Correlation of the Growth Rate of the Hydrate Layer at a Guest/Liquid-Water Interface to Mass Transfer Resistance
Abstract
:1. Introduction
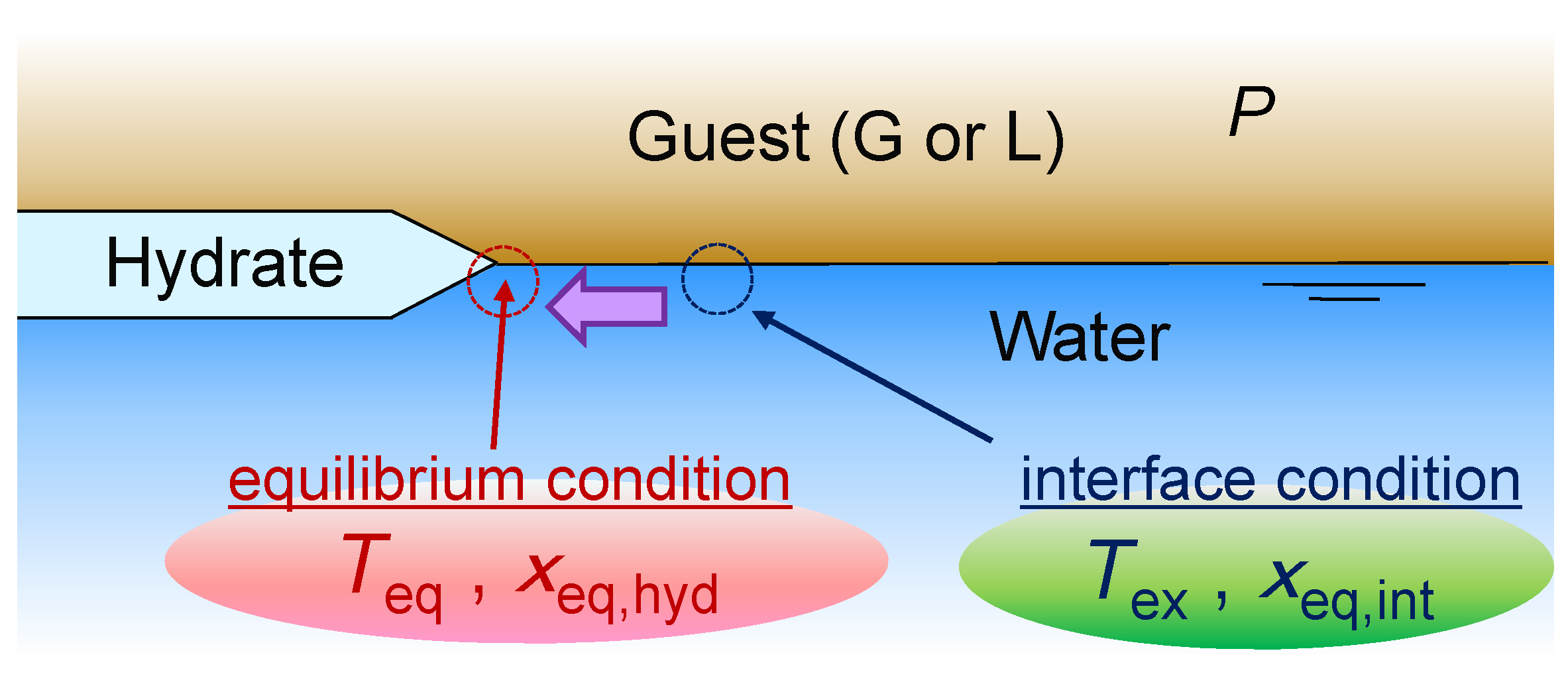
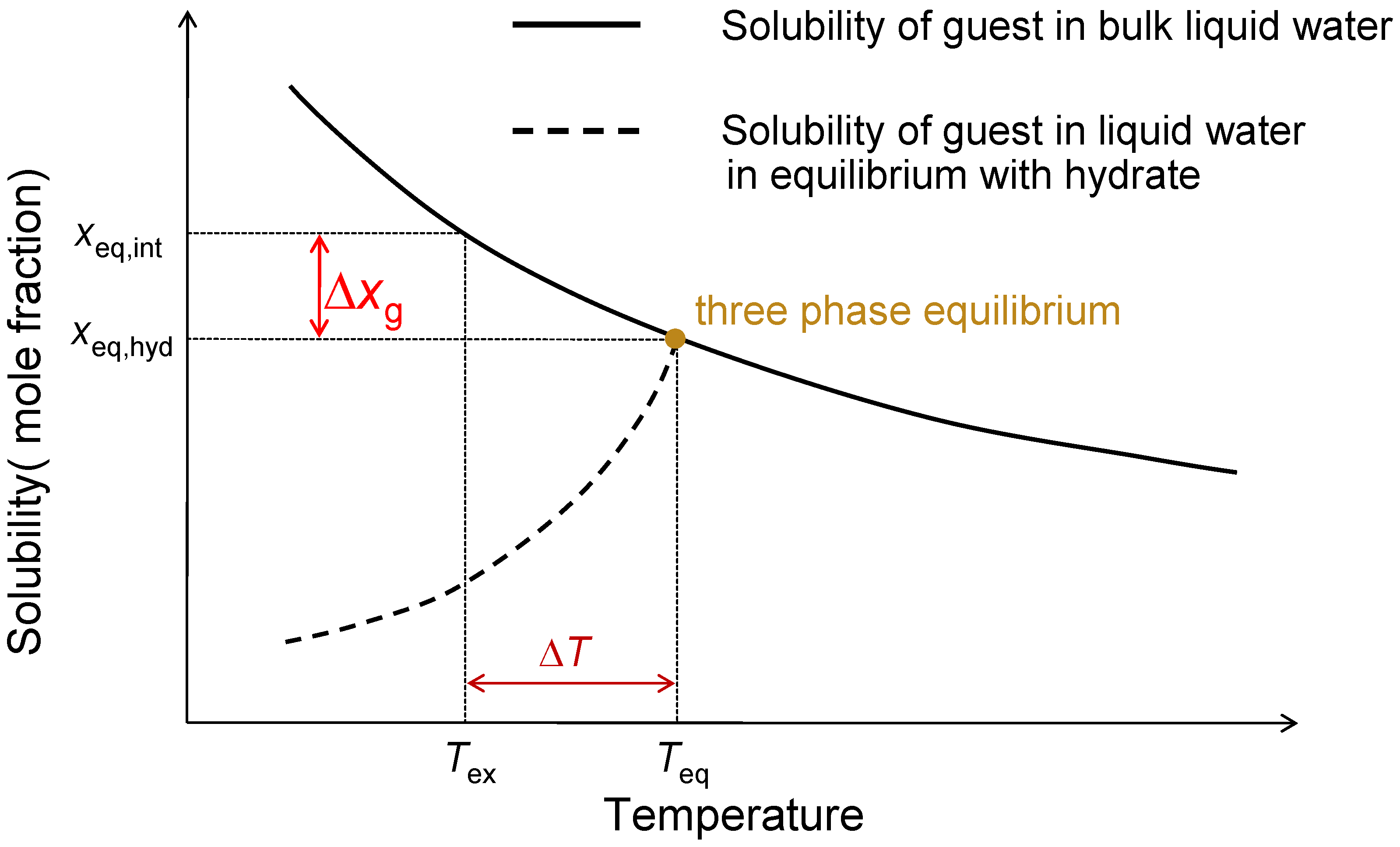
2. Formulations for the Conjugate Process of Mass Transfer and Hydrate Crystal Growth
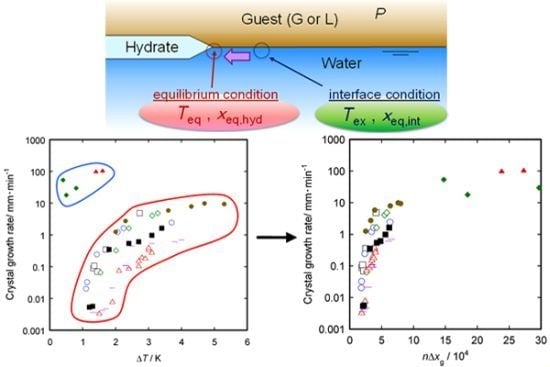
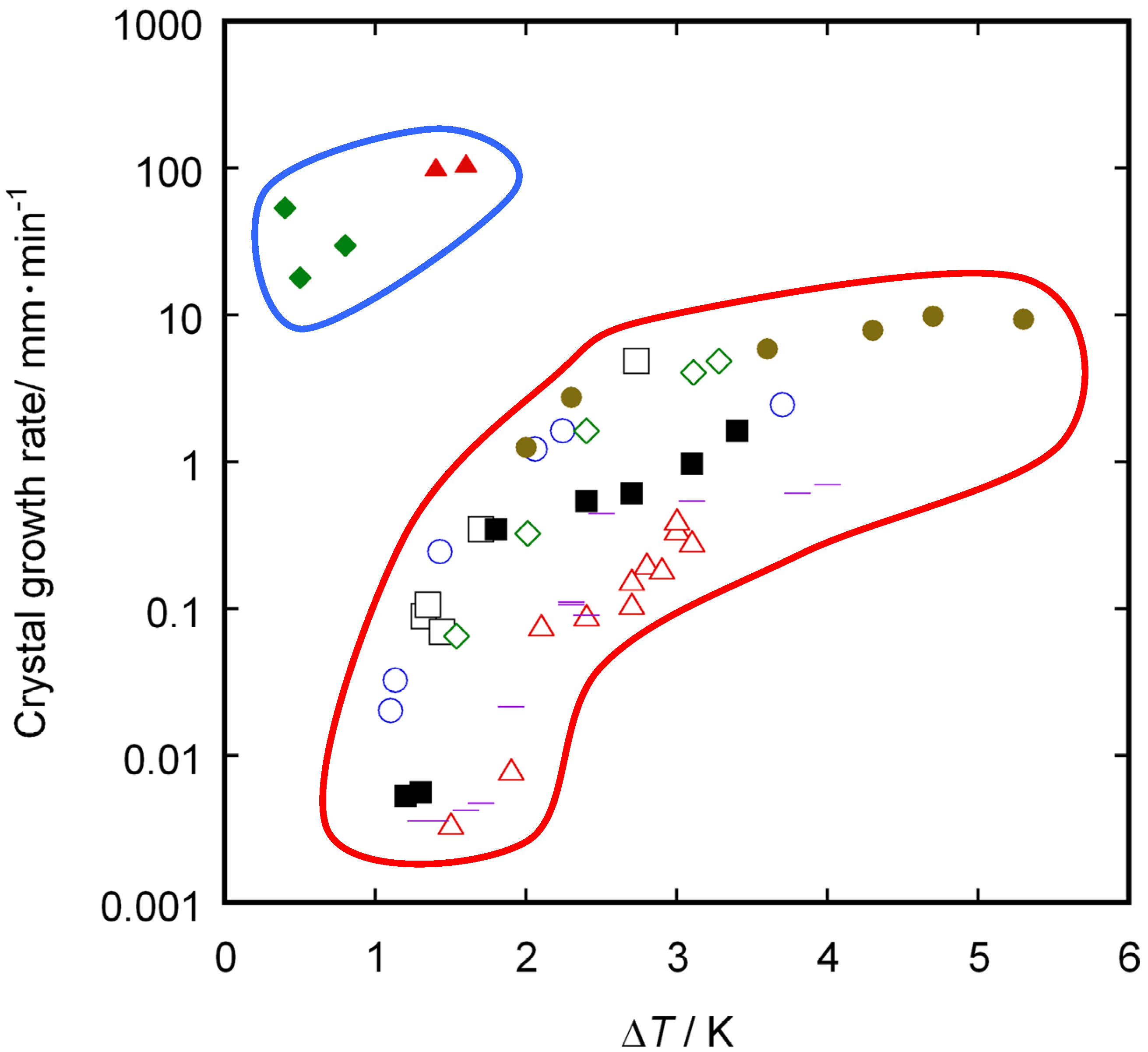
3. Results and Discussion
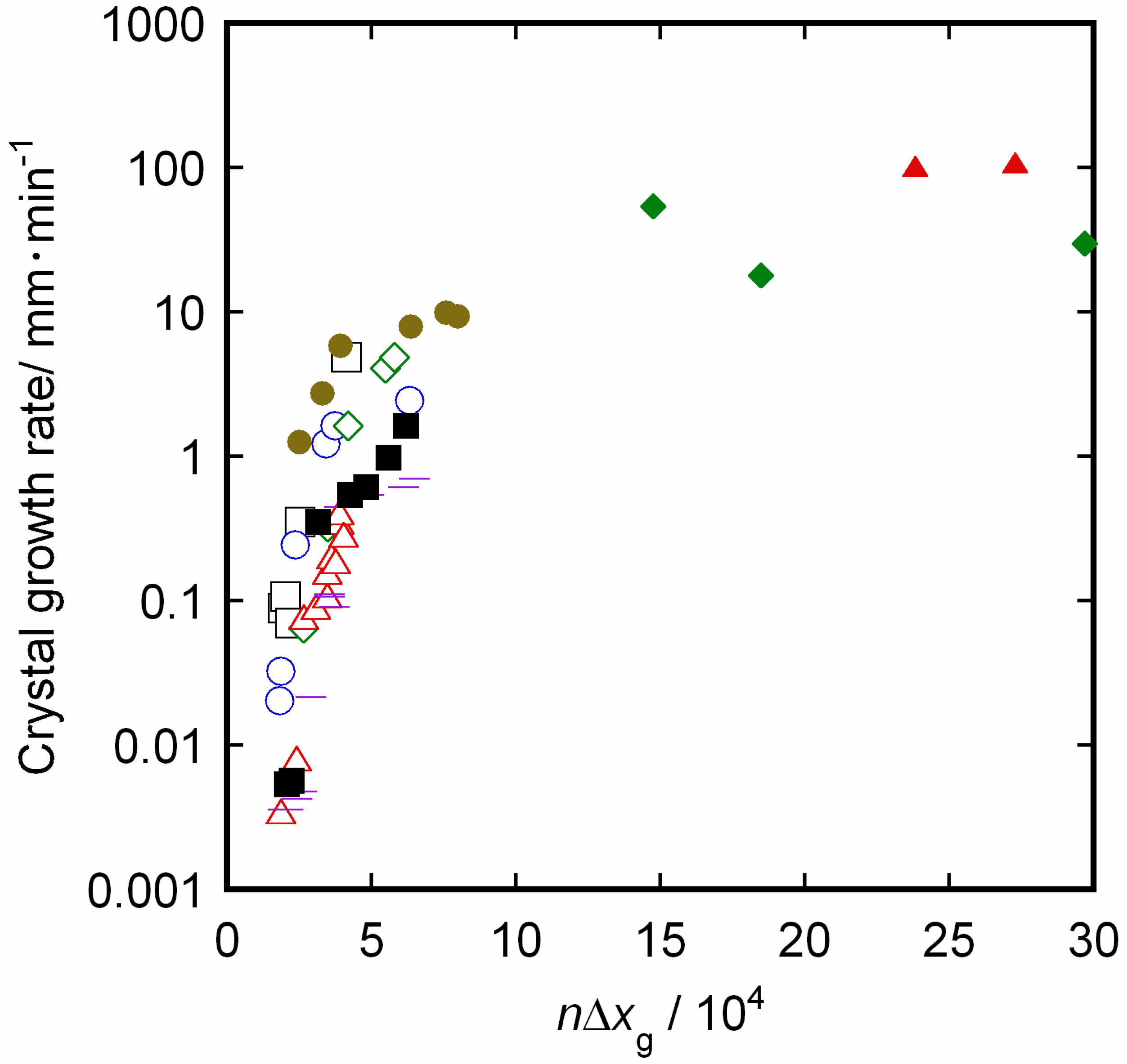
4. Conclusions
Acknowledgments
References
- Mori, Y.H. Recent advances in hydrate-based technologies for natural gas storage—A review. J. Chem. Ind. Eng. (China) 2003, 54, 1–17. [Google Scholar]
- Mao, W.L.; Mao, H. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. USA 2004, 101, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, R.; Mori, Y.H. Critical conditions for CO2 hydrate films to rest on submarine CO2 pond surfaces: A mechanistic study. Environ. Sci. Technol. 1998, 32, 1120–1127. [Google Scholar] [CrossRef]
- Brewer, P.G.; Peltzer, E.; Aya, I.; Haugan, P.; Bellerby, R.; Yaname, K.; Kojima, R.; Waltz, P.; Nakajima, Y.J. Small scale field study of an ocean CO2 plume. J. Oceanogr. 2004, 60, 751–758. [Google Scholar] [CrossRef]
- Brewer, P.G.; Frienderich, G.; Petlter, E.T.; Orr, F.M., Jr. Direct experiments on the ocean disposal of fossil fuel CO2. Science 1999, 284, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ito, T.; Watanabe, K.; Tahara, K.; Hiraoka, R.; Ochiai, J.; Ohmura, R.; Mori, Y.H. Development of a novel hydrate-based refrigeration system: A preliminary overview. Appl. Therm. Eng. 2006, 26, 2157–2167. [Google Scholar] [CrossRef]
- Kamata, Y.; Yamakoshi, Y.; Ebinuma, T.; Oyama, H.; Shimada, W.; Narita, H. Hydrogen sulfide separation using Tetra-n-butyl ammonium bromide semi-clathrate (TBAB) hydrate. Energy Fuels 2005, 19, 1717–1722. [Google Scholar] [CrossRef]
- Seo, Y.-T.; Moudravski, I.L.; Ripmeester, J.A.; Lee, J.-W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, K.; Yamamoto, Y.; Takahashi, M.; Komai, T. Interferometric observation of mass transport processes adjacent to tetrahydrofuran clathrate hydrates under nonequilibrium conditions. Fluid Phase Equilib. 2003, 214, 11–24. [Google Scholar] [CrossRef]
- Sabase, Y.; Nagashima, K. Growth mode transition of tetrahydrofuran clathrate hydrates in the guest/host concentration boundary layer. J. Phys. Chem. B 2009, 113, 15304–15311. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Mori, H.; Mochizuki, T.; Mori, Y.H. Formation and dissociation of clathrate hydrate in stoichiometric tetrahydrofuran–water mixture subjected to one-dimensional cooling or heating. Chem. Eng. Sci. 2001, 56, 4747–4758. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakemoto, R.; Ohmura, R. Crystal growth of clathrate hydrates formed at the interface of liquid water and gaseous methane, ethane, or propane: Variations in crystal morphology. Cryst. Growth Des. 2009, 9, 2529–2536. [Google Scholar] [CrossRef]
- Freer, E.M.; Selim, M.S.; Sloan, E.D. Methane hydrate film growth kinetics. Fluid Phase Equilib. 2001, 185, 65–75. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Kawabata, J.; Narita, H. Microscopic observations of formation processes of clathrate-hydrate films at an interface between water and carbon dioxide. J. Cryst. Growth 1999, 204, 348–356. [Google Scholar] [CrossRef]
- Ohmura, R.; Shimada, W.; Uchida, T.; Mori, Y.H.; Takya, S; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H. Clathrate hydrate crystal growth in liquid water saturated with a hydrate-forming substance: variations in crystal morphology. Philos. Mag. 2004, 84, 1–16. [Google Scholar] [CrossRef]
- Taylor, C.J.; Miller, K.T.; Koh, A.; Sloan, E.D. Macroscopic investigation of hydrate film growth at the hydrocarbon/water interface. Chem. Eng. Sci. 2007, 62, 6542–6533. [Google Scholar] [CrossRef]
- Peng, B.Z.; Dandekar, A.; Sun, C.Y.; Ma, Q.L.; Pang, W.X.; Chen, G.J. Hydrate film growth on the surface of a gas bubble suspended in water. J. Phys. Chem. B 2007, 111, 12485–12493. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Sum, A.K.; Ohmura, R. Correlation of hydrate-film growth rate at the guest/liquid-water interface to mass transfer resistance. Ind. Eng. Chem. Res. 2010, 49, 7102–7103. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Hyduk, W.; Laudie, H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChE J. 1974, 20, 611–615. [Google Scholar] [CrossRef]
- HWHydrateGUI (version 1.1). Phase-equilibrium calculation program developed at the Centre for Gas Hydrate Research. Heriot-Watt University: Edinburgh, UK, 2005.
- Dodds, W.S.; Stutzman, L.F.; Sollami, B.J. Carbon dioxide solubility in water. Ind. Eng. Chem., Chem. Eng. Data Ser. 1956, 1, 92–95. [Google Scholar] [CrossRef]
- Lekvam, K.; Bishnoi, P.R. Dissolution of methane in water at low temperature and intermediate pressures. Fluid Phase Equilib. 1997, 131, 297–309. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Measurement of dissolved methane in water in equilibrium with its hydrate. J. Chem. Eng. 2002, 47, 87–90. [Google Scholar]
- Gaudette, J.; Servio, P. Measurement of dissolved propane in water in the presence of gas hydrate. J. Chem. Eng. Data 2007, 52, 1449–1451. [Google Scholar] [CrossRef]
- Sum, A.K.; Burruss, R.C.; Sloan, E.D. Measurement of clathrate hydrates via raman spectroscopy. J. Phys. Chem. B 1997, 101, 7371–7377. [Google Scholar] [CrossRef]
- Uchida, T.; Hirano, T.; Ebinuma, T.; Narita, H.; Gohara, K.; Mae, S.; Matsumoto, R. Raman spectroscopic determination of hydration number of methane hydrates. AIChE J. 1999, 45, 2641–2645. [Google Scholar] [CrossRef]
- Udachin, K.A.; Ratcliffe, C.I.; Ripmeester, J.A. Structure, composition, and thermal expansion of CO2 hydrate from single crystal X-ray diffraction measurements. J. Phys. Chem. B 2001, 105, 4200–4204. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Brouwer, E.B.; Maclaurin, G.; Lee, M.; Parks, D.; Ripmeester, J.A. Some new halogen-containing hydrate-formers for structure I and II clathrate hydrates. Supramolec. Chem. 1997, 8, 361–367. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kishimoto, M.; Ohmura, R. Correlation of the Growth Rate of the Hydrate Layer at a Guest/Liquid-Water Interface to Mass Transfer Resistance. Energies 2012, 5, 92-100. https://doi.org/10.3390/en5010092
Kishimoto M, Ohmura R. Correlation of the Growth Rate of the Hydrate Layer at a Guest/Liquid-Water Interface to Mass Transfer Resistance. Energies. 2012; 5(1):92-100. https://doi.org/10.3390/en5010092
Chicago/Turabian StyleKishimoto, Masatoshi, and Ryo Ohmura. 2012. "Correlation of the Growth Rate of the Hydrate Layer at a Guest/Liquid-Water Interface to Mass Transfer Resistance" Energies 5, no. 1: 92-100. https://doi.org/10.3390/en5010092