Simulation of Synthesis Gas Production from Steam Oxygen Gasification of Colombian Coal Using Aspen Plus®
Abstract
:1. Introduction
| Parameter | w/w (%) |
|---|---|
| Proximate analysis | |
| Moisture | 4.12 |
| Ash | 5.61 |
| Fixed carbon | 67.84 |
| Volatiles | 22.43 |
| Ultimate analysis * | |
| Carbon | 75.69 |
| Hydrogen | 5.29 |
| Nitrogen | 1.58 |
| Chloride | 2.35 |
| Sulfur | 1.57 |
| Ash | 5.61 |
| Oxygen | 7.91 |
Gasification Technologies
| Gasifier type | Main features |
|---|---|
| Entrained-flow | Particle size below 0.1 mm |
| High operating temperature (>1473 K) | |
| High operating pressure (3 to 12 MPa) | |
| High oxidant demand | |
| Short residence time (0.5 to 10 s) | |
| Ash is removed as molten slag | |
| Fluidized-bed | Particle size between 6 and 10 mm |
| Uniform temperature distribution | |
| High operating temperature (1073 to 1323 K) | |
| Lower carbon conversion | |
| Ash is removed as slag or dry | |
| Fixed-bed | Coarse particles (6 to 50 mm) |
| Low operating temperature (698 to 1088 K) | |
| Low oxidant demand | |
| Residence time above 600 s | |
| Ash is removed as slag or dry |
2. Process Description
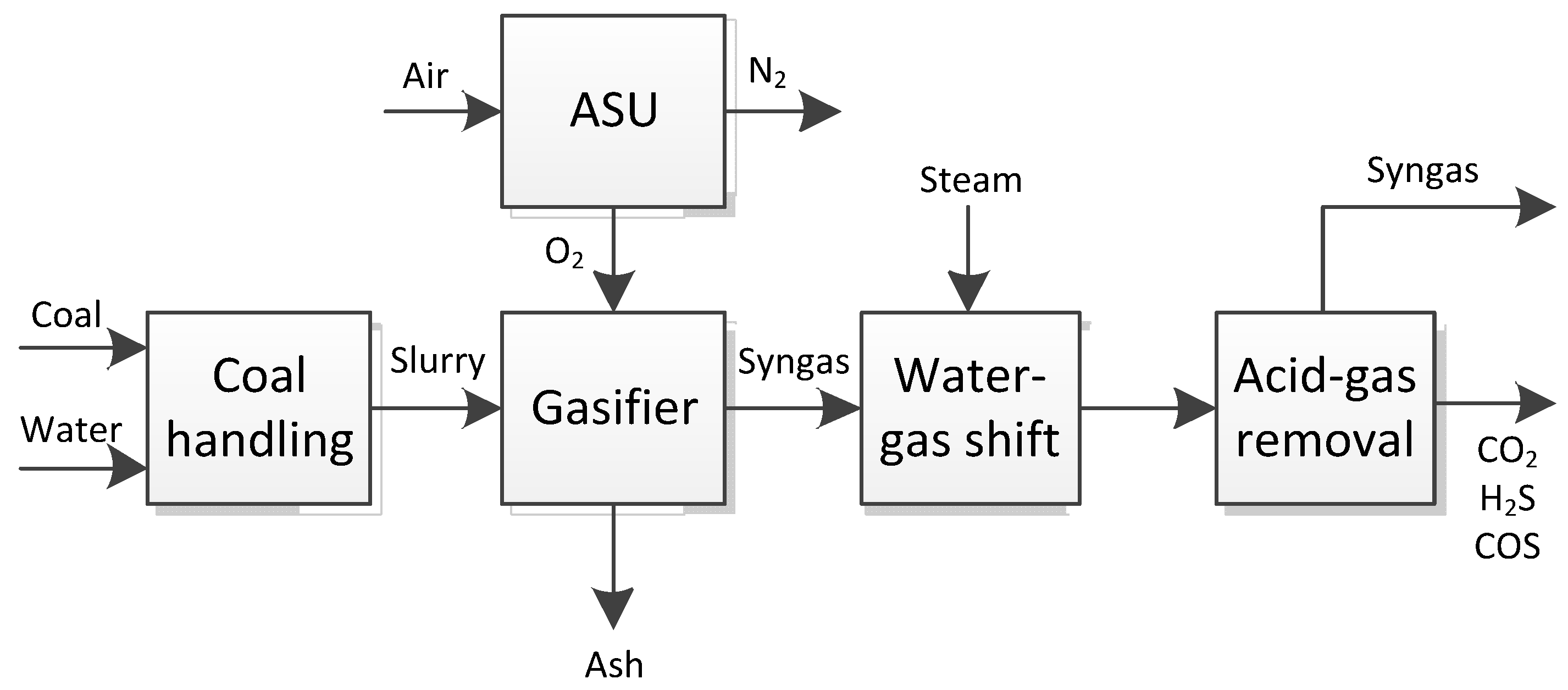
3. Simulation Model
| Unit operation | Aspen Plus model | Comments/specifications |
|---|---|---|
| ASU | RadFrac | LPC: Rigurous distillation model, first stage to separate N2 and O2. SN 40, RR 12.3, BR 41.3, partial-vapor condenser, TSP 0.14 MPa, CPD 0.005 MPa. |
| HPC: Rigurous distillation model, second stage to separate N2 and O2. SN 26, RR 0.5, BR 1.0, partial-vapor condenser, TSP 0.6 MPa, CPD 0.05 MPa. | ||
| Coal Gasification | RGibbs | Specification of the possible products: CO, CO2, C, H2, H2O, CH4, SO2, H2S, S, CS2, COS, N2, NH3, HCN, O2, NO2, NO3. |
| HTS reactor | REquil | Specification of the stoichometric reactions. OP 3.8 MPa, OT 623 K. |
| LTS reactor | REquil | Specification of the stoichometric reactions. OP 0.5 MPa, OT 473 K. |
| CH3OH absorber | Radfrac | Rigorous absorption of H2S, SO2, COS, NH3, HCN. SN 10, TSP 3.2 MPa. |
3.1. Physical Property Method
3.2. Chemical Reactions
| Reaction | Reaction name | Heat of reaction (kJ mol−1) | Reaction number |
|---|---|---|---|
| Carbon combustion | −393 | R1 | |
| Carbon combustion | −221 | R2 | |
| Boudouard | +173 | R3 | |
| Steam gasification | +131 | R4 | |
| Water gas shift | −412 | R5 | |
| Steam reforming | −206 | R6 | |
| Methanation | −165 | R7 | |
| Sulfur combustion | −297 | R8 | |
| H2S formation | −207 | R9 | |
| CS2 formation | +115 | R10 | |
| COS formation | +63 | R11 | |
| NH3 formation | −46 | R12 | |
| NO2 formation | +66 | R13 | |
| COS hydrolysis | −34 | R14 |
4. Results and Discussion
4.1. Thermal Efficiency
| Variable | ηTE, % | LHV, MJ kg−1 | LHV, MJ Nm−3 | H2 molar fraction in H2-rich syngas |
|---|---|---|---|---|
| O2 to carbon ratio † | ||||
| 0.160 | 34.1 | 55.5 | 20.9 | 0.561 |
| 0.320 | 42.2 | 69.0 | 15.1 | 0.806 |
| 0.480 | 52.0 | 79.8 | 13.0 | 0.895 |
| 0.640 | 62.6 | 83.4 | 12.0 | 0.922 |
| 0.800 | 60.1 | 92.7 | 10.8 | 0.977 |
| 0.960 | 54.5 | 97.3 | 10.7 | 0.983 |
| Coal slurry concentration (% w/w) ‡ | ||||
| 86.21 | 61.3 | 61.8 | 10.8 | 0.926 |
| 75.47 | 60.4 | 87.0 | 10.8 | 0.971 |
| 65.01 | 59.9 | 95.1 | 10.8 | 0.979 |
| 56.34 | 59.2 | 97.0 | 11.0 | 0.974 |
| 50.00 | 58.4 | 93.4 | 11.5 | 0.958 |
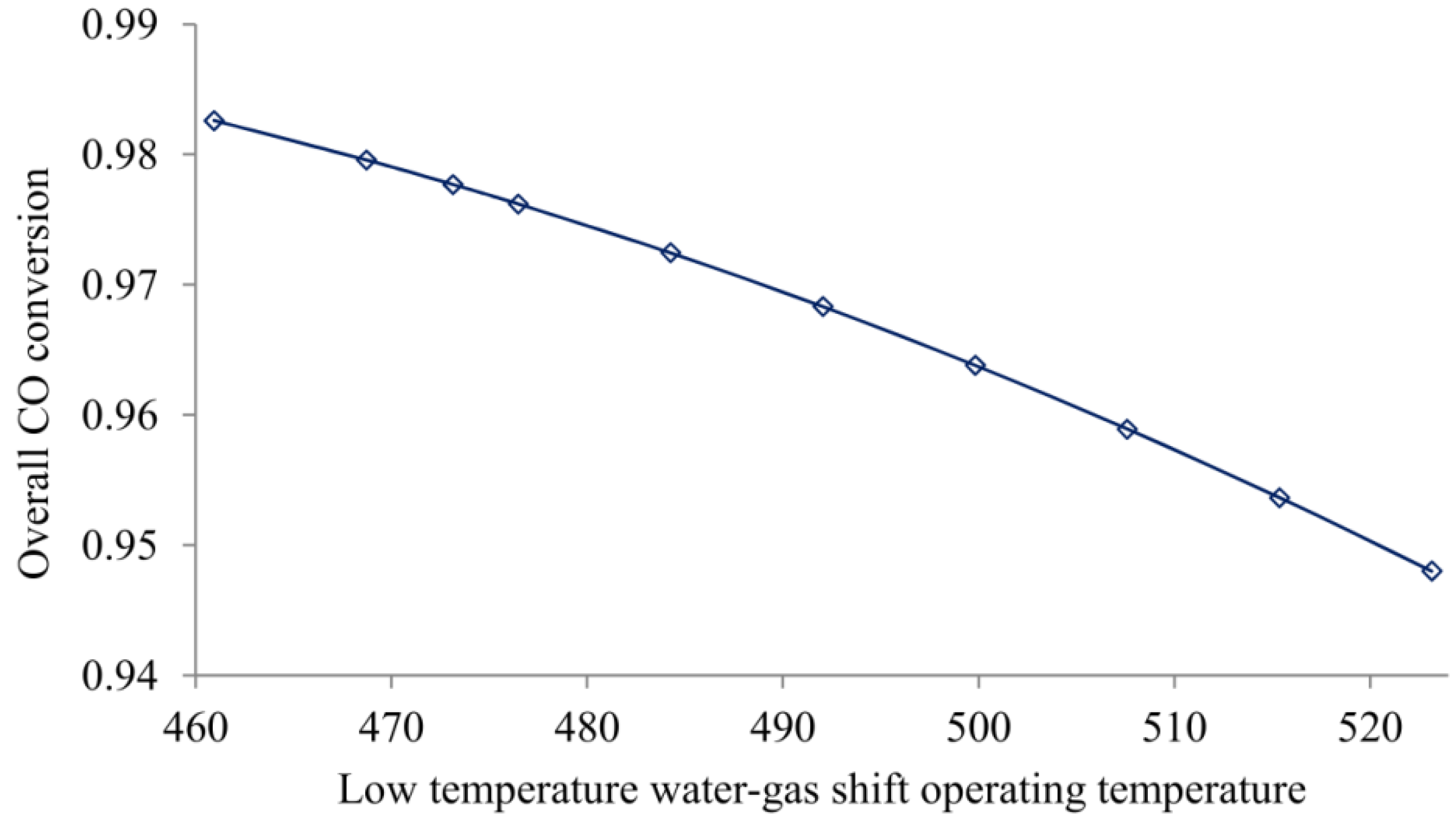
| LTS reactor temperature (K) ** | ||||
| 453 | 59.5 | 99.3 | 10.7 | 0.983 |
| 473 | 59.9 | 95.1 | 10.8 | 0.979 |
| 498 | 60.4 | 88.6 | 10.8 | 0.971 |
| 523 | 61.5 | 81.5 | 10.8 | 0.962 |
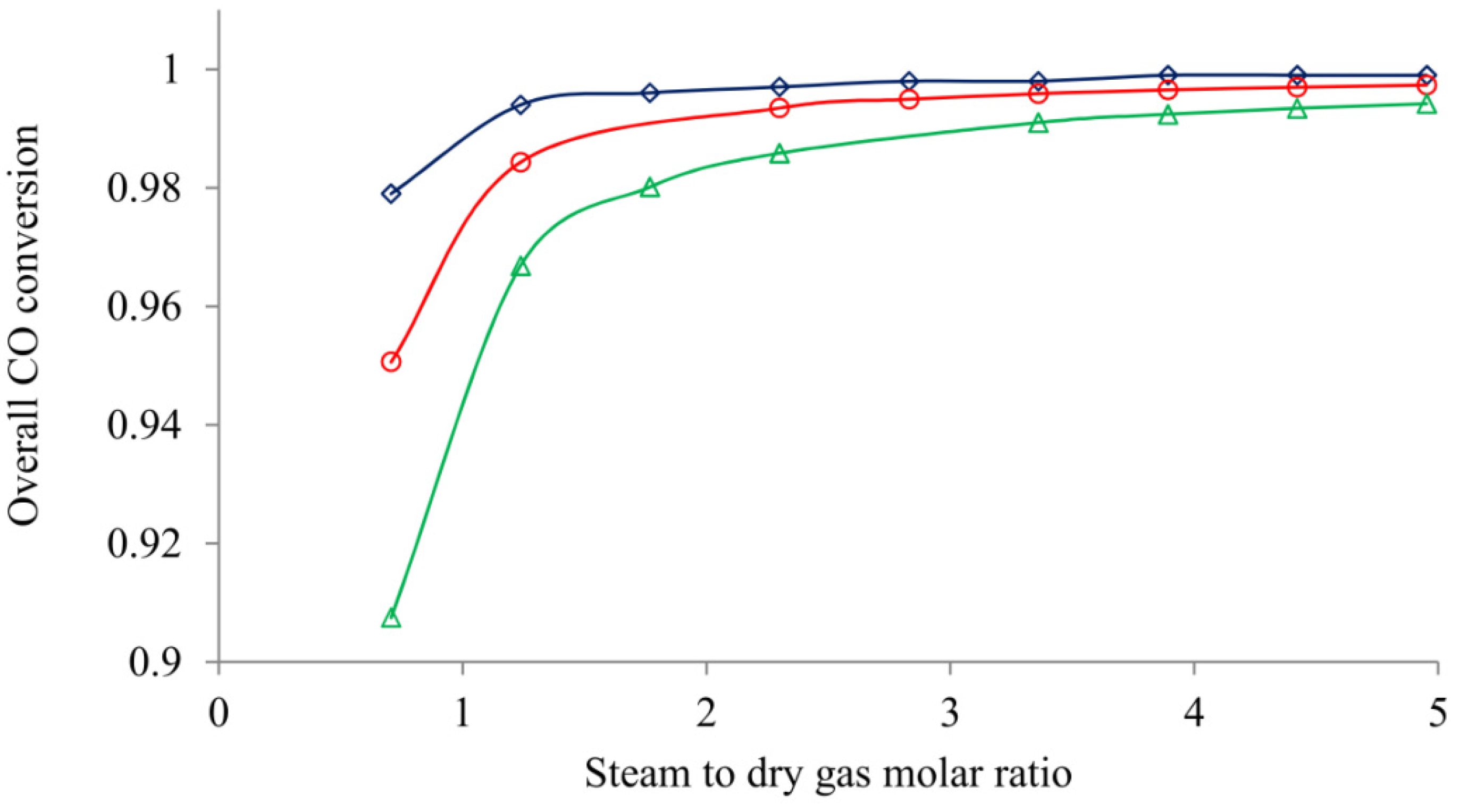
| SDG gas molar ratio in WGS †† | ||||
| 0.694 | 59.9 | 95.1 | 10.8 | 0.979 |
| 0.972 | 58.6 | 102.5 | 10.8 | 0.986 |
| 1.768 | 55.3 | 106.3 | 10.8 | 0.989 |
| 2.564 | 52.4 | 107.3 | 10.8 | 0.990 |
| 3.360 | 49.8 | 107.7 | 10.8 | 0.990 |
| 3.917 | 48.2 | 107.9 | 10.8 | 0.991 |
4.2. Oxygen to Carbon Mass Ratio Effect

4.3. Coal Slurry Concentration Effect
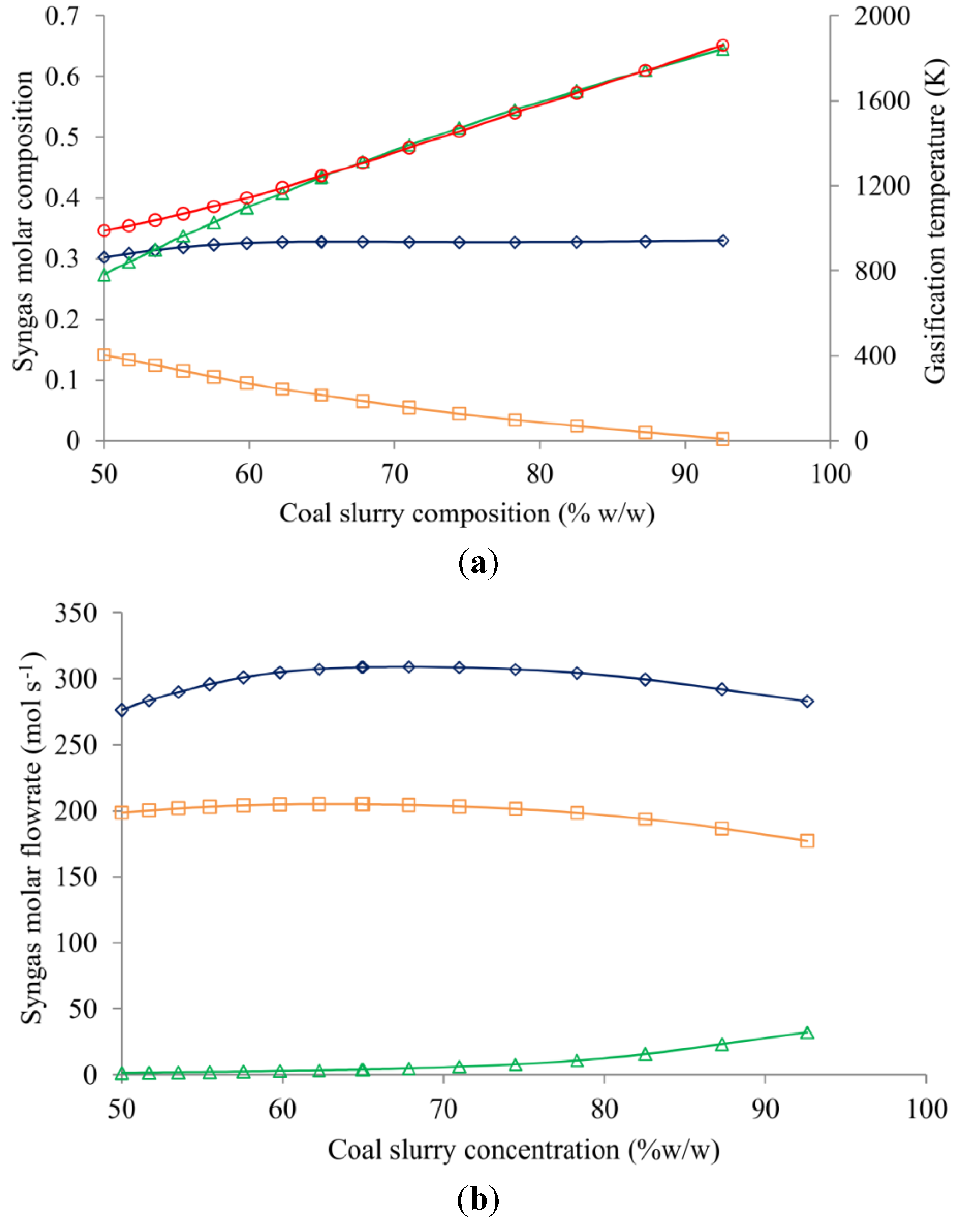
4.4. WGS Reactor Operating Temperature Effect
4.5. Effect of Steam to Dry Gas Molar Ratio
4.6. Optimal Syngas Composition
| Component | H2-rich syngas molar fraction |
|---|---|
| H2O | 6.47 × 10−11 |
| H2 | 0.922 |
| N2 | 7.13 × 10−3 |
| Cl2 | 0.00 |
| CO | 9.58 × 10−3 |
| CO2 | 6.00 × 10−6 |
| CH4 | 0.062 |
| H2S | 0.00 |
| COS | 0.00 |
| NH3 | 1.74 × 10−7 |
| HCN | 0.00 |
| CH4OH | 4.15 × 10−6 |
5. Conclusions
Abbreviations
| ASU | Air separation unit |
| HPC | High pressure column |
| HTS | High temperature reactor |
| LHV | Lower heating value, MJ Nm−3 |
| LPC | Low pressure column |
| LTS | Low temperature reactor |
| SOG | Steam-oxygen gasification |
| SDG | Steam to dry gas ratio |
| Syn | Syngas |
| WGS | Water-gas shift |
Nomenclature
| M | Mass flow rate (kg h−1) |
| n | Mass fraction |
| QAux | Auxiliary power required (MJ h−1) |
| ηTE | Thermal efficiency (%) |
| ρ | Syngas density (kg m−3) |
References
- Jin, H.; Lu, Y.; Liao, B.; Guo, L.; Zhang, X. Hydrogen production by coal gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2010, 35, 7151–7160. [Google Scholar] [CrossRef]
- Ramos, I.A.C.; Montini, T.; Lorenzut, B.; Troiani, H.; Gennari, F.C.; Graziani, M.; Fornasiero, P. Hydrogen production from ethanol steam reforming on M/CeO2/YSZ (M = Ru, Pd, Ag) nanocomposites. Catal. Today 2012, 180, 96–104. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M. Gasification, 2nd ed.; Gulf Professional Publishing: Burlington, VT, USA, 2008. [Google Scholar]
- Liu, K.; Cui, Z.; Fletcher, T.H. Coal Gasification. In Hydrogen and Syngas Production and Purification Technologies; Liu, K., Song, C., Subramani, V., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 156–218. [Google Scholar]
- Giunta, P.; Amadeo, N.; Laborde, M. Simulation of a low temperature water gas shift reactor using the heterogeneous model/application to a pem fuel cell. J. Power Sources 2006, 156, 489–496. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Statistical Review of World Energy 2012; Technical Reports for BP Global: London, UK, June 2012; Available online: http://www.bp.com/statisticalreview (accessed on 1 September 2012).
- Stańczyk, K.; Kapusta, K.; Wiatowski, M.; Świądrowski, J.; Smoliński, A.; Rogut, J.; Kotyrba, A. Experimental simulation of hard coal underground gasification for hydrogen production. Fuel 2012, 91, 40–50. [Google Scholar] [CrossRef]
- Jones, R.H.; Thomas, G.J. Materials for the Hydrogen Economy; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- U.S. Department of Energy. Pioneering Gasification Plants. Available online: http://www.fossil.energy.gov/programs/powersystems/gasification/gasificationpioneer.html (accessed on 1 September 2012).
- Ministerio de Minas y Energı́a de Colombia; Instituto Colombiano de Geología y Minería. El Carbón Colombiano: Recursos, Reservas y Calidad; Ingenominas: Bogotá, Colombia, 2004. [Google Scholar]
- Minchener, A.J. Coal gasification for advanced power generation. Fuel 2005, 84, 2222–2235. [Google Scholar] [CrossRef]
- Emun, F.; Gadalla, M.; Majozi, T.; Boer, D. Integrated gasification combined cycle (IGCC) process simulation and optimization. Comput. Chem. Eng. 2010, 34, 331–338. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Ramzan, N.; Ashraf, A.; Naveed, S.; Malik, A. Simulation of hybrid biomass gasification using Aspen plus: A comparative performance analysis for food, municipal solid and poultry waste. Biomass Bioenergy 2011, 35, 3962–3969. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Y.; Xiao, J. Simulation of hydrogen production from biomass gasification in interconnected fluidized beds. Biomass Bioenergy 2008, 32, 120–127. [Google Scholar] [CrossRef]
- Liu, B.; Yang, X.; Song, W.; Lin, W. Process simulation development of coal combustion in a circulating fluidized bed combustor based on aspen plus. Energy Fuels 2011, 25, 1721–1730. [Google Scholar] [CrossRef]
- Robinson, P.J.; Luyben, W.L. Simple dynamic gasifier model that runs in aspen dynamics. Ind. Eng. Chem. Res. 2008, 47, 7784–7792. [Google Scholar] [CrossRef]
- Chiesa, P.; Consonni, S.; Kreutz, T.; Robert, W. Co-production of hydrogen, electricity and CO2 from coal with commercially ready technology. Part A: Performance and emissions. Int. J. Hydrogen Energy 2005, 30, 747–767. [Google Scholar] [CrossRef]
- Davison, T.Y. GE Gasification Project Update. In Presented at Gasification 11 Conference, Cagliari, Italy, 8–11 May 2012.
- Smith, A.R.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Vinson, D.R. Air separation control technology. Comput. Chem. Eng. 2006, 30, 1436–1446. [Google Scholar] [CrossRef]
- Cryogenic Air Separation: History and Technological Progress; The Linde Group: Vresova, Czech Republic; Available online: http://www.linde-le.de/process_plants/air_separation_plants/documents/L_2_1_e_09_150dpi.pdf (accessed on 1 September 2012).
- Castle, W.F. Air separation and liquefaction: Recent developments and prospects for the beginning of the new millennium. Int. J. Refrig. 2002, 25, 158–172. [Google Scholar] [CrossRef]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design—A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Haldor Topsøe. Sulphur Resistant/Sour Water-Gas Shift Catalyst. Available online: http://www.topsoe.com/business_areas/gasification_based/Processes/~/media/PDF%20files/SSK/topsoe_SSK%20brochure_aug09.ashx (accessed on 1 September 2012).
- Bell, D.; Towler, B. Coal Gasification and Its Applications; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Korens, N.; Simbeck, D.; Wilhelm, D. Process Screening Analysis of Alternative Gas Treating and Sulfur Removal for Gasification; Technical Report for National Energy Technology Laboratory, U.S. Department of Energy: Pittsburgh, PA, USA, December 2002.
- Heil, S.; Brunhuber, C.; Link, K.; Kittel, J.; Meyer, B. Dynamic Modelling of CO2-Removal Units for an IGCC power plant. In Proceedings of the 7th Modelica Conference, Como, Italy, 20–22 September 2009; The Modelica Association: Como, Italy; pp. 77–85.
- Larson, E.D.; Consonni, S.; Katofsky, R.E.; Consulting, N.; Burlington, I.; Iisa, K.; Frederick, W.J., Jr. A Cost-Benefit Assessment of Gasification-Based Biorefining in the Kraft Pulp and Paper Industry; Technical Report for Princeton University: Princeton, NJ, USA, 21 December 2006. [Google Scholar]
- Kabadi, V.N.; Danner, R.P. A modified Soave-Redlich-Kwong equation of state for water-hydrocarbon phase equilibria. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 537–541. [Google Scholar] [CrossRef]
- Yuehong, Z.; Hao, W.; Zhihong, X. Conceptual design and simulation study of a co-gasification technology. Energy Convers. Manag. 2006, 47, 1416–1428. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.-Q.; Yan, J.-H.; Chi, Y. Simulation of municipal solid waste gasification in two different types of fixed bed reactors. Fuel 2011, in press. [Google Scholar]
- Wang, Y.; Dong, W.; Dong, L.; Yue, J.; Gao, S.; Suda, T.; Xu, G. Production of middle caloric fuel gas from coal by dual-bed gasification technology. Energy Fuels 2010, 24, 2985–2990. [Google Scholar] [CrossRef]
- Wu, Y.X.; Zhang, J.S.; Smith, P.J.; Zhang, H.; Reid, C.; Lv, J.F.; Yue, G.X. Three-dimensional simulation for an entrained flow coal slurry gasifier. Energy Fuels 2010, 24, 1156–1163. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, T.J.; Kim, J.H.; Lee, J.G.; Hong, J.C.; Kim, Y.G. Experimental studies of 1 ton/day coal slurry feed type oxygen blown, entrained flow gasifier. Korean J. Chem. Eng. 2001, 18, 493–498. [Google Scholar] [CrossRef]
- Seo, H.K.; Park, S.; Lee, J.; Kim, M.; Chung, S.W.; Chung, J.H.; Kim, K. Effects of operating factors in the coal gasification reaction. Korean J. Chem. Eng. 2011, 28, 1851–1858. [Google Scholar] [CrossRef]
- Grol, E.; Yang, W.-C. Evaluation of Alternate Water Gas Shift Configurations for IGCC Systems; Technical Report for National Energy Technology Laboratory, U.S. Department of Energy: Pittsburgh, PA, USA, 5 August 2009.
- Walton, S.M.; He, X.; Zigler, B.T.; Wooldridge, M.S. An experimental investigation of the ignition properties of hydrogen and carbon monoxide mixtures for syngas turbine applications. Proc. Combust. Inst. 2007, 31, 3147–3154. [Google Scholar] [CrossRef]
- Davison, J.; Bressan, L.; Domenichini, R. CO2 capture in coal-based igcc power plants. In Proceedings of the 7th International Conference in Greenhouse Gas Control Technologies, Vancouver, Canada, 5–9 September 2004.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Preciado, J.E.; Ortiz-Martinez, J.J.; Gonzalez-Rivera, J.C.; Sierra-Ramirez, R.; Gordillo, G. Simulation of Synthesis Gas Production from Steam Oxygen Gasification of Colombian Coal Using Aspen Plus®. Energies 2012, 5, 4924-4940. https://doi.org/10.3390/en5124924
Preciado JE, Ortiz-Martinez JJ, Gonzalez-Rivera JC, Sierra-Ramirez R, Gordillo G. Simulation of Synthesis Gas Production from Steam Oxygen Gasification of Colombian Coal Using Aspen Plus®. Energies. 2012; 5(12):4924-4940. https://doi.org/10.3390/en5124924
Chicago/Turabian StylePreciado, Jorge E., John J. Ortiz-Martinez, Juan C. Gonzalez-Rivera, Rocio Sierra-Ramirez, and Gerardo Gordillo. 2012. "Simulation of Synthesis Gas Production from Steam Oxygen Gasification of Colombian Coal Using Aspen Plus®" Energies 5, no. 12: 4924-4940. https://doi.org/10.3390/en5124924
APA StylePreciado, J. E., Ortiz-Martinez, J. J., Gonzalez-Rivera, J. C., Sierra-Ramirez, R., & Gordillo, G. (2012). Simulation of Synthesis Gas Production from Steam Oxygen Gasification of Colombian Coal Using Aspen Plus®. Energies, 5(12), 4924-4940. https://doi.org/10.3390/en5124924




