Designs and Architectures for the Next Generation of Organic Solar Cells
Abstract
:1. Introduction
2. Basic Principles of Operation for OSCs
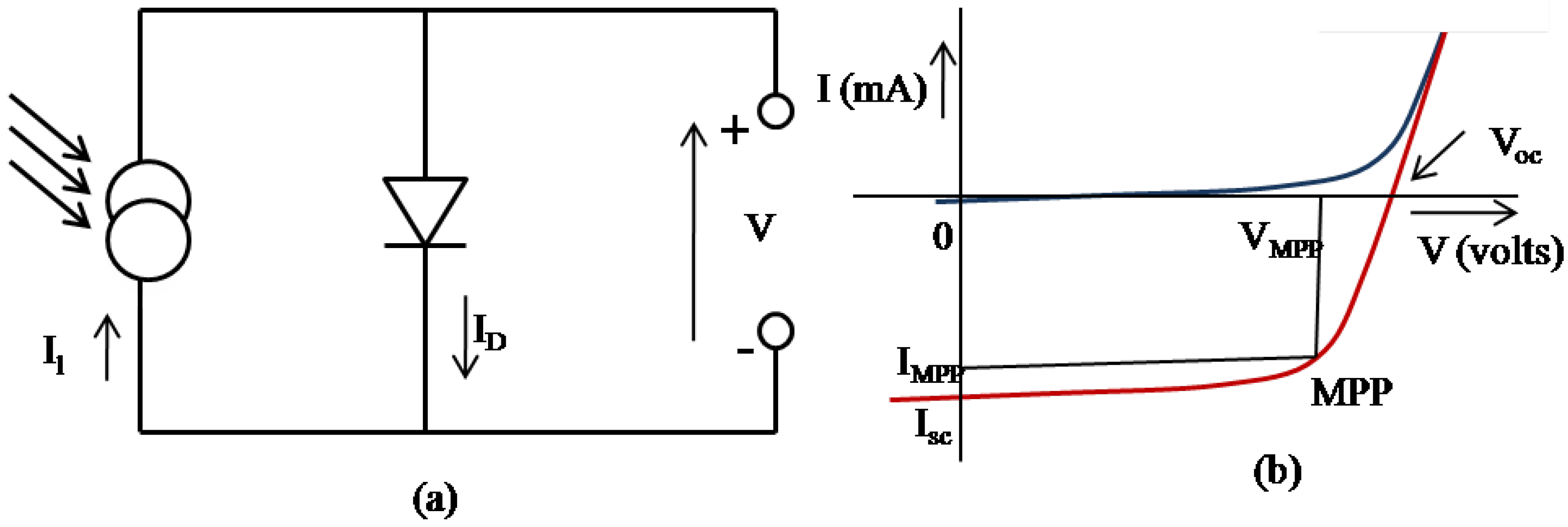
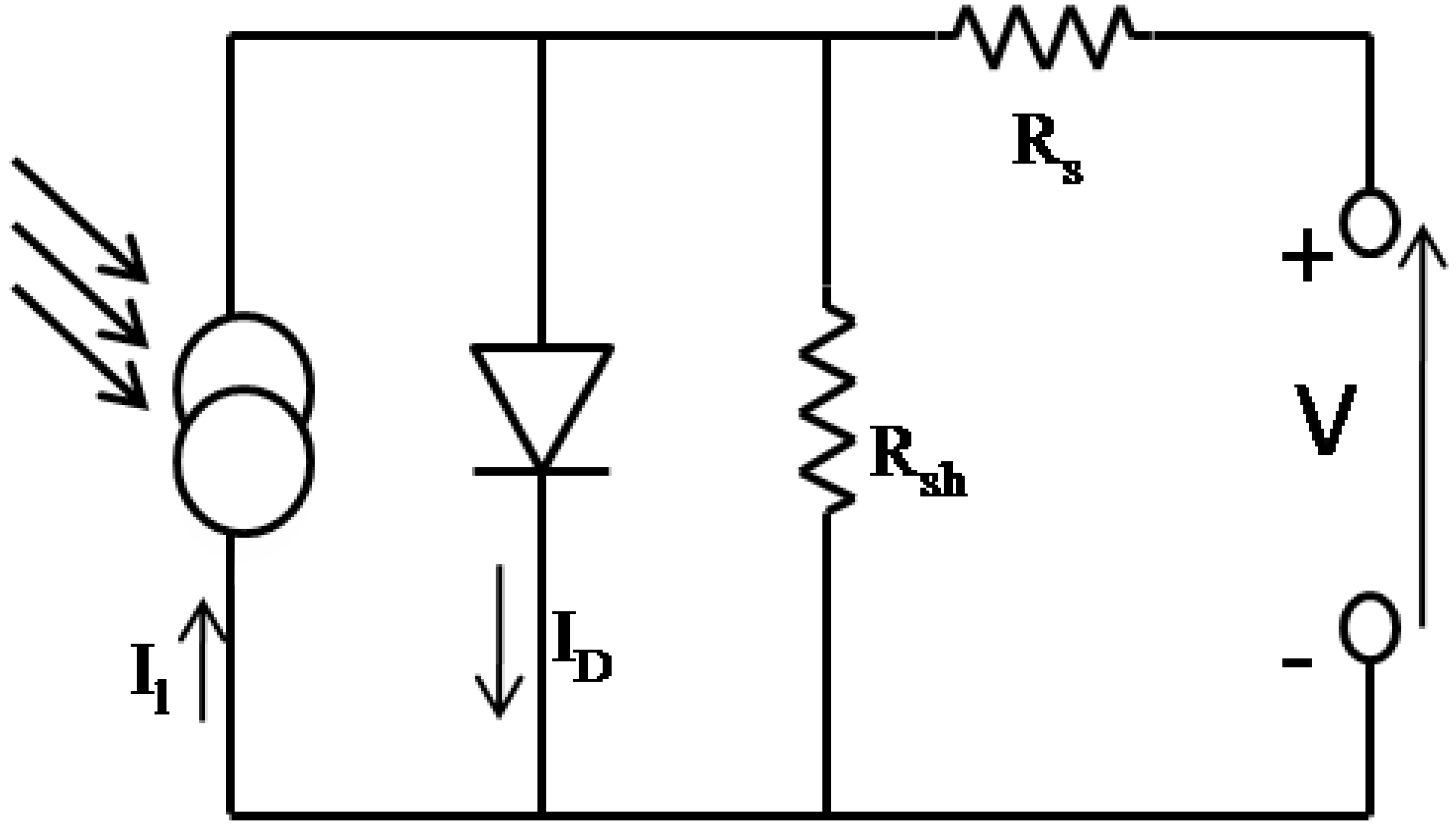
2.1. The OSC active layer
2.1.1. Single layer
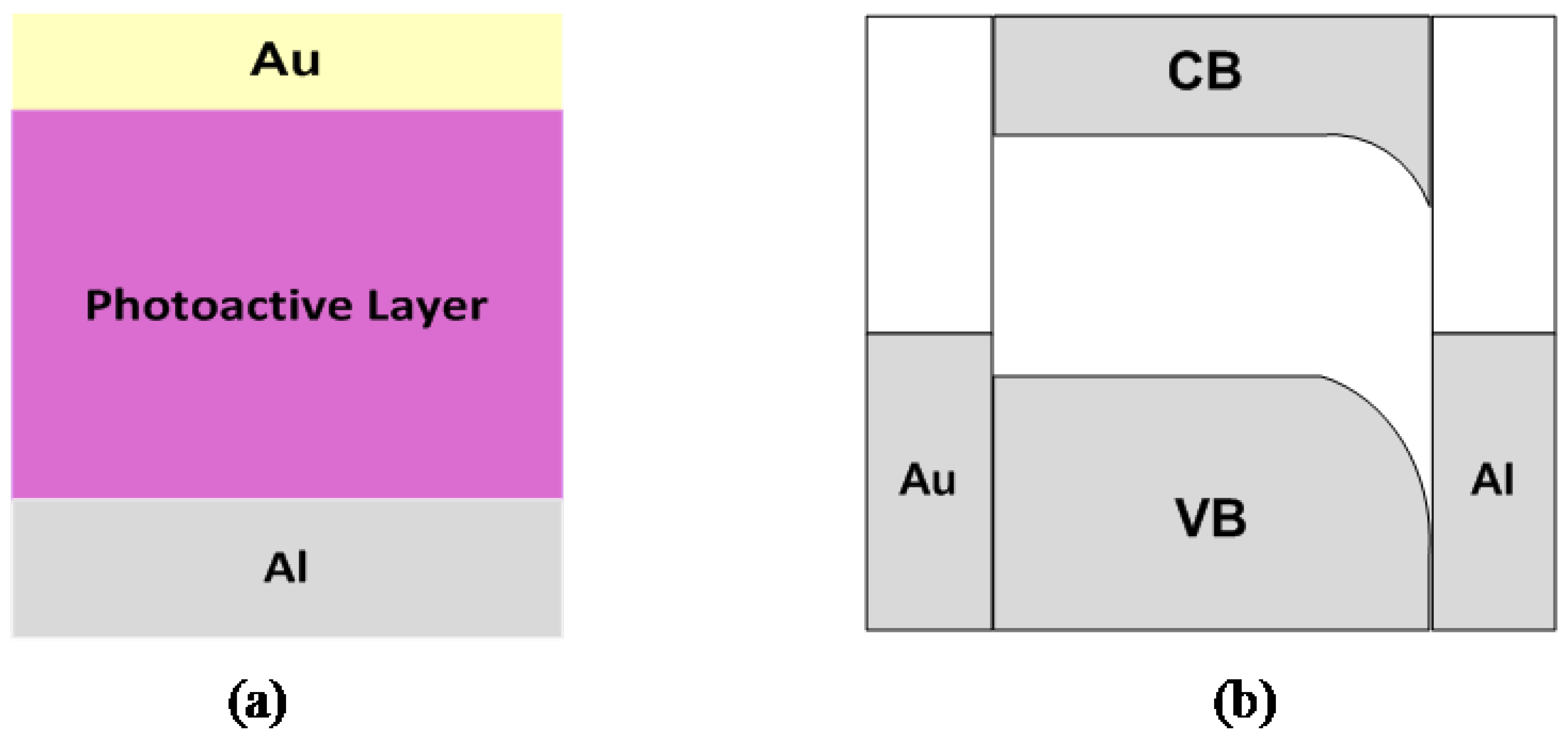
2.1.2. Bilayer heterojunction
2.1.3. Bulk heterojunction (BHJ)

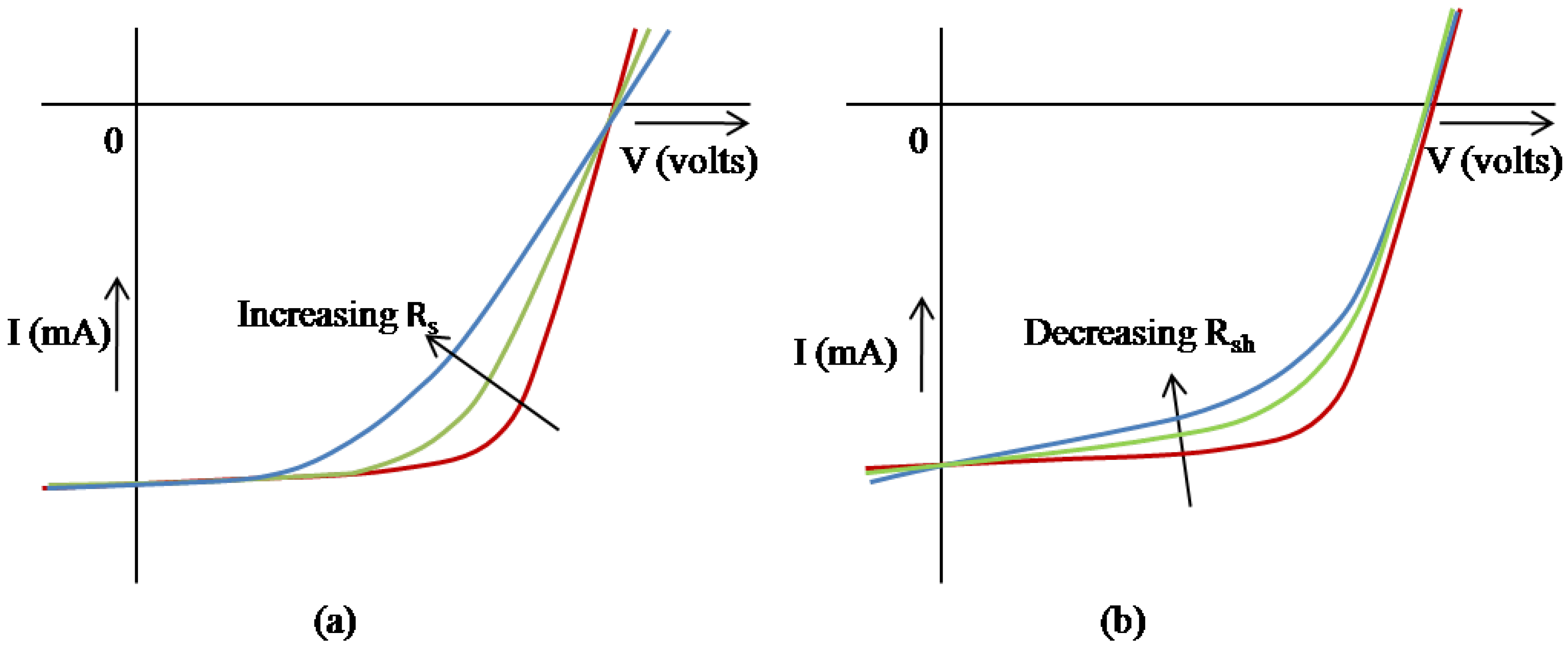
2.2. Critical parameters of an OSC
2.3. Anodes
2.3.1. Doped metal oxides
2.3.2. Organic polymers
2.3.3. Metal grids
2.3.4. Carbon nanotubes
2.3.5. Graphene
2.4. Cathodes
2.5. Inverted solar cells
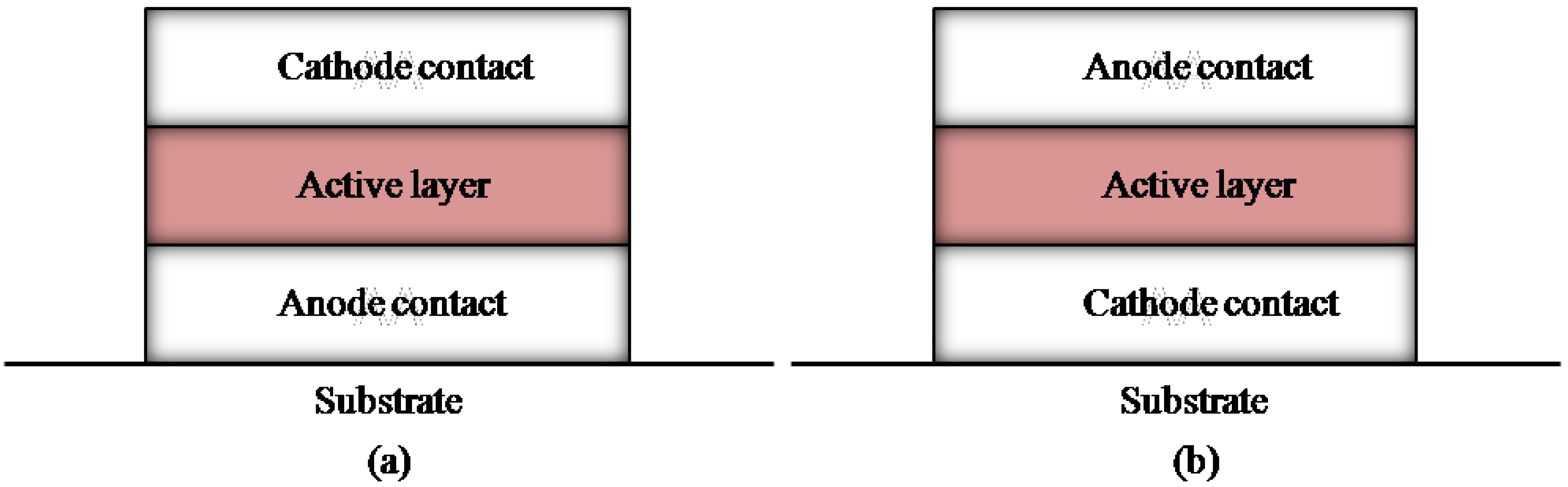
3. Progress of Module Designs and Processing Technologies to Realize Large-Area OSCs
3.1. Device area
3.2. Effect of electrode geometry
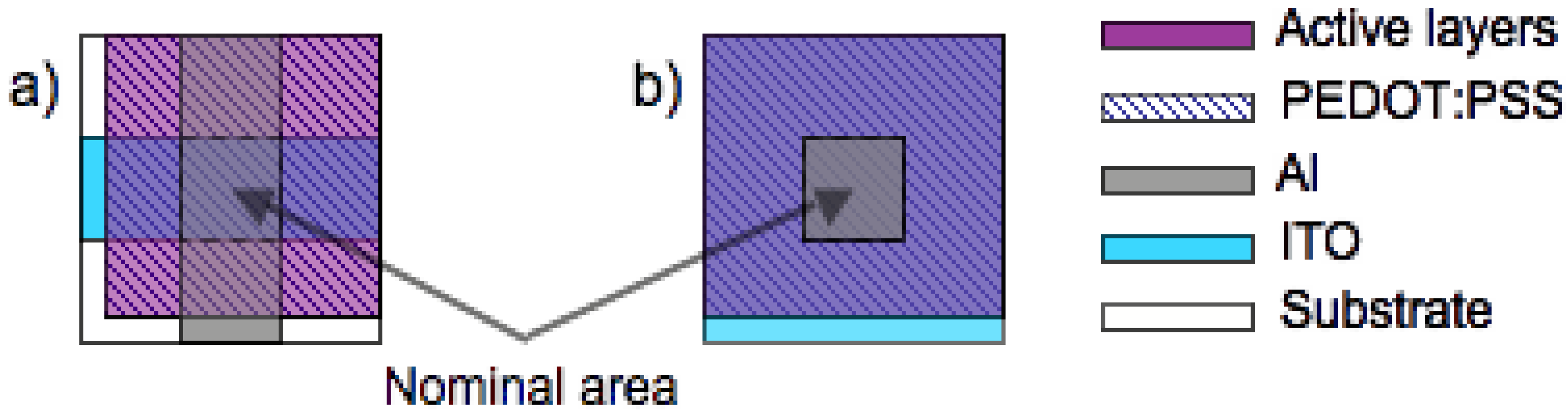

3.3. Edge effects
3.4. Large area solar cells
| Device structurea | Connection | Areab (cm2) | JSC (μA/cm2) | ISC (mA) | VOC (V) | FF (%) | η (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITO-PET/MEH-PPV/C60/Al | 3 Sd | ~30e | 20 | --- | 0.73 | 49 | 0.0046 | [91] | ||||
| ITO-PET/MEH-PPV/Al | 13S Χ 7Pg | 655 | 0.1 | --- | 10.5 | 13 | 0.00001f,c | [90] | ||||
| ITO-PET/MEH-PPV/C60/Al | 13S Χ 7Pg | 655 | 3.5 | --- | 3.6 | 19 | 0.0002 f,c | [90] | ||||
| ITO-Glass/PEDOT:PSS/P3HT:PC70BM/Al | 4 Sd | 1.04 | --- | 1.4 | 2.48 | 44 | 1.7c | [96] | ||||
| ITO-Glass/PEDOT:PSS/P3HT:PCBM/Al | 2 Sd | 17.1 | --- | 56.5 | 1.14 | 42 | 1.5c | [83] | ||||
| ITO-Glass/PEDOT:PSS/P3HT:PCBM /Al | 3 Sd | 11.3 | --- | 27.2 | 1.66 | 39 | 1.5c | [83] | ||||
| Flexible Foil/ITO/PEDOT:PSS/P3HT:PCBM /Al | 3 Sd | 11.3 | --- | 13.9 | 1.38 | 29 | 0.5c | [83] | ||||
| ITO-Glass/ PEDOT:PSS/ P3HT:PCBM/Al | 22 Sd | 46.2 | --- | 16.56 | 12.45 | 43 | 2c | [97] | ||||
| ITO-PET/ZnO/P3HT:PCBM/PEDOT:PSS/Ag | 8 Sd | 120 | --- | 159.97 | 3.76 | 35 | 1.78c | [75] | ||||
| ITO-PET/ZnO/P3HT-PCBM/PEDOT:PSS/Ag | 8 Sd | 120 | --- | 60 | 3.6 | 44 | 2.08 g,c | [75] | ||||
| ITO-PET/ZnO/P3HT-PCBM/PEDOT:PSS/Ag | 8 Sd | 4.8 | --- | 6.87 | 3.62 | 44 | 2.33c | [75] | ||||
| ITO-PET/ZnO/P3CT:ZnO/PEDOT:PSS/Ag | 8 Sd | 120 | --- | 6.82 | 2.99 | 29 | 0.05c | [98] | ||||
| ITO-PET/ ZnO /P3CT:ZnO/PEDOT:PSS/Ag | 5 Si | 75 | --- | 0.1 | 3 | 16j | 0.013c | [92] | ||||
| ITO-Glass/ HTL/P3HT: PCBM/Ca-Al | 54 Sd | 108 | --- | 16.91 | 29.35 | 51 | 3.4c | [99] | ||||
| ITO-Glass/PEDOT:PSS/P3HT:PCBM/Al | 3Sd | 16.2 | 9000 | --- | 1.94 | 52 | 3.0c | [100] | ||||
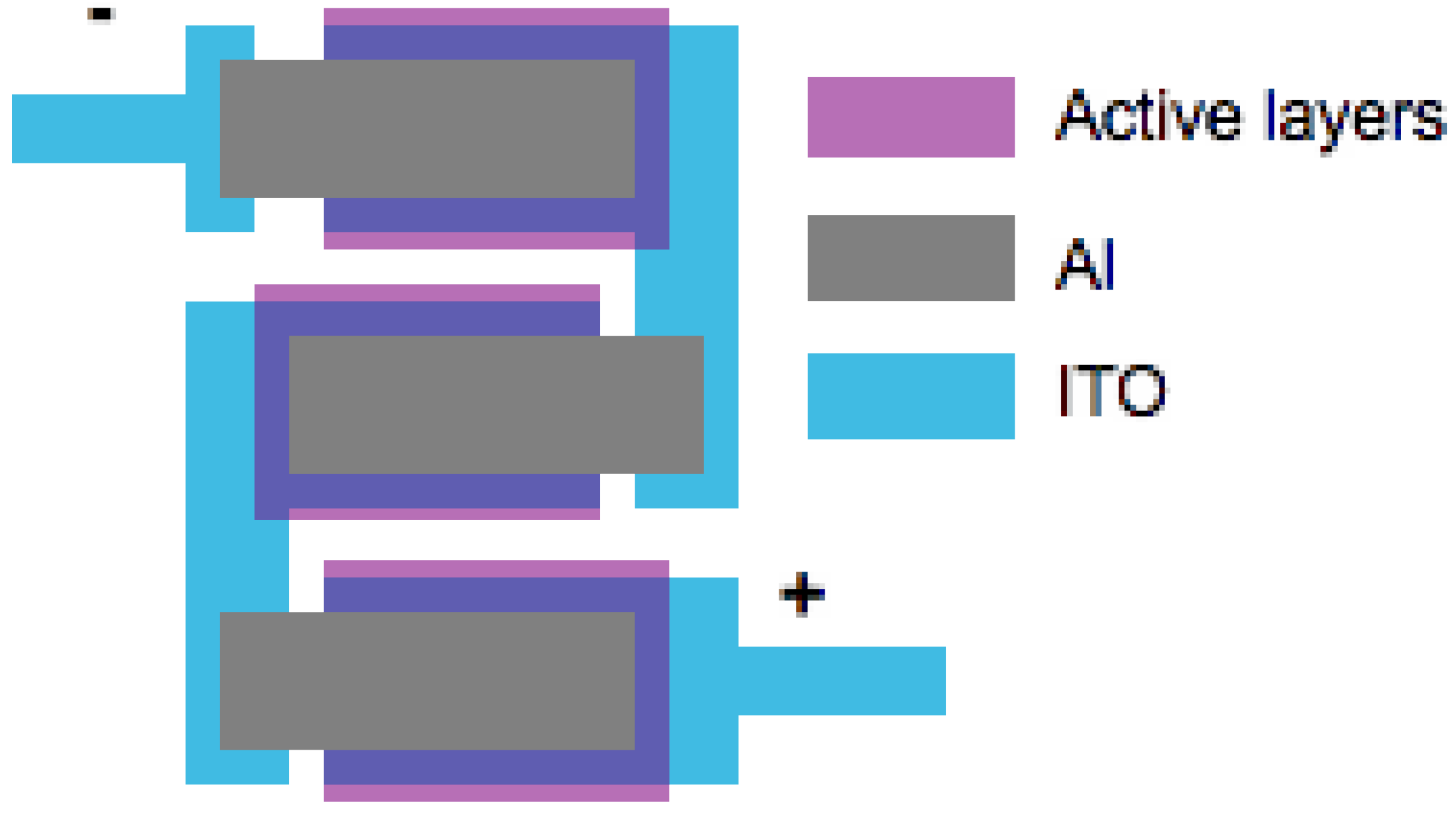
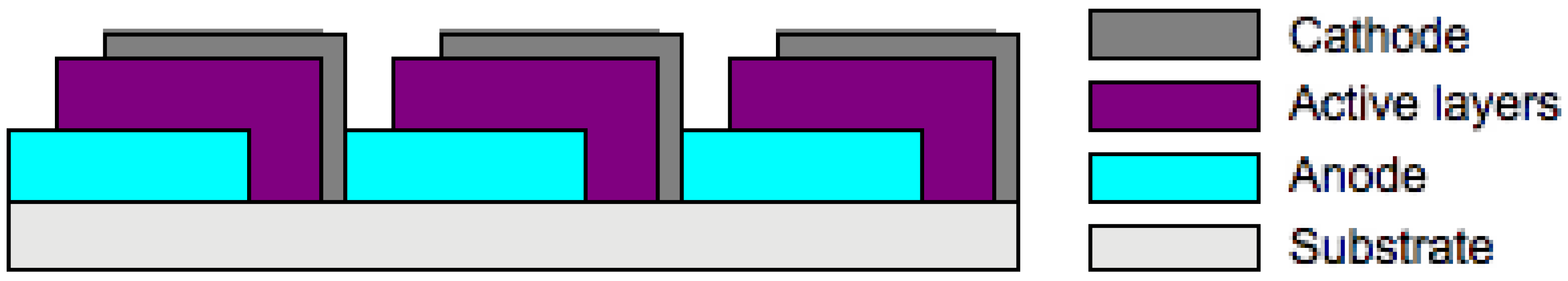
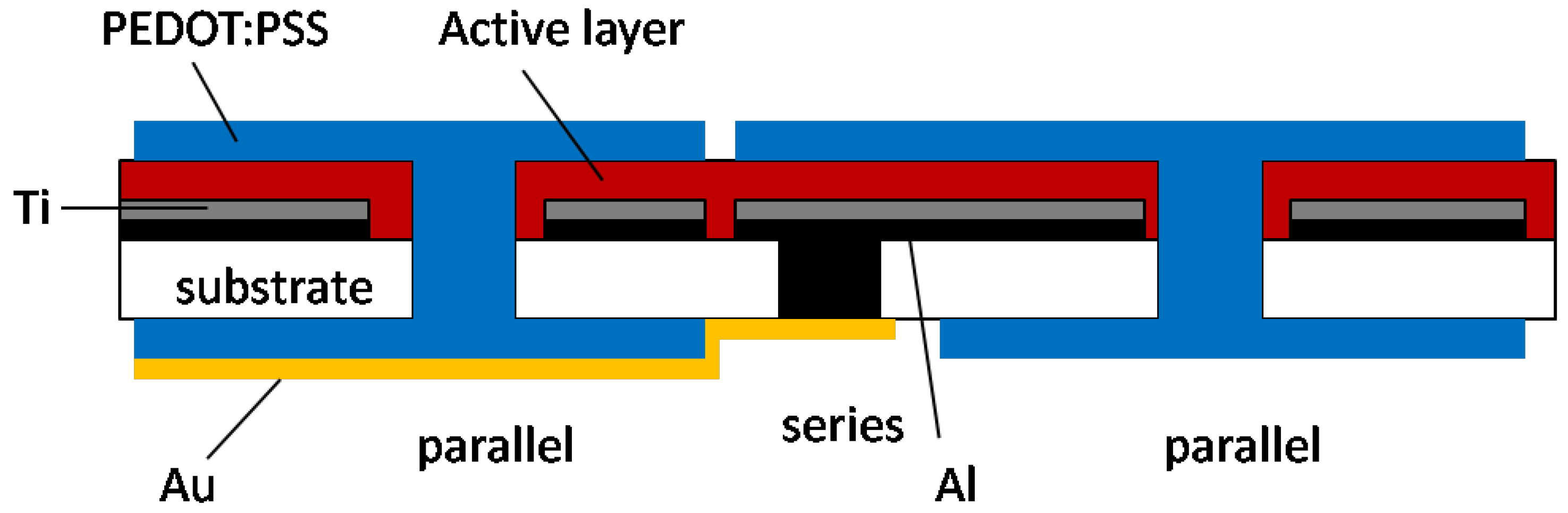
4. Optimization of OSC Performance through Device Engineering and New Architectures
4.1. Modification and optimization of flat panel architectures

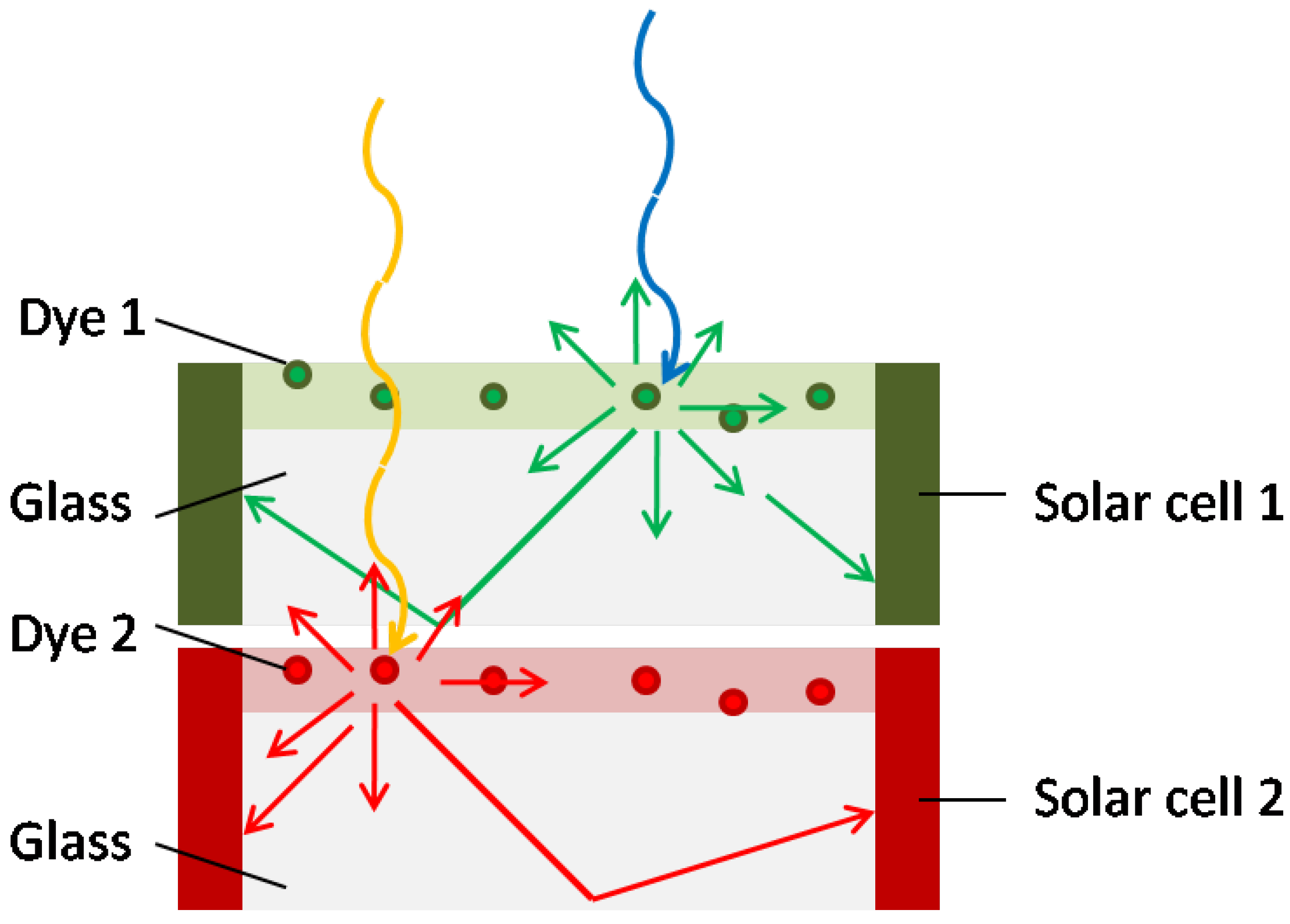
4.2. Progression to new architectures
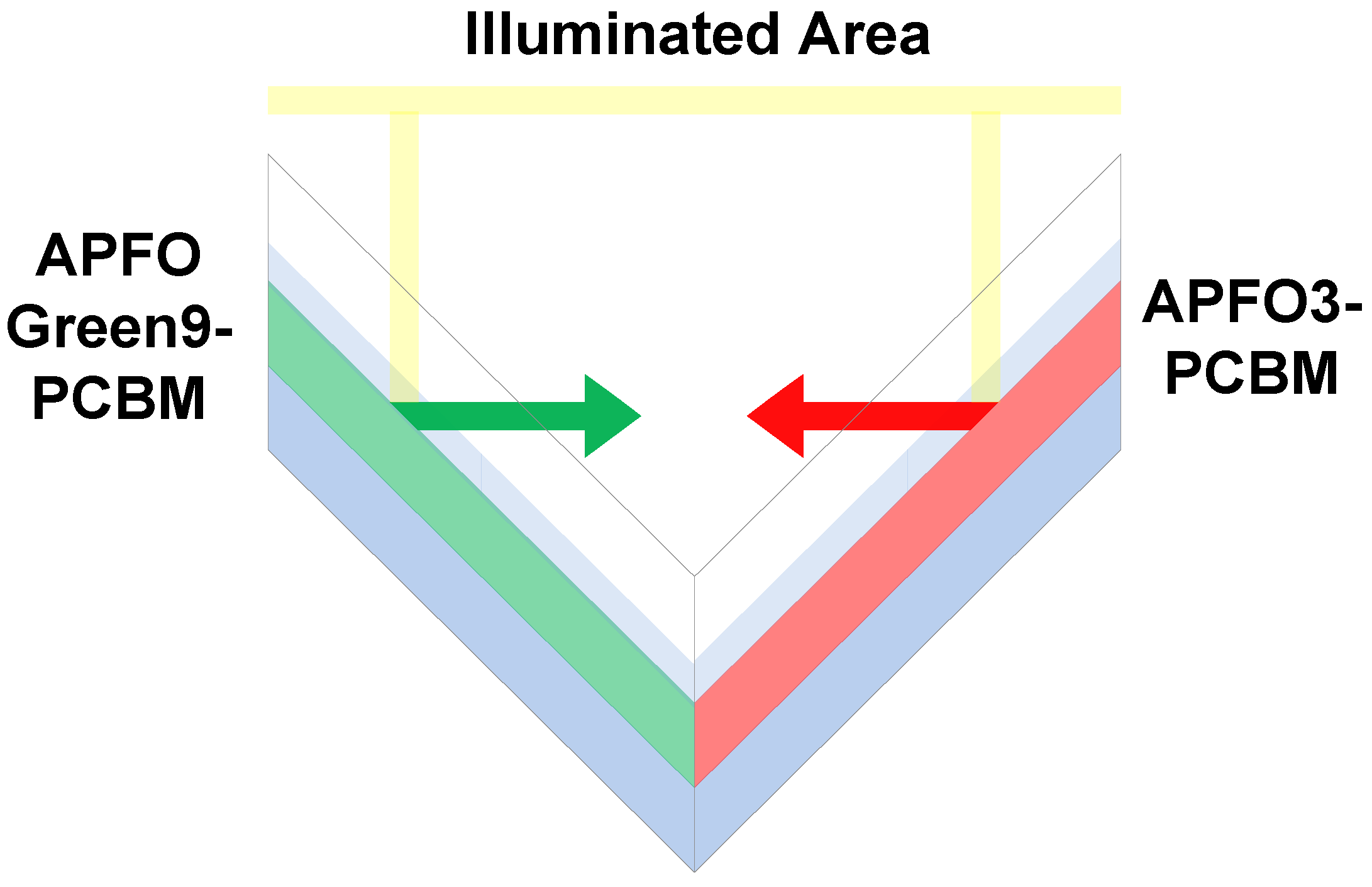
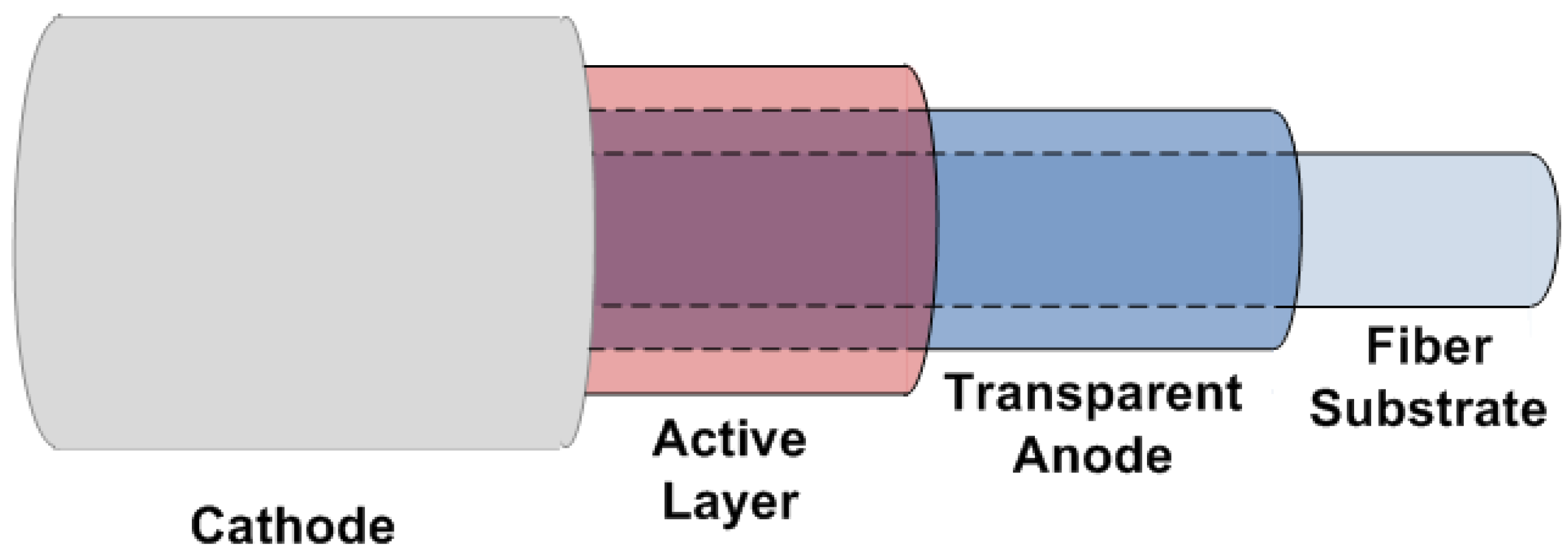
5. Conclusions
Acknowledgements
References and Notes
- Heeger, A.J.; MacDiarmid, A.G.; Shirakawa, H. Nobel lectures for the Nobel Prize in Chemistry 2000. Available online: http://nobelprize.org/nobel_prizes/chemistry/laureates/2000/index.html (accessed on 9 June 2010).
- Tang, C.W. 2-Layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Roes, A.L.; Alsema, E.A.; Blok, K.; Patel, M.K. Ex-ante environmental and economic evaluation of polymer photovoltaics. Prog. Photovolt. Res. Appl. 2009, 17, 372–393. [Google Scholar] [CrossRef]
- Industrial reports of high efficiency organic solar cells certified by the US Department of Energy’s National Renewable Energy Laboratory: 7.9% by Solarmer (December 2009) http://www.solarmer.com; 5.4% by Plextronics (August 2007) http://www.plextronics.com/
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Martin, A.G.; Keith, E.; Yoshihiro, H.; Wilhelm, W. Solar cell efficiency tables (Version 34). Prog. Photovoltaics Res. Appl. 2009, 17, 320–326. [Google Scholar] [CrossRef]
- Canizo, C.; Coso, G.; Sinke, W.C. Crystalline silicon solar module technology: Towards the 1 Euro per watt-peak goal. Prog. Photovoltaics Res. Appl. 2009, 17, 199–209. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Roth, S.; Blumentritt, S.; Burghard, M.; Cammi, E.; Carrol, D.; Curran, S.; Düsberg, G.; Liu, K.; Muster, J.; Philipp, G.; Rabenau, T. Molecular rectifiers and transistors based on π-conjugated materials. Synth. Met. 1998, 94, 105. [Google Scholar] [CrossRef]
- Sargent, E.H. Infrared photovoltaics made by solution processing. Nat. Photonics 2009, 3, 325–331. [Google Scholar] [CrossRef]
- Gueymard, C.; Myers, D.; Emery, K. Proposed reference irradiance spectra for solar energy systems testing. Solar Energy 2002, 73, 443–467. [Google Scholar] [CrossRef]
- Spanggaard, H.; Krebs, F.C. A brief history of the development of organic and polymeric photovoltaics. Sol. Energy Mater. Sol. Cells 2004, 83, 125–146. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Morel, D.L.; Feng, T.; Shaw, R.F.; Rowe, C.A. Photovoltaic and rectification properties of Al/Mg Phthalocyanine/Ag Schottky-barrier cells. J. Appl. Phys. 1974, 45, 230–236. [Google Scholar] [CrossRef]
- Chamberlain, G.A. Organic solar cells: A review. Solar Cells 1983, 8, 47–83. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: an overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Xue, J.G.; Uchida, S.; Rand, B.P.; Forrest, S.R. 4.2% efficient organic photovoltaic cells with low series resistances. Appl. Phys. Lett. 2004, 84, 3013–3015. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, X.; Wang, H.; Yan, D. Organic photovoltaic cells with near infrared absorption spectrum. Appl. Phys. Lett. 2007, 91, 253503. [Google Scholar] [CrossRef]
- Diogenes, P.; Weining, W.; Shallcross, R.C.; Kenneth, W.N.; Michael, B.; Neal, R.A. Organic photovoltaic cells based on solvent-annealed, textured Titanyl Phthalocyanine/C60 heterojunctions. Adv. Funct. Mater. 2009, 19, 1913–1921. [Google Scholar] [CrossRef]
- Tong, X.; Lassiter, B.E.; Forrest, S.R. Inverted organic photovoltaic cells with high open-circuit voltage. Org. Electron. 2010, 11, 705–709. [Google Scholar] [CrossRef]
- Ma, B.; Woo, C.H.; Miyamoto, Y.; Frechet, J.M.J. Solution processing of a small molecule, Subnaphthalocyanine, for efficient organic photovoltaic cells. Chem. Mater. 2009, 21, 1413–1417. [Google Scholar] [CrossRef]
- Gorodetsky, A.A.; Cox, M.; Tremblay, N.J.; Kymissis, I.; Nuckolls, C. Solar cells from a solution processable pentacene with improved air stability. Chem. Mater. 2009, 21, 4090–4092. [Google Scholar] [CrossRef]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.; Sariciftci, N.S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 2006, 16, 45–61. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Helgesen, M.; Søndergaard, R.; Krebs, F.C. Advanced materials and processes for polymer solar cell devices. J. Mater. Chem. 2010, 20, 36–60. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.J.; Yang, Y. Solvation-induced morphology effects on the performance of polymer-based photovoltaic devices. Adv. Funct. Mater. 2001, 11, 420–424. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Blom, P.W.M.; Hummelen, J.C.; Rispens, M.T. Cathode dependence of the open-circuit voltage of polymer: Fullerene bulk heterojunction solar cells. J. Appl. Phys. 2003, 94, 6849–6854. [Google Scholar] [CrossRef]
- Ramsdale, C.M.; Barker, J.A.; Arias, A.C.; MacKenzie, J.D.; Friend, R.H.; Greenham, N.C. The origin of the open-circuit voltage in polyfluorene-based photovoltaic devices. J. Appl. Phys. 2002, 92, 4266–4270. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Scharber, M.C.; Muhlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells—Towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Arias, A.C.; MacKenzie, J.D.; Stevenson, R.; Halls, J.J.M.; Inbasekaran, M.; Woo, E.P.; Richards, D.; Friend, R.H. Photovoltaic performance and morphology of polyfluorene blends: A combined microscopic and photovoltaic investigation. Macromolecules 2001, 34, 6005–6013. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Xu, Z.; Xia, J.B.; Tsai, S.T.; Wu, Y.; Li, G.; Ray, C.; Yu, L.P. For the bright future—Bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010. Early View. [Google Scholar]
- Bhosle, V.; Prater, J.T.; Yang, F.; Burk, D.; Forrest, S.R.; Narayan, J. Gallium-doped zinc oxide films as transparent electrodes for organic solar cell applications. J. Appl. Phys. 2007, 102, 5. [Google Scholar]
- Schulze, K.; Maennig, B.; Leo, K.; Tomita, Y.; May, C.; Hupkes, J.; Brier, E.; Reinold, E.; Bauerle, P. Organic solar cells on indium tin oxide and aluminum doped zinc oxide anodes. Appl. Phys. Lett. 2007, 91, 073521. [Google Scholar] [CrossRef]
- Bernede, J.C.; Berredjem, Y.; Cattin, L.; Morsli, M. Improvement of organic solar cell performances using a zinc oxide anode coated by an ultrathin metallic layer. Appl. Phys. Lett. 2008, 92, 083304. [Google Scholar] [CrossRef]
- Murdoch, G.B.; Hinds, S.; Sargent, E.H.; Tsang, S.W.; Mordoukhovski, L.; Lu, Z.H. Aluminum doped zinc oxide for organic photovoltaics. Appl. Phys. Lett. 2009, 94, 213301. [Google Scholar] [CrossRef]
- Park, H.K.; Jeong, J.A.; Park, Y.S.; Na, S.I.; Kim, D.Y.; Kim, H.K. Room-temperature Indium-free Ga:ZnO/Ag/Ga:ZnO multilayer electrode for organic solar cell applications. Electrochem. Solid-State Lett. 2009, 12, H309–H311. [Google Scholar] [CrossRef]
- Ouyang, B.Y.; Chi, C.W.; Chen, F.C.; Xi, Q.F.; Yang, Y. High-conductivity poly (3,4-ethylenedioxythiophene): poly(styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Admassie, S.; Zhang, F.L.; Manoj, A.G.; Svensson, M.; Andersson, M.R.; Inganas, O. A polymer photodiode using vapour-phase polymerized PEDOT as an anode. Sol. Energy Mater. Sol. Cells 2006, 90, 133–141. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, S.S.; Jo, J.; Kim, D.Y. Efficient and flexible ITO-free organic solar cells using highly conductive polymer anodes. Adv. Mater. 2008, 20, 4061–4067. [Google Scholar] [CrossRef]
- Na, S.I.; Wang, G.; Kim, S.S.; Kim, T.W.; Oh, S.H.; Yu, B.K.; Lee, T.; Kim, D.Y. Evolution of nanomorphology and anisotropic conductivity in solvent-modified PEDOT:PSS films for polymeric anodes of polymer solar cells. J. Mater. Chem. 2009, 19, 9045–9053. [Google Scholar] [CrossRef]
- Ahlswede, E.; Muhleisen, W.; Wahi, M.; Hanisch, J.; Powalla, M. Highly efficient organic solar cells with printable low-cost transparent contacts. Appl. Phys. Lett. 2008, 92, 143307. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, F.L.; Tvingstedt, K.; Barrau, S.; Li, F.H.; Tian, W.J.; Inganas, O. Investigation on polymer anode design for flexible polymer solar cells. Appl. Phys. Lett. 2008, 92, 233308. [Google Scholar] [CrossRef]
- Aernouts, T.; Vanlaeke, P.; Geens, W.; Poortmans, J.; Heremans, P.; Borghs, S.; Mertens, R.; Andriessen, R.; Leenders, L. Printable anodes for flexible organic solar cell modules. Thin Solid Films 2004, 451, 22–25. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Inganas, O. Electrode grids for ITO-free organic photovoltaic devices. Adv. Mater. 2007, 19, 2893–2897. [Google Scholar] [CrossRef]
- Kang, M.G.; Kim, M.S.; Kim, J.S.; Guo, L.J. Organic solar cells using nanoimprinted transparent metal electrodes. Adv. Mater. 2008, 20, 4408–4413. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, A.D.; Unalan, H.E.; Kanwal, A.; Miller, S.; Chhowalla, M. Conducting and transparent single-wall carbon nanotube electrodes for polymer-fullerene solar cells. Appl. Phys. Lett. 2005, 87, 203511. [Google Scholar] [CrossRef]
- Rowell, M.W.; Topinka, M.A.; McGehee, M.D.; Prall, H.J.; Dennler, G.; Sariciftci, N.S.; Hu, L.B.; Gruner, G. Organic solar cells with carbon nanotube network electrodes. Appl. Phys. Lett. 2006, 88, 233506. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Barnes, T.M.; Rumbles, G.; Shaheen, S.E.; Coutts, T.J.; Weeks, C.; Levitsky, I.; Peltola, J.; Glatkowski, P. Organic solar cells with carbon nanotubes replacing In2O3: Sn as the transparent electrode. Appl. Phys. Lett. 2006, 88, 233503. [Google Scholar] [CrossRef]
- Tenent, R.C.; Barnes, T.M.; Bergeson, J.D.; Ferguson, A.J.; To, B.; Gedvilas, L.M.; Heben, M.J.; Blackburn, J.L. Ultrasmooth, large-area, high-uniformity, conductive transparent single-walled-carbon-nanotube films for photovoltaics produced by ultrasonic spraying. Adv. Mater. 2009, 21, 3210–3216. [Google Scholar] [CrossRef]
- Wu, J.B.; Becerril, H.A.; Bao, Z.N.; Liu, Z.F.; Chen, Y.S.; Peumans, P. Organic solar cells with solution-processed graphene transparent electrodes. Appl. Phys. Lett. 2008, 92, 263302. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Landolt-Börnstein - Group III Condensed Matter Numerical Data and Functional Relationships in Science and Technology; Madelung, O.; Rössler, U.; Schulz, M. (Eds.) Springer: New York, NY, USA, 1999; Volume 41B.
- Blochwitz, J.; Fritz, T.; Pfeiffer, M.; Leo, K.; Alloway, D.M.; Lee, P.A.; Armstrong, N.R. Interface electronic structure of organic semiconductors with controlled doping levels. Org. Electron. 2001, 2, 97–104. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Koster, L.J.A.; Blom, P.W.M. Effect of metal electrodes on the performance of polymer: Fullerene bulk heterojunction solar cells. Appl. Phys. Lett. 2004, 85, 970–972. [Google Scholar] [CrossRef]
- Ahlswede, E.; Hanisch, J.; Powalla, M. Comparative study of the influence of LiF, NaF, and KF on the performance of polymer bulk heterojunction solar cells. Appl. Phys. Lett. 2007, 90, 163504. [Google Scholar] [CrossRef]
- Zhang, F.; Ceder, M.; Inganas, O. Enhancing the photovoltage of polymer solar cells by using a modified cathode. Adv. Mater. 2007, 19, 1835–1838. [Google Scholar] [CrossRef]
- Reese, M.O.; White, M.S.; Rumbles, G.; Ginley, D.S.; Shaheen, S.E. Optimal negative electrodes for poly(3-hexylthiophene): [6,6]-phenyl C61-butyric acid methyl ester bulk heterojunction photovoltaic devices. Appl. Phys. Lett. 2008, 92, 053307. [Google Scholar] [CrossRef]
- Eo, Y.S.; Rhee, H.W.; Chin, B.D.; Yu, J.-W. Influence of metal cathode for organic photovoltaic device performance. Synth. Met. 2009, 159, 1910–1913. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Y.; Zhang, F.; Liu, X.; Zhan, Y.; Fahlman, M. Tuning work function of noble metals as promising cathodes in organic electronic devices. Chem. Mater. 2009, 21, 2798–2802. [Google Scholar] [CrossRef]
- Zhang, F.L.; Jespersen, K.G.; Bjorstrom, C.; Svensson, M.; Andersson, M.R.; Sundstrom, V.; Magnusson, K.; Moons, E.; Yartsev, A.; Inganas, O. Influence of solvent mixing on the morphology and performance of solar cells based on polyfluorene copolymer/fullerene blends. Adv. Funct. Mater. 2006, 16, 667–674. [Google Scholar] [CrossRef]
- Sahin, Y.; Alem, S.; de Bettignies, R.; Nunzi, J.M. Development of air stable polymer solar cells using an inverted gold on top anode structure. Thin Solid Films 2005, 476, 340–343. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.L.; Baek, N.S.; Zou, J.Y.; O'Malley, K.; Jen, A.K.Y. Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer. Appl. Phys. Lett. 2008, 92, 233304. [Google Scholar] [CrossRef]
- Li, C.Y.; Wen, T.C.; Lee, T.H.; Guo, T.F.; Huang, J.C.A.; Lin, Y.C.; Hsu, Y.J. An inverted polymer photovoltaic cell with increased air stability obtained by employing novel hole/electron collecting layers. J. Mater. Chem. 2009, 19, 1643–1647. [Google Scholar] [CrossRef]
- Zimmermann, B.; Würfel, U.; Niggemann, M. Longterm stability of efficient inverted P3HT:PCBM solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 491–496. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.L.; Acton, O.; Baek, N.S.; Ma, H.; Jen, A.K.Y. Interfacial modification to improve inverted polymer solar cells. J. Mater. Chem. 2008, 18, 5113–5119. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.L.; Ma, H.; Jen, A.K.Y. High performance ambient processed inverted polymer solar cells through interfacial modification with a fullerene self-assembled monolayer. Appl. Phys. Lett. 2008, 93, 253301. [Google Scholar] [CrossRef]
- Glatthaar, M.; Niggemann, M.; Zimmermann, B.; Lewer, P.; Riede, M.; Hinsch, A.; Luther, J. Organic solar cells using inverted layer sequence. Thin Solid Films 2005, 491, 298–300. [Google Scholar] [CrossRef]
- Al-Ibrahim, M.; Sensfuss, S.; Uziel, J.; Ecke, G.; Ambacher, O. Comparison of normal and inverse poly(3-hexylthiophene)/fullerene solar cell architectures. Sol. Energy Mater. Sol. Cells 2005, 85, 277–283. [Google Scholar] [CrossRef]
- Huang, J.S.; Li, G.; Yang, Y. A semi-transparent plastic solar cell fabricated by a lamination process. Adv. Mater. 2008, 20, 415–419. [Google Scholar] [CrossRef]
- Nakamura, M.; Yang, C.H.; Tajima, K.; Hashimoto, K. High-performance polymer photovoltaic devices with inverted structure prepared by thermal lamination. Sol. Energy Mater. Sol. Cells 2009, 93, 1681–1684. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, F.H.; Barrau, S.; Tian, W.J.; Inganas, O.; Zhang, F.L. Inverted and transparent polymer solar cells prepared with vacuum-free processing. Sol. Energy Mater. Sol. Cells 2009, 93, 497–500. [Google Scholar] [CrossRef]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Krebs, F.C.; Gevorgyan, S.A.; Alstrup, J. A roll-to-roll process to flexible polymer solar cells: Model studies, manufacture and operational stability studies. J. Mater. Chem. 2009, 19, 5442–5451. [Google Scholar] [CrossRef]
- Al-Ibrahim, M.; Roth, H.K.; Zhokhavets, U.; Gobsch, G.; Sensfuss, S. Flexible large area polymer solar cells based on poly(3-hexylthiophene)/fullerene. Sol. Energy Mater. Sol. Cells 2005, 85, 13–20. [Google Scholar] [CrossRef]
- Pandey, A.K.; Nunzi, J.-M. Efficient flexible and thermally stable pentacene/C60 small molecule based organic solar cells. Appl. Phys. Lett. 2006, 89, 213506. [Google Scholar] [CrossRef]
- Choi, S.; Potscavage, J.W.J.; Kippelen, B. Area-scaling of organic solar cells. J. Appl. Phys. 2009, 106, 054507–054510. [Google Scholar] [CrossRef]
- Kang, N.S.; Ju, B.K.; Lee, C.; Ahn, J.P.; Chin, B.D.; Yu, J.W. Partitioning of the organic layers for the fabrication of high efficiency organic photovoltaic devices. Org. Electron. 2009, 10, 1091–1096. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. Accurate measurement and characterization of organic solar cells. Adv. Funct. Mater. 2006, 16, 2016–2023. [Google Scholar] [CrossRef]
- Cravino, A.; Schilinsky, P.; Brabec, C.J. Characterization of organic solar cells: the importance of device layout. Adv. Funct. Mater. 2007, 17, 3906–3910. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, M.-G.; Guo, L.J.; Kim, J. Choice of electrode geometry for accurate measurement of organic photovoltaic cell performance. Appl. Phys. Lett. 2008, 92, 133301. [Google Scholar] [CrossRef]
- Lungenschmied, C.; Dennler, G.; Neugebauer, H.; Sariciftci, S.N.; Glatthaar, M.; Meyer, T.; Meyer, A. Flexible, long-lived, large-area, organic solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 379–384. [Google Scholar] [CrossRef]
- Muhsin, B.; Renz, J.; Drue, K.-H.; Gobsch, G.; Hoppe, H. Influence of polymer solar cell geometry on series resistance and device efficiency. Phys. Status Solidi A 2009, 206, 2771–2774. [Google Scholar]
- Yu, G.; Heeger, A.J. Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 1995, 78, 4510. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells—Enhanced efficiencies via a network of internal donor-acceptor heterojuctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Brabec, C.J.; Padinger, F.; Hummelen, J.C.; Janssen, R.A.J.; Sariciftci, N.S. Realization of large area flexible fullerene—Conjugated polymer photocells: A route to plastic solar cells. Synth. Met. 1999, 102, 861–864. [Google Scholar] [CrossRef]
- Schilinsky, P.; Waldauf, C.; Brabec, C.J. Performance analysis of printed bulk-heterojunction solar cells. Adv. Funct. Mater. 2006, 16, 1669–1672. [Google Scholar] [CrossRef]
- Reyes-Reyes, M.; Kim, K.; Dewald, J.; Lopez-Sandoval, R.; Avadhanula, A.; Curran, S.; Carroll, D.L. Meso-structure formation for enhanced organic photovoltaic cells. Org. Lett. 2005, 7, 5749–5752. [Google Scholar] [CrossRef] [PubMed]
- Krebs, F.C.; Spanggard, H.; Kjær, T.; Biancardo, M.; Alstrup, J. Large area plastic solar cell modules. Mater. Sci. Eng. B 2007, 138, 106–111. [Google Scholar] [CrossRef]
- Krebs, F.C.; Alstrup, J.; Spanggaard, H.; Larsen, K.; Kold, E. Production of large-area polymer solar cells by industrial silk screen printing, lifetime considerations and lamination with polyethyleneterephthalate. Sol. Energy Mater. Sol. Cells 2004, 83, 293–300. [Google Scholar] [CrossRef]
- Krebs, F.C.; Jørgensen, M.; Norrman, K.; Hagemann, O.; Alstrup, J.; Nielsen, T.D.; Fyenbo, J.; Larsen, K.; Kristensen, J. A complete process for production of flexible large area polymer solar cells entirely using screen printing--First public demonstration. Sol. Energy Mater. Sol. Cells 2009, 93, 422–441. [Google Scholar] [CrossRef]
- Shaheen, S.E.; Radspinner, R.; Peyghambarian, N.; Jabbour, G.E. Fabrication of bulk heterojunction plastic solar cells by screen printing. Appl. Phys. Lett. 2001, 79, 2996–2998. [Google Scholar] [CrossRef]
- Hoth, C.N.; Choulis, S.A.; Schilinsky, P.; Brabec, C.J. High photovoltaic performance of inkjet printed polymer:fullerene blends. Adv. Mater. 2007, 19, 3973–3978. [Google Scholar] [CrossRef]
- Aernouts, T.; Aleksandrov, T.; Girotto, C.; Genoe, J.; Poortmans, J. Polymer based organic solar cells using ink-jet printed active layers. Appl. Phys. Lett. 2008, 92, 033306. [Google Scholar] [CrossRef]
- Yoo, S.; Potscavage, W.J.; Domercq, B.; Kim, J.; Holt, J.; Kippelen, B. Integrated organic photovoltaic modules with a scalable voltage output. Appl. Phys. Lett. 2006, 89, 233516. [Google Scholar] [CrossRef]
- Niggemann, M.; Zimmermann, B.; Haschke, J.; Glatthaar, M.; Gombert, A. Organic solar cell modules for specific applications—From energy autonomous systems to large area photovoltaics. Thin Solid Films 2008, 516, 7181–7187. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Tipnis, R.; Bernkopf, J.; Jia, S.; Krieg, J.; Li, S.; Storch, M.; Laird, D. Large-area organic photovoltaic module—Fabrication and performance. Sol. Energy Mater. Sol. Cells 2009, 93, 442–446. [Google Scholar] [CrossRef]
- Muhsin, B.; Renz, J.; Drue, K.H.; Gobsch, G.; Hoppe, H. Efficient polymer solar cell modules. Synth. Met. 2009, 159, 2358–2361. [Google Scholar] [CrossRef]
- Krebs, F.C. A simple nanostructured polymer/ZnO hybrid solar cell preparation and operation in air. Nanotechnol. 2008, 19, 424013. [Google Scholar] [CrossRef]
- Zimmermann, B.; Glatthaar, M.; Niggemann, M.; Riede, M.K.; Hinsch, A.; Gombert, A. ITO-free wrap through organic solar cells—A module concept for cost-efficient reel-to-reel production. Sol. Energy Mater. Sol. Cells 2007, 91, 374–378. [Google Scholar] [CrossRef]
- Steim, R.; Schilinsky, P.; Choulis, S.A.; Brabec, C.J. Flexible polymer photovoltaic modules with incorporated organic bypass diodes to address module shading effects. Sol. Energy Mater. Sol. Cells 2009, 93, 1963–1967. [Google Scholar] [CrossRef]
- Balenzategui, J.L.; Chenlo, F. Measurement and analysis of angular response of bare and encapsulated silicon solar cells. Sol. Energy Mater. Sol. Cells 2005, 86, 53–83. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Organic solar cells: An overview. J. Mater. Res. 2004, 19, 1924–1945. [Google Scholar] [CrossRef]
- Hoppe, H.; Sariciftci, N.S. Morphology of polymer/fullerene bulk heterojunction solar cells. J. Mater. Chem. 2006, 16, 45–61. [Google Scholar] [CrossRef]
- Agrawal, M.; Peumans, P. Broadband optical absorption enhancement through coherent light trapping in thin-film photovoltaic cells. Opt. Exp. 2008, 16, 5385–5396. [Google Scholar] [CrossRef]
- Gunes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Winder, C.; Sariciftci, N.S.; Hummelen, J.C.; Dhanabalan, A.; van Hal, P.A.; Janssen, R.A.J. A low-bandgap semiconducting polymer for photovoltaic devices and infrared emitting diodes. Adv. Funct. Mater. 2002, 12, 709–712. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Kroon, R.; Lenes, M.; Hummelen, J.C.; Blom, P.W.M.; de Boer, B. Small bandgap polymers for organic solar cells (Polymer material development in the last 5 years). Polym. Rev. 2008, 48, 531–582. [Google Scholar] [CrossRef]
- Wienk, M.M.; Struijk, M.P.; Janssen, R.A.J. Low band gap polymer bulk heterojunction solar cells. Chem. Phys. Lett. 2006, 422, 488–491. [Google Scholar] [CrossRef]
- Winder, C.; Sariciftci, N.S. Low bandgap polymers for photon harvesting in bulk heterojunction solar cells. J. Mater. Chem. 2004, 14, 1077–1086. [Google Scholar] [CrossRef]
- Pettersson, L.A.A.; Roman, L.S. Modeling photocurrent action spectra of photovoltaic devices based on organic thin films. J. Appl. Phys. 1999, 86, 487. [Google Scholar] [CrossRef]
- Ameri, T.; Dennler, G.; Waldauf, C.; Denk, P.; Forberich, K.; Scharber, M.C.; Brabec, C.J.; Hingerl, K. Realization, characterization, and optical modeling of inverted bulk-heterojunction organic solar cells. J. Appl. Phys. 2008, 103. [Google Scholar]
- Persson, N.K.; Inganäs, O. Organic tandem solar cells—Modeling and predictions. Sol. Energy Mater. Sol. Cells 2006, 90, 3491–3507. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, A.; Yoshikawa, O.; Fujieda, T.; Uehara, K.; Yoshikawa, S. High performance polythiophene/fullerene bulk-heterojunction solar cell with a TiOx hole blocking layer. Appl. Phys. Lett. 2007, 90, 163517. [Google Scholar] [CrossRef]
- Gilot, J.; Barbu, I.; Wienk, M.M.; Janssen, R.A.J. The use of ZnO as optical spacer in polymer solar cells: Theoretical and experimental study. Appl. Phys. Lett. 2007, 91, 113520. [Google Scholar] [CrossRef]
- Andersson, B.V.; Huang, D.M.; Moule, A.J.; Inganas, O. An optical spacer is no panacea for light collection in organic solar cells. Appl. Phys. Lett. 2009, 94, 043302. [Google Scholar] [CrossRef]
- Gilot, J.; Wienk, M.M.; Janssen, R.A.J. Double and triple junction polymer solar cells processed from solution. Appl. Phys. Lett. 2007, 90, 143512. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.; Ameri, T.; Denk, P.; Forberich, K.; Waldauf, C.; Brabec, C. Design rules for donors in bulk-heterojunction tandem solar cells—Towards 15% energy-conversion efficiency. Adv. Mater. 2008, 20, 579–583. [Google Scholar] [CrossRef]
- Hadipour, A.; de Boer, B.; Blom, P.W.M. Organic tandem and multi-junction solar cells. Adv. Funct. Mater. 2008, 18, 169–181. [Google Scholar] [CrossRef]
- Peumans, P.; Yakimov, A.; Forrest, S.R. Small molecular weight organic thin-film photodetectors and solar cells. J. Appl. Phys. 2003, 93, 3693–3723. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Dal Zilio, S.; Inganas, O.; Tormen, M. Trapping light with micro lenses in thin film organic photovoltaic cells. Opt. Exp. 2008, 16, 21608–21615. [Google Scholar] [CrossRef]
- Roman, L.S.; Inganäs, O.; Granlund, T.; Nyberg, T.; Svensson, M.; Andersson, M.R.; Hummelen, J.C. Trapping light in polymer photodiodes with soft embossed gratings. Adv. Mater. 2000, 12, 189–195. [Google Scholar] [CrossRef]
- Na, S.I.; Kim, S.S.; Jo, J.; Oh, S.H.; Kim, J.; Kim, D.Y. Efficient polymer solar cells with surface relief gratings fabricated by simple soft lithography. Adv. Funct. Mater. 2008, 18, 3956–3963. [Google Scholar] [CrossRef]
- Niggemann, M.; Glatthaar, M.; Gombert, A.; Hinsch, A.; Wittwer, V. Diffraction gratings and buried nano-electrodes--architectures for organic solar cells. Thin Solid Films 2004, 451-452, 619–623. [Google Scholar]
- Zilio, S.D.; Tvingstedt, K.; Inganäs, O.; Tormen, M. Fabrication of a light trapping system for organic solar cells. Microelectron. Eng. 2009, 86, 1150–1154. [Google Scholar] [CrossRef]
- Koeppe, R.; Sariciftci, N.S.; Buchtemann, A. Enhancing photon harvesting in organic solar cells with luminescent concentrators. Appl. Phys. Lett. 2007, 90, 181126. [Google Scholar] [CrossRef]
- Currie, M.J.; Mapel, J.K.; Heidel, T.D.; Goffri, S.; Baldo, M.A. High-efficiency organic solar concentrators for photovoltaics. Science 2008, 321, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Rim, S.-B.; Zhao, S.; Scully, S.R.; McGehee, M.D.; Peumans, P. An effective light trapping configuration for thin-film solar cells. Appl. Phys. Lett. 2007, 91, 243501. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Andersson, V.; Zhang, F.; Inganas, O. Folded reflective tandem polymer solar cell doubles efficiency. Appl. Phys. Lett. 2007, 91, 123514. [Google Scholar] [CrossRef]
- Andersson, V.; Tvingstedt, K.; Inganas, O. Optical modeling of a folded organic solar cell. J. Appl. Phys. 2008, 103, 7. [Google Scholar] [CrossRef]
- Andersson, B.V.; Persson, N.K.; Inganas, O. Comparative study of organic thin film tandem solar cells in alternative geometries. J. Appl. Phys. 2008, 104, 124508. [Google Scholar] [CrossRef]
- Curran, S.; Talla, J.; Dias, S.; Dewald, J. Microconcentrator photovoltaic cell (the m-C cell): Modeling the optimum method of capturing light in an organic fiber based photovoltaic cell. J. Appl. Phys. 2008, 104, 064305. [Google Scholar] [CrossRef]
- Liu, J.W.; Namboothiry, M.A.G.; Carroll, D.L. Optical geometries for fiber-based organic photovoltaics. Appl. Phys. Lett. 2007, 90, 133515. [Google Scholar] [CrossRef]
- O'Connor, B.; Pipe, K.P.; Shtein, M. Fiber based organic photovoltaic devices. Appl. Phys. Lett. 2008, 92, 193306. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, K.-S.; Yambem, S.D.; Haldar, A.; Alley, N.J.; Curran, S.A. Designs and Architectures for the Next Generation of Organic Solar Cells. Energies 2010, 3, 1212-1250. https://doi.org/10.3390/en3061212
Liao K-S, Yambem SD, Haldar A, Alley NJ, Curran SA. Designs and Architectures for the Next Generation of Organic Solar Cells. Energies. 2010; 3(6):1212-1250. https://doi.org/10.3390/en3061212
Chicago/Turabian StyleLiao, Kang-Shyang, Soniya D. Yambem, Amrita Haldar, Nigel J. Alley, and Seamus A. Curran. 2010. "Designs and Architectures for the Next Generation of Organic Solar Cells" Energies 3, no. 6: 1212-1250. https://doi.org/10.3390/en3061212




