive molecules in clathrate cavities. Science 1963, 142, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.H.; Seol, J.; Park, Y.; Koh, D.Y.; Kang, Y.S.; Lee, H. Spectroscopic observation of atomic hydrogen radicals entrapped in icy hydrogen hydrate. J. Am. Chem. Soc. 2008, 130, 9208–9209. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Ripmeester, J.A. Migration of hydrogen radicals through clathrate hydrate cages. Chem. Phys. Lett. 2009, 479, 234–237. [Google Scholar] [CrossRef]
- Gotoh, K.; Miyazaki, T.; Fueki, K.; Lee, K.P. ESR study of radiolysis of solid rare gas-alkane mixtures at 4.2k. Ionic fragmentation and initial energy of hot H atoms. Int. J. Radiat. Phys. Chem. 1987, 30, 89–93. [Google Scholar]
- Sugahara, T.; Kobayashi, Y.; Tani, A.; Inoue, T.; Ohgaki, K. Intermolecular hydrogen transfer between guest species in small and large cages of methane + propane mixed gas hydrates. J. Phys. Chem. A 2012, 116, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Fessenden, R.W.; Schuler, R.H. Electron spin resonance studies of transient alkyl radicals. J. Chem. Phys. 1963, 39, 2147–2195. [Google Scholar] [CrossRef]
- Kochi, J.K.; Krusic, P.J. Electron spin resonance of primary alkyl radicals. Photolysis of acyl peroxides. J. Am. Chem. Soc. 1969, 91, 3940–3942. [Google Scholar] [CrossRef]
- Rouher, O.S.; Barduhn, A.J. Hydrates of iso- and normal butane and their mixtures. Desalination 1969, 6, 57–73. [Google Scholar] [CrossRef]
- Holder, G.D.; Godbole, S.P. Measurement and prediction of dissociation pressures of isobutane and propane hydrates below the ice point. AIChE J. 1982, 28, 930–934. [Google Scholar] [CrossRef]
- Hanley, H.J.M.; Meyers, G.J.; White, J.W.; Sloan, E.D. The melting curve of tetrahydrofuran hydrate in D2O. Int. J. Thermophys. 1989, 10, 903–909. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kobayashi, N.; Minami, T.; Tani, A.; Nakagoshi, M.; Sugahara, T.; Takeya, K.; Ohgaki, K. Intermolecular Hydrogen Transfer in Isobutane Hydrate. Energies 2012, 5, 1705-1712. https://doi.org/10.3390/en5061705
Kobayashi N, Minami T, Tani A, Nakagoshi M, Sugahara T, Takeya K, Ohgaki K. Intermolecular Hydrogen Transfer in Isobutane Hydrate. Energies. 2012; 5(6):1705-1712. https://doi.org/10.3390/en5061705
Chicago/Turabian StyleKobayashi, Naohiro, Takashi Minami, Atsushi Tani, Mikio Nakagoshi, Takeshi Sugahara, Kei Takeya, and Kazunari Ohgaki. 2012. "Intermolecular Hydrogen Transfer in Isobutane Hydrate" Energies 5, no. 6: 1705-1712. https://doi.org/10.3390/en5061705




