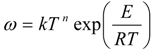A Phenomenological Model for Prediction Auto-Ignition and Soot Formation of Turbulent Diffusion Combustion in a High Pressure Common Rail Diesel Engine
Abstract
:1. Introduction
2. TP Model
2.1. Auto-Ignition
2.1.1. Modified Model
| Reaction | k (1/s) | n | E (J/mol) |
|---|---|---|---|
| C7H16 + O2 → C7H15 + HO2 (initiation) | 4.500 × 1013 | 0.0 | 48810.0 |
| C7H16 + OH → C7H15 + H2O (initiation) | 8.610 × 109 | 1.10 | 1815.0 |
| C7H15 + O2 ↔ C7H15O2 (first O2-addition) | 4.000 × 1012 | 0.0 | 0.0 |
| C7H15O2 → C7H14O2H (internal H-abstraction) | 6.000 × 1011 | 0.0 | 20380.0 |
| C7H14O2H + O2 → C7H14O4H (second O2-addition ) | 6.000 × 1011 | 0.0 | 0.0 |
| C7H14O4H → C7H14O3 + OH (chain reaction) | 1.000 × 109 | 0.0 | 7480.0 |
| Reaction | k (1/s) | n | E (J/mol) |
|---|---|---|---|
| C7H16 + 2O2 → HO2C7H13O + H2O | 3.500 × 1013 | 0.0 | 37810.0 |
| HO2C7H13O + 9O2 → 7CO2 + 7H2O | 8.610 × 109 | 0.0 | 1815.0 |
2.1.2. Implementation in CFD Code
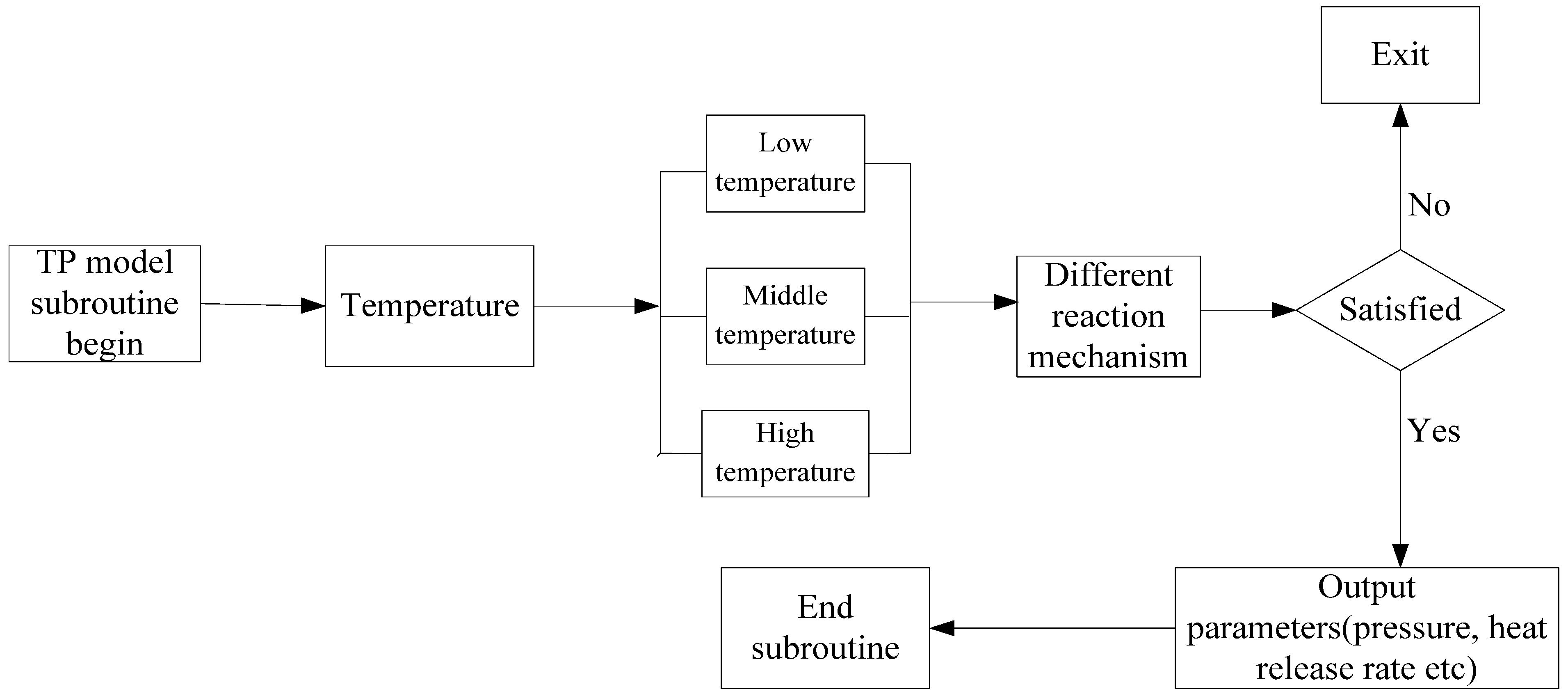
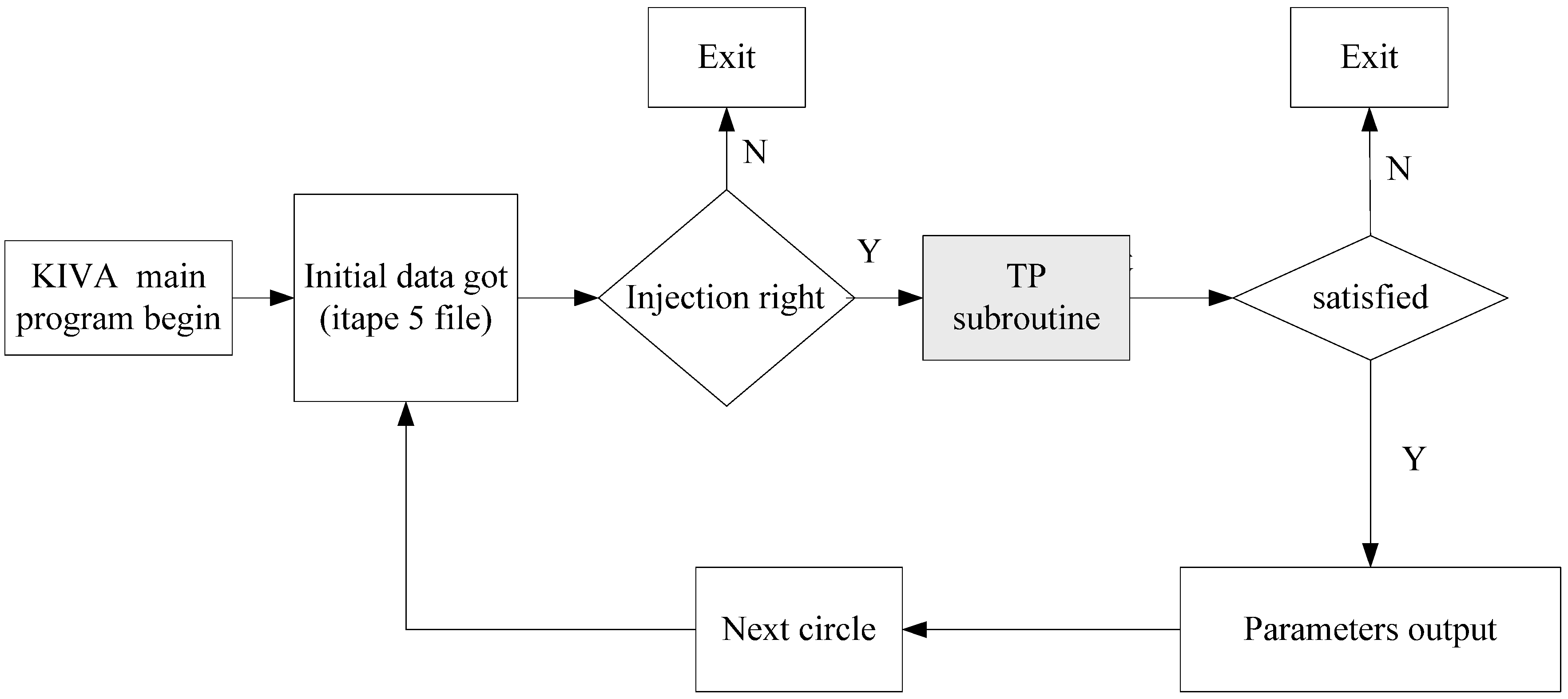
2.2. Soot Model
2.2.1. PAH Calculation





2.2.2. Soot Source Term
kA2 = kC4H2 = 1.0 × 107 exp(−5000/RT)











3. Simulations
3.1. Numerical Implementation
3.2. Basis for the Numerical Simulation

3.3. Experiment Set-up

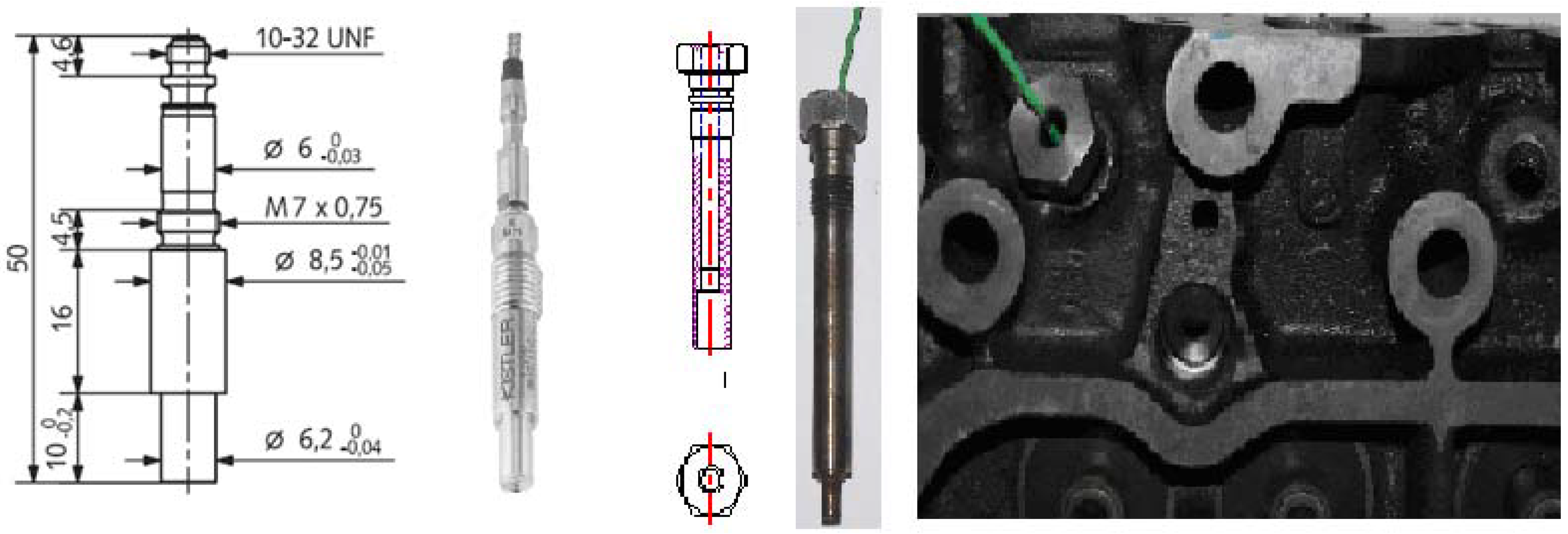
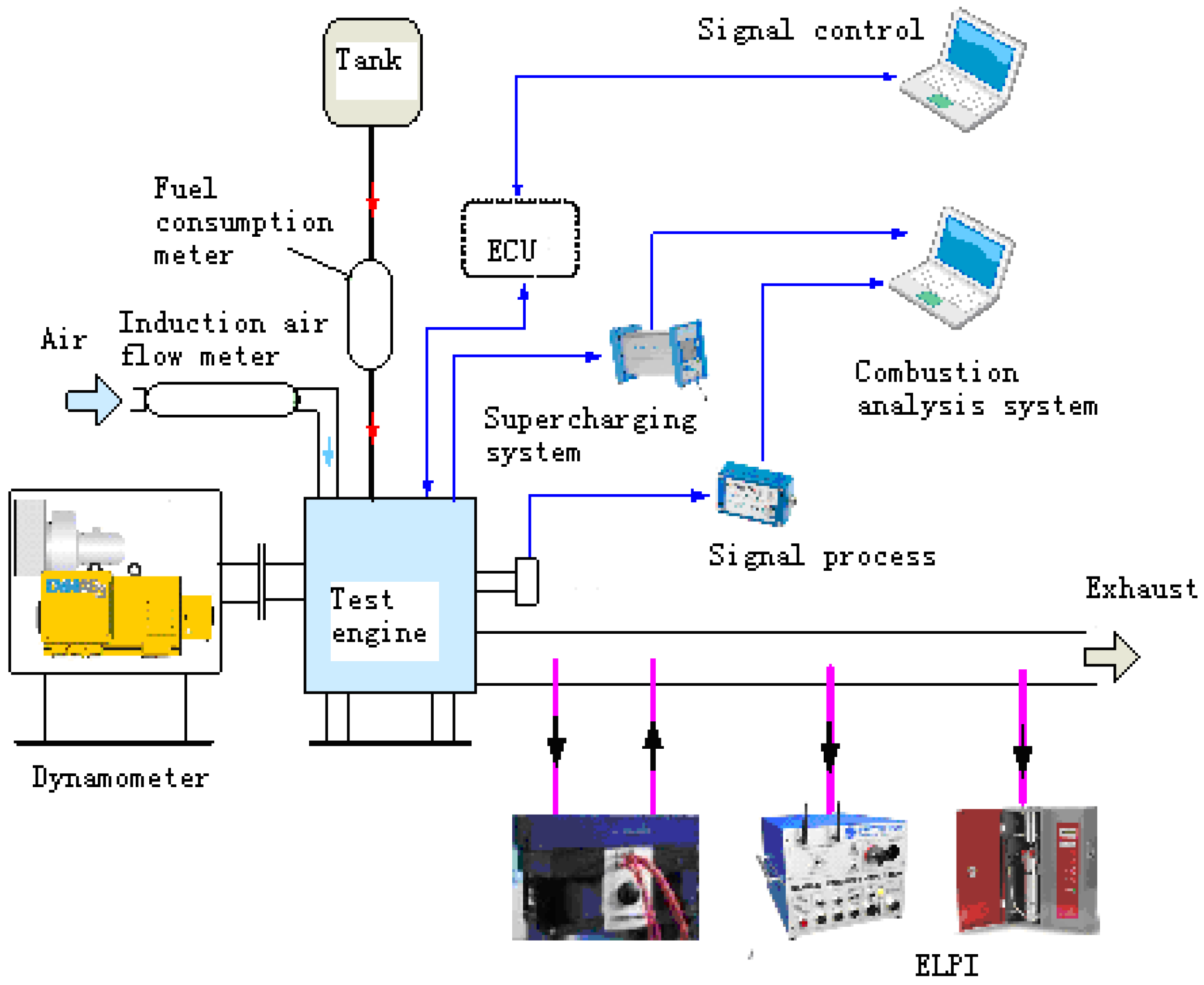
| Speed (r/min) | Torque (N.m) | Power (kW) | Rail Pressure (bar) |
|---|---|---|---|
| 750 (Idle) | 0 | 0 | 330 |
| 2200 (100% load) | 276 | 64.424 | 1200 |
| 2200 (75% load) | 211 | 49.23 | 980 |
| 2200 (50% load) | 135 | 31.498 | 809 |
| 2200 (25% load) | 64 | 14.926 | 723 |
| 2700 (100% load) | 250 | 71.367 | 1200 |
| 2700 (75% load) | 195 | 53.363 | 1190 |
| 2700 (50% load) | 127 | 36.241 | 900 |
| 2700 (25% load) | 64 | 18.256 | 750 |
| 3200 (100% load) | 239 | 81.592 | 1300 |
| 3200 (75% load) | 179 | 61.108 | 1200 |
| 3200 (50% load) | 121 | 41.308 | 1100 |
| 3200 (25% load) | 59 | 20.136 | 1000 |

4. Results and Discussion
4.1. Cylinder Pressure and Heat Release Rate
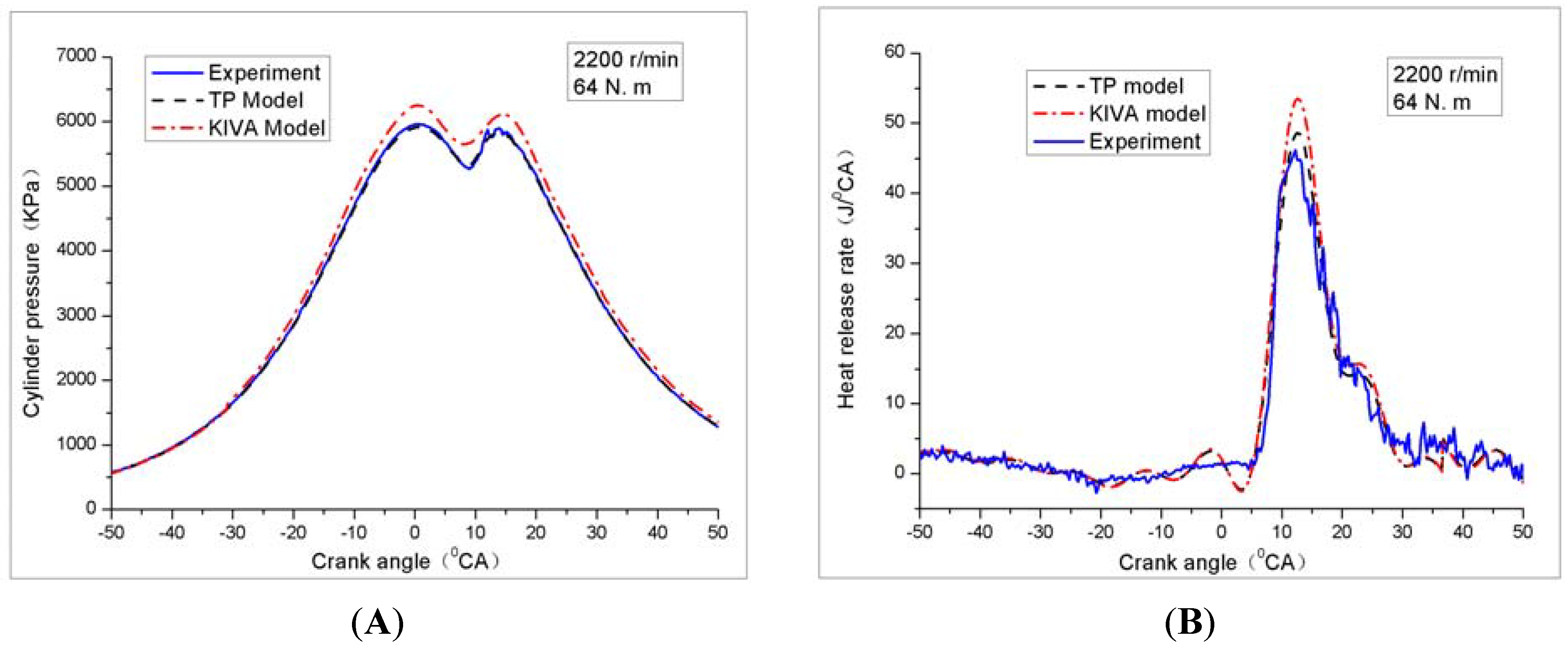
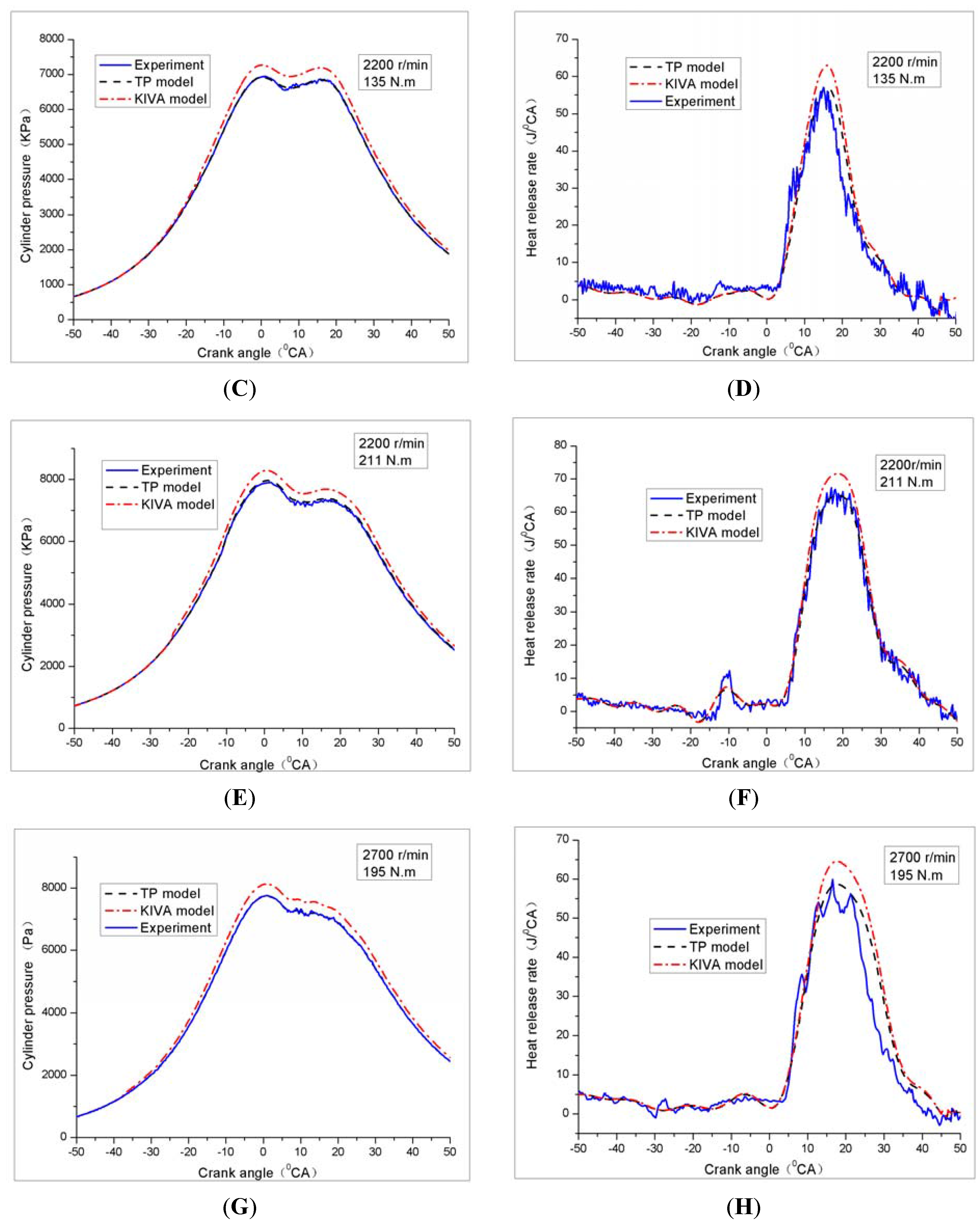
4.2. Soot
4.2.1. Soot versus Crank Angle
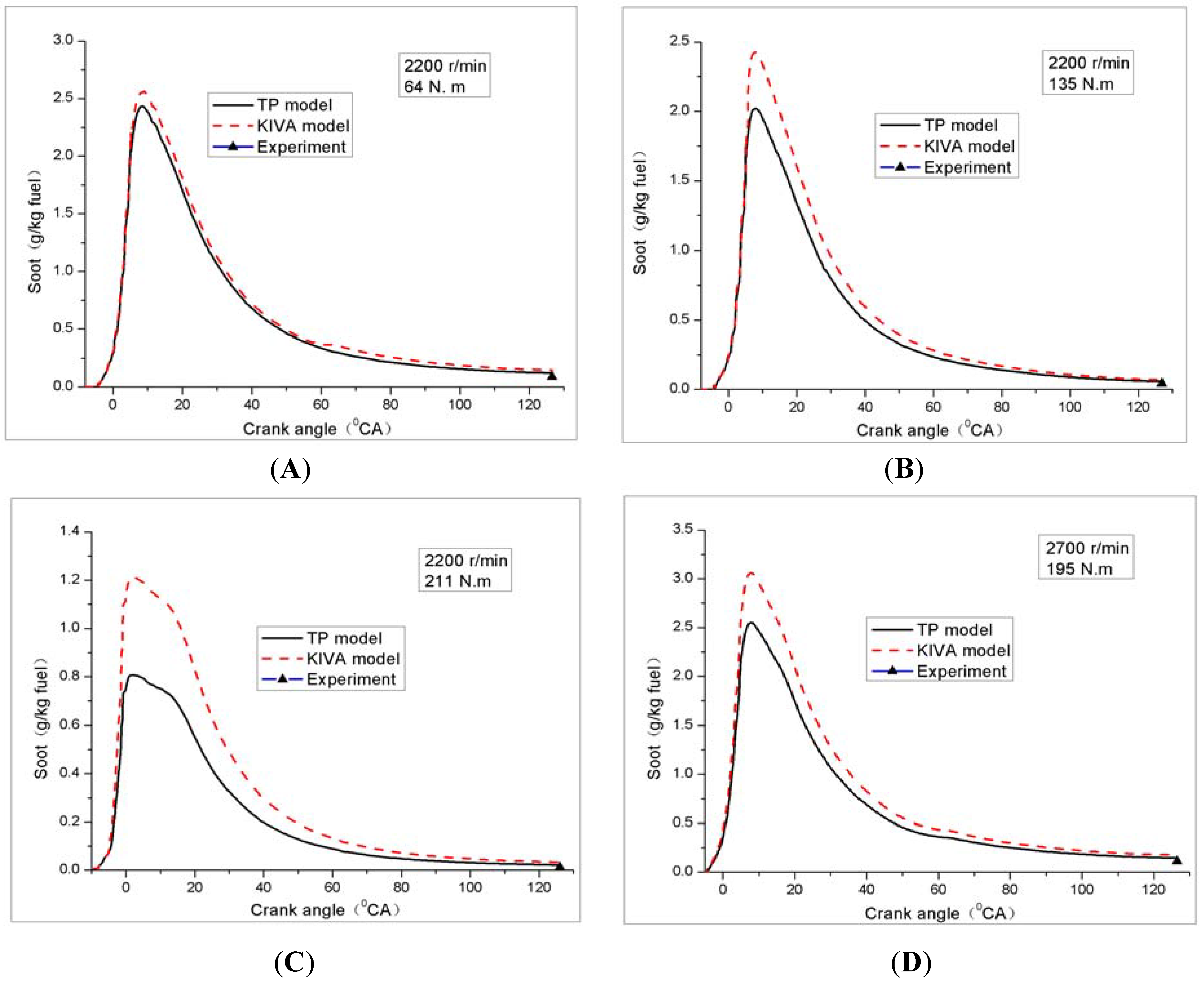
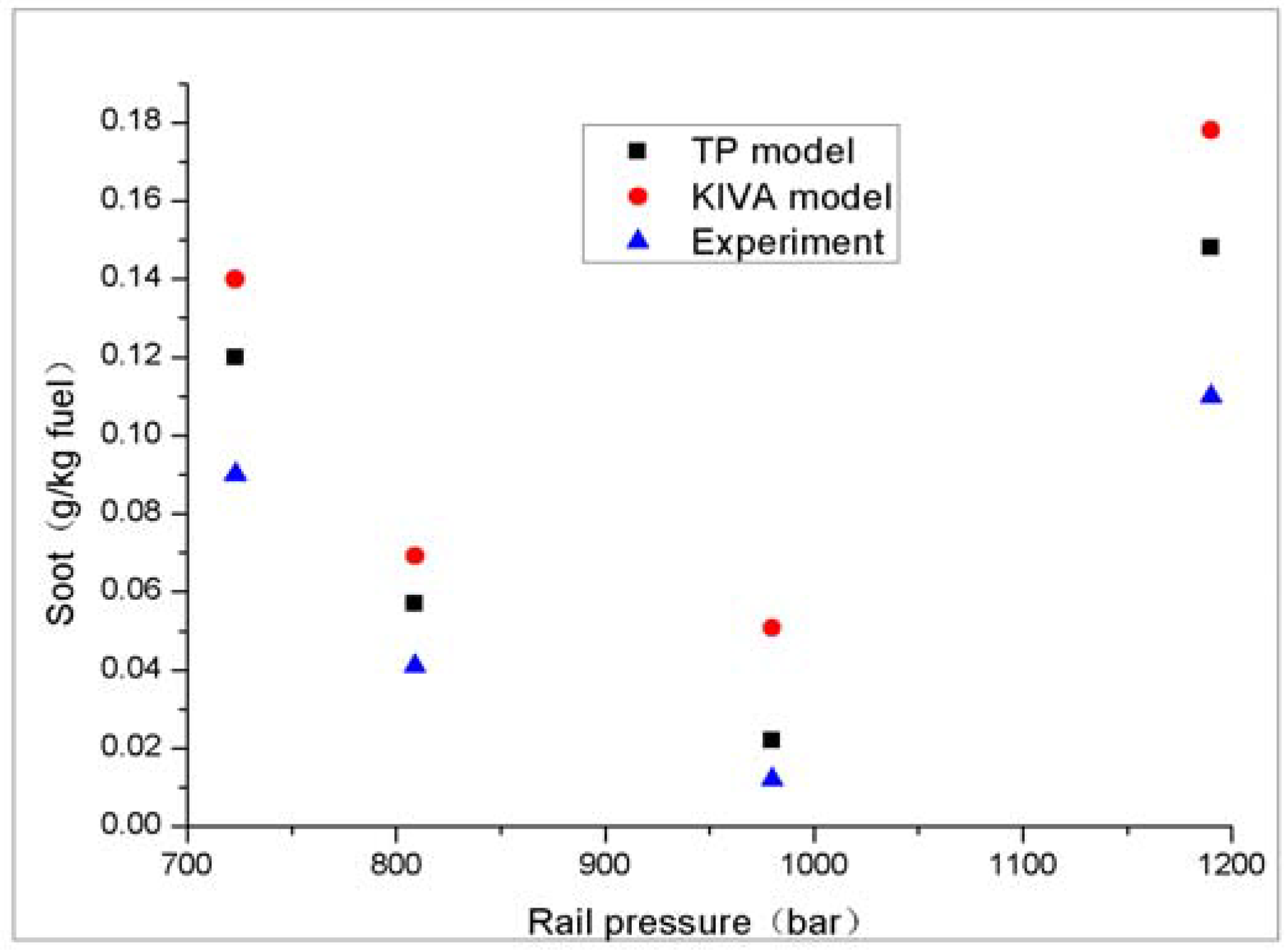
4.2.2. Summary of Investigated Load Conditions
5. Conclusions
Acknowledgements
References
- United Nations Development Programme (UNDP). World Energy Assessment: Energy and the Challenge of Sustainability; UNDP: New York, NY, USA, 2008. [Google Scholar]
- Peters, N. Turbulent Combustion; Cambridge University Press: Cambridge, UK, 2004; pp. 1–5. [Google Scholar]
- Frenklach, M.; Wang, H. Soot Formation in Combustion-Mechanisms and Models; Springer Verlag: Berlin-Heidelberg, Germany, 2004; pp. 162–164. [Google Scholar]
- Mauss, F. Entwicklung eines Kinetischen Modells der Russbildung mit Schneller Polymerisation. Ph.D. Thesis, RWTH Aachen, Aachen, Germany, 1997. [Google Scholar]
- Balthasar, M.; Heyl, A.; Mauss, F. Flamelet modeling of soot formation in laminar ethyne/air diffusion flames. In Proceedings of the Twenty-Sixth Symposium (International) on Combustion, Naples, Italy; 1996; pp. 2396–2405. [Google Scholar]
- Barths, H. Simulation of Diesel Engine and Gas Turbine Combustion Using Multiple Flamelets with Detailed Chemistry. Ph.D. Thesis, RWTH-Aachen, Aachen, Germany, 2001. [Google Scholar]
- Liu, Y.; Pei, P. Analysis on ignition and extinction of n-heptane in homogeneous systems. Sci. China 2005, 48, 556–569. [Google Scholar] [CrossRef]
- Liu, Y. Analysis on three dimensional computational fluid dynamics software based on KIVA-3V code. In Proceedings of the 3rd International Conference on Computer Science & Education, Henan, China; 2008; pp. 259–263. [Google Scholar]
- Liu, Y. Optimization research for a high pressure common rail diesel engine based on simulation. Int. J. Automot. Technol. 2010, 11, 625–636. [Google Scholar] [CrossRef]
- Liu, Y. Mesh Generation and Dynamic Mesh Management for KIVA-3V. J. Beijing Inst. Technol. 2009, 17, 41–45. [Google Scholar]
- Amsden, A.A. KIVA-3V, Release 2, Improvements to KIVA-3V; Technical Report LA-13608-MS; Los Alamos National Laboratory: Los Alamos, NM, USA, 1999.
- Zimmer, L.; Tachibana, S. Laser induced plasma spectroscopy for local equivalence ratio measurements in an oscillating combustion environment. Proc. Combust. Inst. 2007, 31, 737–745. [Google Scholar] [CrossRef]
- Wang, Y.; Rutland, C.J. Direct numerical simulation of ignition in turbulent n-heptane liquid-fuel spray jets. Combust. Flame 2007, 149, 353–365. [Google Scholar] [CrossRef]
- Hergart, C.A. Modeling Combustion and Soot Emissions in a Small-Bore Direct-Injection Diesel Engine; Shaker Verlag: Aachen, Germany, 2001; pp. 10–22. [Google Scholar]
- Balthasar, M.; Frenklach, M. Detailed kinetic modeling of soot aggregate formation in laminar premixed flames. Combust. Flame 2005, 140, 130–145. [Google Scholar] [CrossRef]
- Netzell, K.; Lehtiniemi, H.; Mauss, F. Calculating the soot particle size distribution function in turbulent diffusion flames using a sectional method. Proc. Combust. Inst. 2007, 31, 667–674. [Google Scholar] [CrossRef]
- Morgan, N.; Kraft, M.; Balthasar, M.; Wong, D.; Frenklach, M.; Mitchell, P. Pablo Mitchell Numerical simulations of soot aggregation in premixed laminar flames. Proc. Combust. Inst. 2007, 31, 693–700. [Google Scholar] [CrossRef]
- Tao, F.; Golovitchev, V.I.; Chomiak, J. A phenomenological model for the prediction of soot formation in diesel spray combustion. Combust. Flame 2004, 136, 270–282. [Google Scholar] [CrossRef]
- Dec, J.E.; Coy, E.B. OH Radical Imaging in a D.I. Diesel Engine and the Structure of the Early Diffusion Flame. In Proceedings of the International Congress and Exposition of the SOCIETY of Automotive Engineers (SAE), Detroit, MI, USA, 26–29 February 1996.
- Dec, J.E.; Canaan, R.E. PLIF Imaging of NO Formation in a DI Diesel Engine. SAE Paper No. 980147. SAE Trans. 1998, 107, 176–204. [Google Scholar]
- Dec, J.E.; Kelly-Zion, P.L. The Effects of Injection Timing and Diluent Addition on Late-Combustion Soot Burnout in a DI Diesel Engine based on Simultaneous 2-D Imaging of OH and Soot. SAE Paper No. 2000-01-0238. SAE Trans. 2002, 109, 1–10. [Google Scholar]
- Bouvier, Y.; Mihesan, C.; Ziskind, M.; Therssen, E.; Focsa, C.; Pauwels, J.F.; Desgroux, P. Molecular species adsorbed on soot particles issued from low sooting methane and acetylene laminar flames: A laser-based experiment. Proc. Combust. Inst. 2007, 31, 841–849. [Google Scholar] [CrossRef]
- Marjamaki, M.; Keskenin, J.; Chen, D.R.; Pui, D.Y.H. Performance Evaluation of the Electrical Low Pressure Impactor (ELPI). J. Aerosol Sci. 2000, 31, 249–261. [Google Scholar] [CrossRef]
- Harris, S.J.; Maricq, M.M. Signature size distributions for diesel and gasoline engine exhaust particulate matter. J. Aerosol Sci. 2001, 32, 749–764. [Google Scholar] [CrossRef]
- Schneider, J.; Hock, N.; Weimer, S.; Borrmann, S. Nucleation Particles in Diesel Exhaust: Composition Inferred from in Situ Mass Spectrometric Analysis. Environ. Sci. Technol. 2005, 39, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Yang, J.; Sun, J.; Zhu, A.; Zhou, Q. A Phenomenological Model for Prediction Auto-Ignition and Soot Formation of Turbulent Diffusion Combustion in a High Pressure Common Rail Diesel Engine. Energies 2011, 4, 894-912. https://doi.org/10.3390/en4060894
Liu Y, Yang J, Sun J, Zhu A, Zhou Q. A Phenomenological Model for Prediction Auto-Ignition and Soot Formation of Turbulent Diffusion Combustion in a High Pressure Common Rail Diesel Engine. Energies. 2011; 4(6):894-912. https://doi.org/10.3390/en4060894
Chicago/Turabian StyleLiu, Yongfeng, Jianwei Yang, Jianmin Sun, Aihua Zhu, and Qinghui Zhou. 2011. "A Phenomenological Model for Prediction Auto-Ignition and Soot Formation of Turbulent Diffusion Combustion in a High Pressure Common Rail Diesel Engine" Energies 4, no. 6: 894-912. https://doi.org/10.3390/en4060894