A High Capacity Li-Ion Cathode: The Fe(III/VI) Super-Iron Cathode
Abstract
:1. Introduction
| Year | Development | Reference |
|---|---|---|
| 1999 | introduction of super-iron charge storage & super-iron alkaline battery | [5] |
| 2000** | introduction of super-iron lithium primary (single discharge) battery | [7] |
| 2001 | demonstration of the solid state stability of the hexavalent iron | [8] |
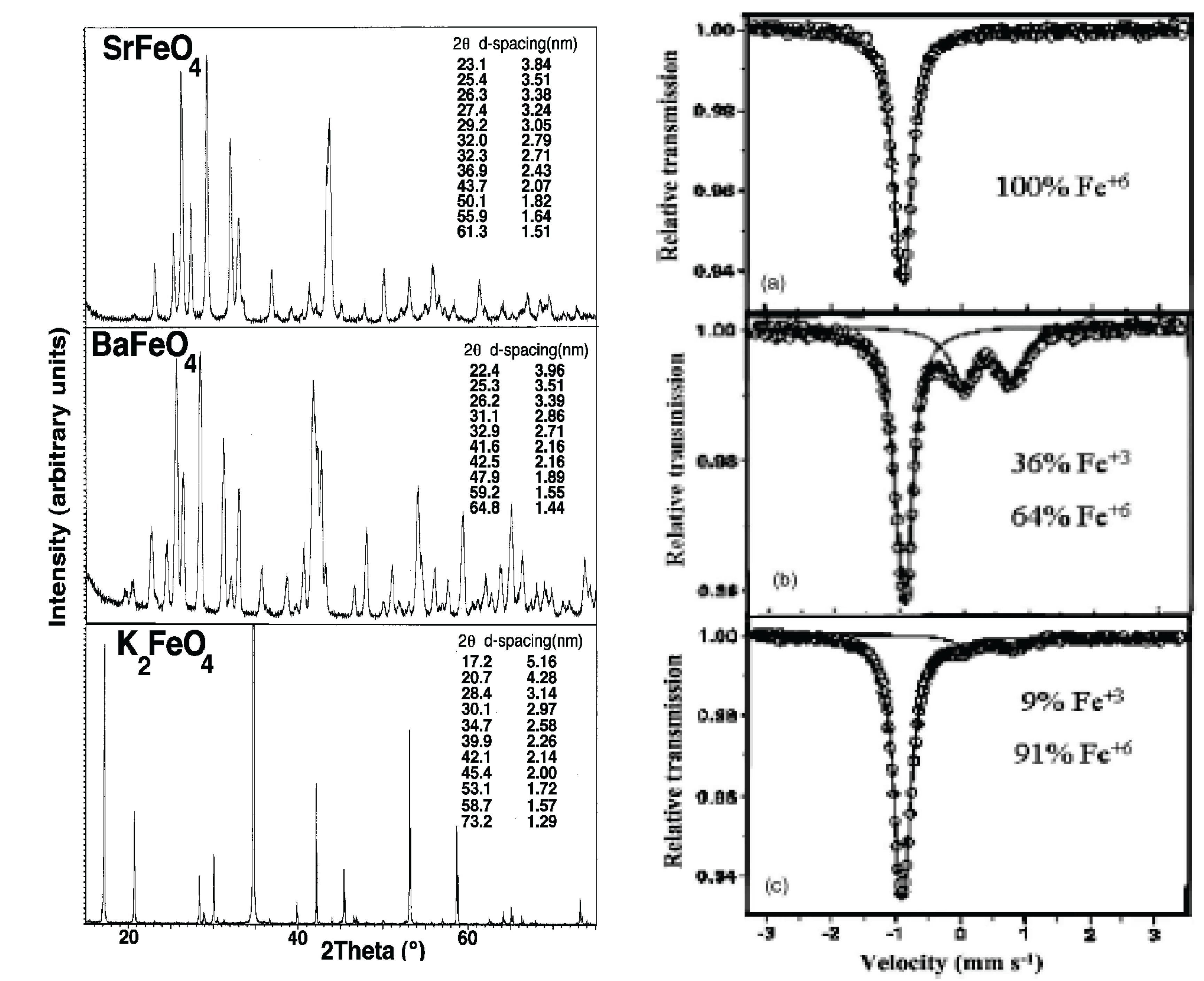
| 1999-5 | chemical syntheses of an array of super-iron salts | [5,7,9,10,11,12,13,14,15,16] |
| 2000-4 | inexpensive, electrochemical syntheses of super-iron salts | [17,18,19,20,21,22,23,24,25,26] |
| 2003-5** | electrolyte optimization for super-iron lithium batteries | [27,28] |
| 2003 | reversibility of alkaline, nanothick (3 nm) Fe(VI) cathodes | [29] |
| 2006 | rechargeable alkaline super-iron battery | [30] |
| 2006** | reversibility of non-aqueous, nanothick (3 nm) Fe(VI) cathodes | [31] |
| 2007-8 | zirconia encapsulation–stabilization of alkali super-irons | [32,33,34,35] |
| 2009** | rechargeable super-iron lithium battery, 4 V cathode | [6] |
2. Results and Discussion
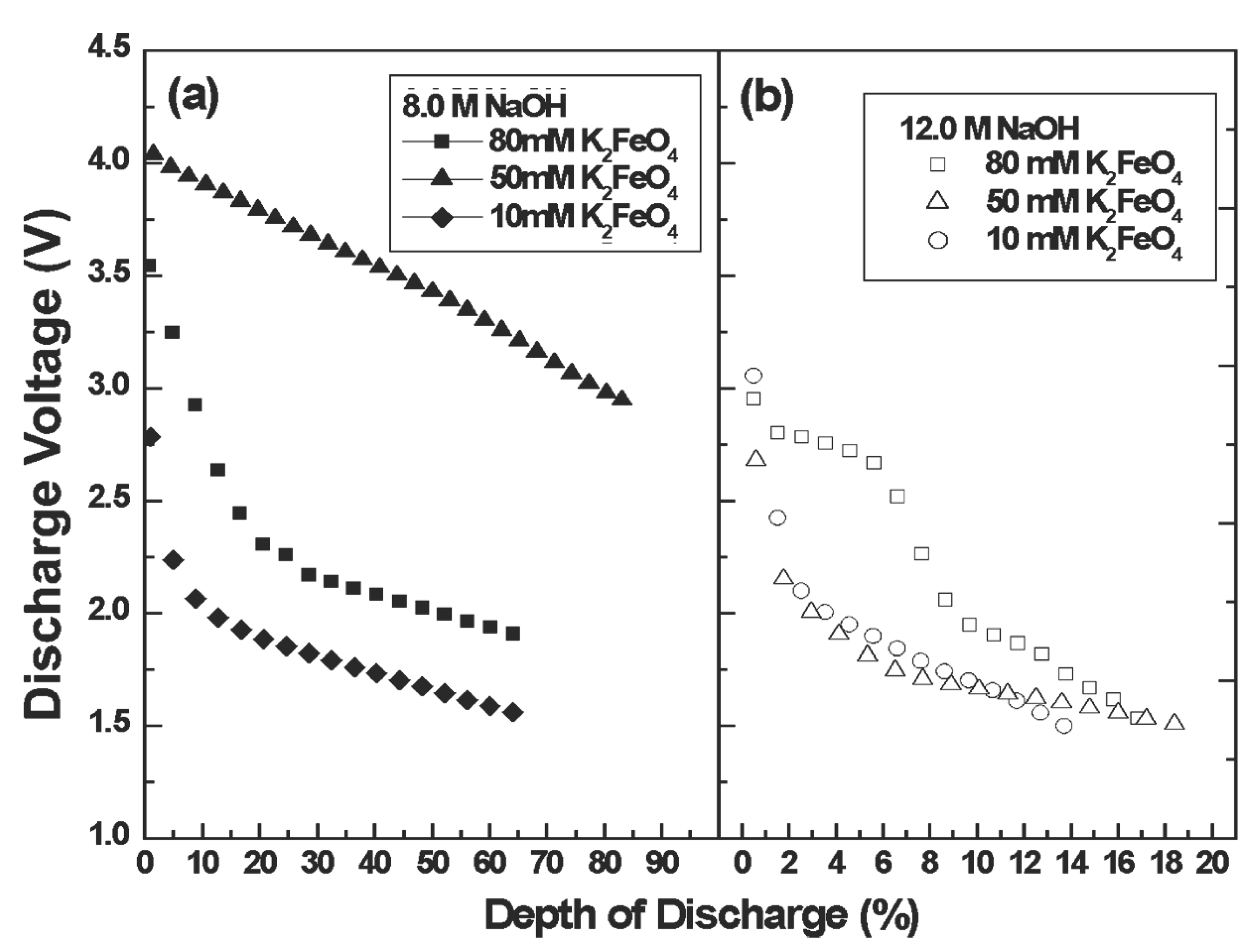
2.1. The challenge of facile Fe(VI) charge transfer

2.2. Reversible non-aqueous 3e- Fe(VI) charge storage
- (i)
- Fe(VI) nonaqueous charge transfer is constrained by reduction, not intercalation.
- (ii)
- Fe(VI) cathodes store the charge equivalent to 3 electrons per iron center,
- (iii)
- an extended conductive matrix facilitates reversible Fe(VI) reduction,
- (iv)
- the redox Fe(VI) potential is 0.25 V larger than that of Li-Mn or Li-Co cathodes.
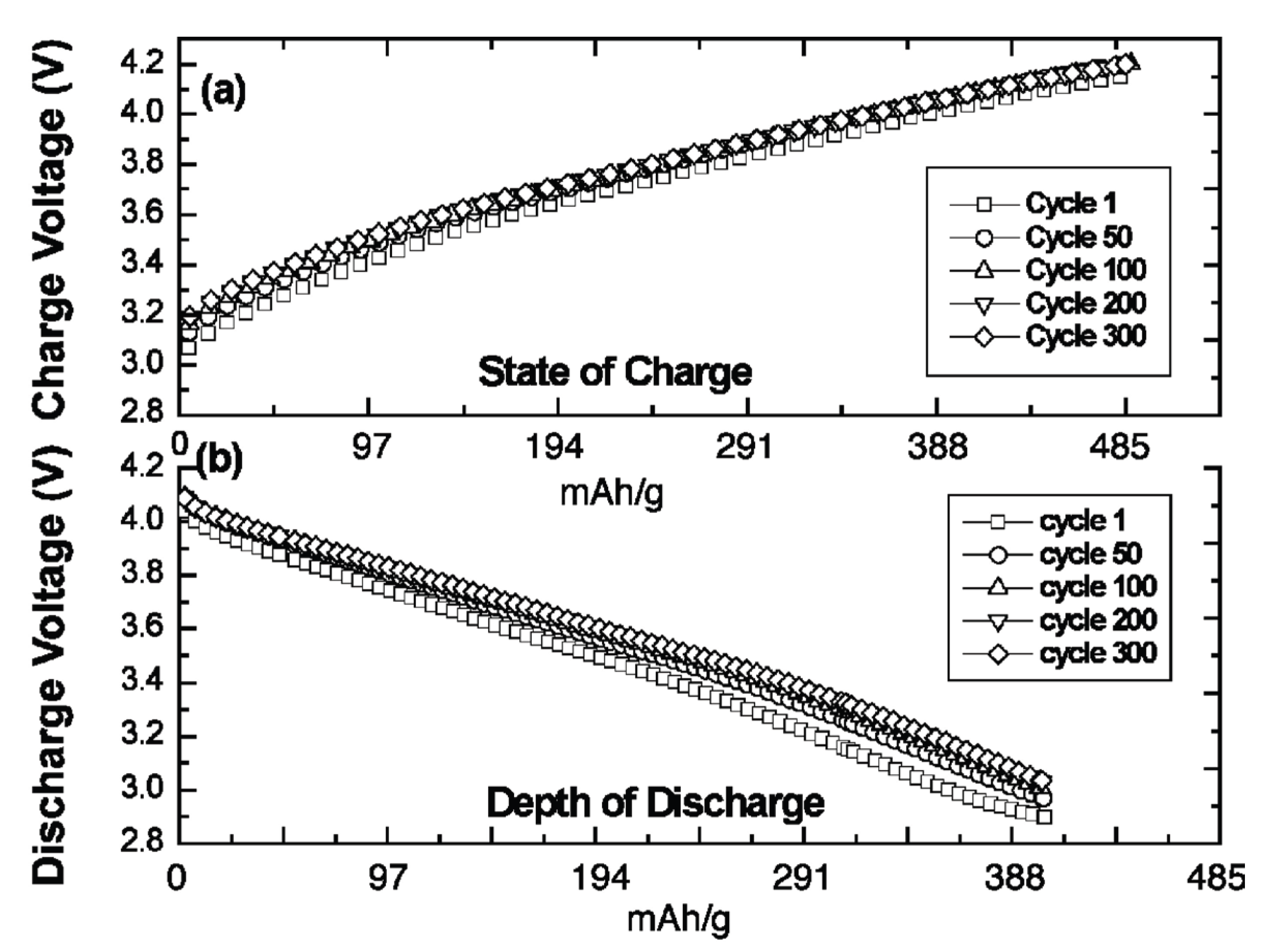
2.3. Preparation of Super-Iron Cathode Films

2.4. Characterization of Super-Iron Cathode Films
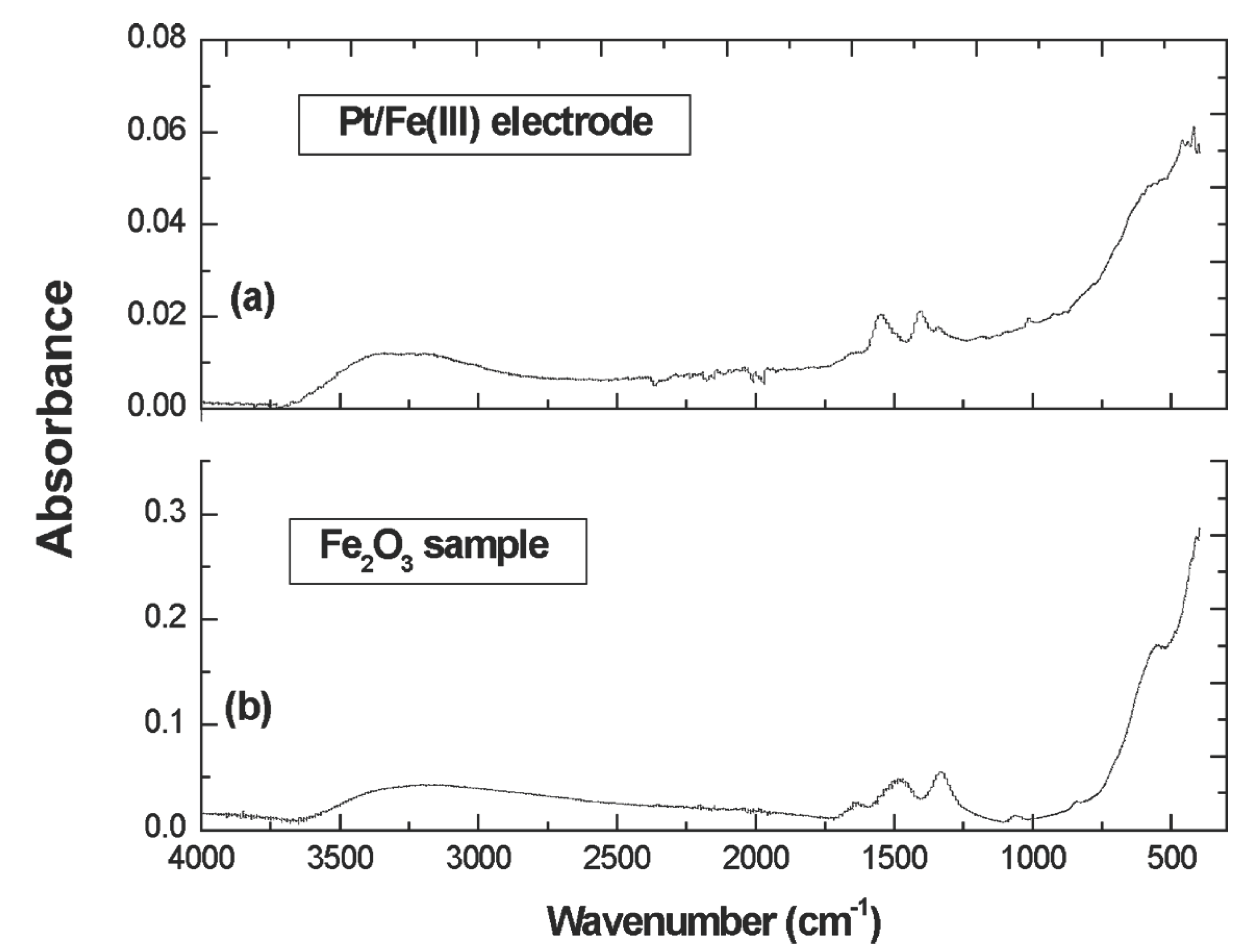

3. Conclusions
Acknowledgements
References and Notes
- Advances in Lithium-ion Batteries; van Schalkwijk, W.A.; Scrosati, B. (Eds.) Springer: Berlin, Germany, 2002.
- Licht, S.; Wu, H.; Yu, X.; Wang, Y. Renewable highest capacity VB2/air energy storage. Chem. Comm. 2008, 28, 3257–3259. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.; Yang, F. Developments of lithium-ion batteries & challenges of LiFePO4 as one promising cathode material. J. Mater. Sci. 2009, 44, 2435–2443. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium-ion batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Licht, S.; Wang, B.; Ghosh, S. Energetic iron (VI) chemistry: The super-iron battery. Science 1999, 285, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; Wang, Y.; Gourdin, G. Enhancement of reversible nonaqueous Fe(III/VI) cathodic charge transfer. J. Phys. Chem. C 2009, 113, 9884–9891. [Google Scholar]
- Licht, S.; Wang, B. Non aqueous Iron(VI) chemistry: The lithium super-iron battery. Electrochem. Solid State Lett. 2000, 3, A 209–A212. [Google Scholar]
- Licht, S.; Naschitz, V.; Halperin, L.; Halperin, L.; Lin, L.; Chen, J.; Ghosh, S.; Lui, B. Analysis of Ferrate(VI) compounds & super-Iron battery cathodes, FTIR, XRD, UV/Vis, ICP, electrochemical & chemical characterization. J. Power Sources 2001, 99, 7–14. [Google Scholar] [CrossRef]
- Licht, S.; Wang, B.; Ghosh, S.; Jun, Li.; Naschitz, V. Insoluble Fe(VI) compounds: Effects on the super-iron battery. Electrochem. Comm. 1999, 1, 522–526. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Ghosh, S.; Liu, B.; Halperin, N.; Halperin, L.; Rozen, D. Chemical synthesis of battery grade super-iron barium and potassium Fe(VI) ferrate compounds. J. Power Sources 2001, 99, 7–14. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Ghosh, S.; Lin, L.; Lui, B. SrFeO4: synthesis, Fe(VI) characterization and the strontium super-iron battery. Electrochem. Comm. 2001, 3, 340–345. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Pan, T.; Xu, J.; Xhang, J.; Cao, C. Studies on electrochem. characteristics of K2Sr(FeO4)2 electrode. Electrochem. Comm. 2002, 4, 710–715. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Wang, B. Rapid chemical synthesis of barium ferrate, BaFe(VI)O4. J. Power Sources 2002, 109, 67–70. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Rozen, D.; Halperin, N. Cathodic charge transfer and analysis of Cs2FeO4, K2FeO4 and mixed alkali Fe(VI), ferrate, super-irons. J. Electrochem. Soc. 2004, 151, A1147–A1151. [Google Scholar] [CrossRef]
- Licht, S.; Yang, L.; Wang, B. Synthesis and analysis of Ag2FeO4 Fe(VI) ferrate super-iron cathodes. Electrochem. Comm. 2005, 7, 931–936. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Shao, H.; Tang, Z.; Zhang, J. Preliminary investigation on the physiochemical properties of calcium ferrate. Electrochem. Comm. 2007, 9, 371–377. [Google Scholar] [CrossRef]
- Licht, S. Electrolytic production of solid Fe(VI) salts, PCT application No. LI00588. Patent No. WO0,121,856, 2000. [Google Scholar]
- Lapique, F.; Valentin, G. Direct electrochemical preparation of solid potassium ferrate. Electrochem. Comm. 2002, 4, 764–766. [Google Scholar]
- Licht, S.; Tel-Vered, R.; Halperin, L. Direct electrochemical preparation of solid Fe(VI) compounds and super-iron battery compounds. Electrochem. Comm. 2002, 4, 933–937. [Google Scholar] [CrossRef]
- Lee, J.; Tryk, D.; Fujishima, A.; Park, S. Electrochemical generation of ferrate in acidic media at boron-doped diamond electrodes. Chem. Comm. 2002, 5, 486–487. [Google Scholar] [CrossRef] [PubMed]
- De Koninck, M.; Brousse, T.; Belanger, D. The electrochemical generation of ferrate at pressed iron powder electrode: Effect of different parameters. Electrochim. Acta 2003, 48, 1425–1433. [Google Scholar] [CrossRef]
- Licht, S.; Tel-Vered, R.; Halperin, L. Towards efficient electrochemical synthesis of Fe(VI) ferrate, and super-iron battery compounds. J. Electrochem. Soc. 2004, 151, A31–A39. [Google Scholar] [CrossRef]
- Zhang, F.C.; Liu, Z.; Wu, F.; Lin, L.; Qi, F. Electrochemical generation of ferrate on SnO2-Sb2O3/Ti electrodes in strong concentration basic condition. Electrochem. Comm. 2005, 6, 1104–1109. [Google Scholar] [CrossRef]
- Cañizares, P.; Arcís, M.; Sáez, C.; Rodrigo, M.A. Electrochemical synthesis of ferrate using boron doped diamond anodes. Electrochem. Comm. 2007, 9, 2286–2290. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Mao, W.; He, W.; Chen, Q.; Zhang, J. The effects of ultrasound on the direct electrosynthesis of solid K2FeO4 and the anodic behaviors of Fe in 14 M KOH solution. J. Solid State Electrochem 2008, 11, 413–420. [Google Scholar] [CrossRef]
- Yu, X.; Licht, S. Advances in electrochemical Fe(VI) synthesis and analysis. J. Appl. Electrochem. 2008, 38, 731–742. [Google Scholar] [CrossRef]
- Licht, S.; Rozen, D.; Tel-Vered, R.; Halperin, L. Enhancement of nonaqueous Fe(VI) super-iron primary cathodic charge transfer. J. Electrochem. Soc. 2003, 150, A1671–A1675. [Google Scholar] [CrossRef]
- Koltypin, M.; Licht, S.; Tel-Vered, R.; Nashitz, V.; Aurbach, D. The Study of Licht, S.: Various ("super iron") MeFeO4 (F6+-super iron) compounds in Li salt solutions. J. Power Sources 2005, 146, 723–726. [Google Scholar] [CrossRef]
- Licht, S.; Tel-Vered, R. Rechargeable Fe(III/VI) super-iron cathodes. Chem. Comm. 2004, 6, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Licht, S.; DeAlwis, C. Conductive matrix mediated charge transfer in Fe(III/VI): Three electron storage, reversible super-iron thin film cathodes. J. Phys. Chem. B 2006, 110, 12394–123404. [Google Scholar] [CrossRef] [PubMed]
- Koltypin, M.; Licht, S.; Nowik, I.; Levi, E.; Gofer, Y.; Aurbach, D. The study of various ("super-iron") MeFeO4 compounds in Li salt solutions as potential cathode materials for Li batteries. J. Electrochem. Soc 2006, 153, A32–A41. [Google Scholar] [CrossRef]
- Licht, S.; Yu, X.; Dhong, Z. Cathodic chemistry of high performance Zr coated alkaline materials. Chem. Comm. 2006, 2006, 4341–4343. [Google Scholar] [CrossRef]
- Licht, S.; Yu, X.; Qu, D. A novel alkaline redox couple: Chemistry of the Fe6+/B2– super-iron boride battery. Chem. Comm. 2007, 2007, 2753–2755. [Google Scholar] [CrossRef]
- Licht, S.; Yu, X.; Wang, Y. Stabilized alkaline Fe(VI) charge transfer: Zirconia coating stabilized super-iron alkaline batteries. J. Electrochem. Soc. 2008, 155, A1–A7. [Google Scholar] [CrossRef]
- Licht, S.; Yu, X.; Wang, Y.; Wu, H. The super-iron boride battery. J.Electrochem. Soc. 2008, 155, A297–A303. [Google Scholar] [CrossRef]
- Ghosh, S.; Wen, W.; Urian, R.C.; Heath, C.; Srinivasamurthi, V.; Reiff, W.M.; Mukerjee, S.; Naschitz, V.; Licht, S. The reversible behavior of K2FeO4. Electrochem. Solid State Lett. 2003, 6, A260–A264. [Google Scholar] [CrossRef]
- Yu, X.; Licht, S. Recent advances in synthesis and analysis of Fe(VI) cathodes: solution phase and solid-state Fe(VI) syntheses, reversible thin-film Fe(VI) synthesis, coating-stabilized Fe(VI) synthesis. J. Solid State Electrochem. 2008, 12, 1523–1540. [Google Scholar] [CrossRef]
- Licht, S.; Ghosh, S.; Naschitz, V.; Halperin, N.; Halperin, L. Fe(VI) catalyzed manganese redox chemistry: Permanganate and super-iron batteries. J. Phys. Chem. B 2001, 105, 11933–11936. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Ghosh, S. Silver mediation of Fe(VI) charge transfer: Activation of the K2FeO4 super-iron cathode. J. Phys. Chem. B 2002, 106, 5947–5955. [Google Scholar] [CrossRef]
- Yu, X.; Licht, S. Advances in Fe(VI) charge storage: Part II. Reversible alkaline super-iron batteries and nonaqueous super-iron batteries. J. Power Sources 2007, 171, 1010–1022. [Google Scholar] [CrossRef]
- Licht, S.; Ghosh, S. High power BaFe(VI)O4 /MnO2 composite cathode alkaline super-iron batteries. J. Power Sources 2002, 109, 465–468. [Google Scholar] [CrossRef]
- Licht, S.; Naschitz, V.; Ghosh, S. Hydroxide activated AgMnO4 alkaline cathodes, alone, and in combination with Fe(VI) super-iron, BaFeO4. Electrochem. Solid State Lett. 2001, 4, A209–A212. [Google Scholar] [CrossRef]
- Anderson, D.L. An evaluation of current and future costs for lithium-ion batteries for use in electrified vehicle powertrains. Master Thesis; Duke University: Durham, NC, USA, May 2009. Available online: http://dukespace.lib.duke.edu/dspace/bitstream/10161/1007/1/Li-Ion%20Battery%20Costs%20-%20Anderson%20-%20MP%20Final.pdf (accessed on 25 April 2010).
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Licht, S. A High Capacity Li-Ion Cathode: The Fe(III/VI) Super-Iron Cathode. Energies 2010, 3, 960-972. https://doi.org/10.3390/en3050960
Licht S. A High Capacity Li-Ion Cathode: The Fe(III/VI) Super-Iron Cathode. Energies. 2010; 3(5):960-972. https://doi.org/10.3390/en3050960
Chicago/Turabian StyleLicht, Stuart. 2010. "A High Capacity Li-Ion Cathode: The Fe(III/VI) Super-Iron Cathode" Energies 3, no. 5: 960-972. https://doi.org/10.3390/en3050960




