1. Introduction
The limited fossil fuel resources along with the need to reduce Green House Gas emissions were a major impulse to the development of alternative fuels. As a result, increased attention has been given to biofuels, such as biodiesel, that can be used as an alternative fuel in compression–ignition engines. Its production from renewable resources, such as vegetable oils and animal fats, makes it biodegradable and non-toxic; also, it contributes to the reduction of CO
2 emissions, because it comprises a closed carbon cycle [
1,
2,
3,
4,
5,
6].
Generally, biodiesel is produced by means of transesterification. Transesterification is the reaction of a lipid with an alcohol to form esters and a byproduct, glycerol. It is, in principle, the action of one alcohol displacing another from an ester, referred to as alcoholysis (cleavage by an alcohol). The reaction is reversible, and thus an excess of alcohol is usually used to force the equilibrium to the product side. The stoichiometry for the reaction is 3:1 alcohol to lipids. However, in practice this is usually increased to 6:1 to raise the product yield. Transesterification consists of a sequence of three consecutive reversible reactions. The first step is the conversion of triglycerides to diglycerides, followed by the conversion of diglycerides to monoglycerides, and finally monoglycerides into glycerol, yielding one ester molecule from each glyceride at each step. The reactions are reversible, although the equilibrium lies towards the production of fatty acid esters and glycerol [
7,
8,
9,
10].
The catalyst used has a significant effect on the reaction, raising the rate notably. It is known that basic catalysts require short times to complete the reaction even at room temperature, while acid catalysts, such as sulfuric acid, require higher temperatures (100 °C) and longer reaction times (3 – 4 h) [
4,
11,
12,
13]. The alkalis that are used generally include sodium and potassium hydroxides, carbonates, and alkoxides such as methoxide, ethoxide, propoxide, and butoxide.
Short-chain alcohols such as methanol, ethanol, and butanol are the most frequently employed. The selection of the alcohol is based on cost and performance consideration. Ethanol can be produced from agricultural renewable resources, thereby attaining total independence from petroleum-based alcohols. Also, ethanol, as an extraction solvent, is preferable to methanol because of its much higher dissolving power for oils. For this cause, ethanol is sometimes used as an suitable alcohol for the transesterification of vegetables oils. Therefore, producing ethyl esters rather than methyl esters is of considerable interest, because, in addition to the entirely agricultural nature of the ethanol, the extra carbon atom provided by the ethanol molecule slightly increases the heat content and the cetane number [
14].
From an environmental point of view, ethyl ester utilization is also more advantageous than the utilization of methyl esters. According to Makareviciene and Janulis [
15], the results showed that when considering emissions of nitrogen oxides (NO
x), carbon monoxide (CO) and smoke density, rapeseed oil ethyl ester had less negative effects on the environment in comparison with that of rapeseed oil methyl ester. When fueled with pure rapeseed oil ethyl ester, HC emissions decreased by 53%, CO emissions by 7.2% and smoke density by 72.6%, if compared with the emissions when fossil diesel fuel was used. Also carbon dioxide (CO
2) emissions, which contribute to the greenhouse effect, decreased by 782.87 g/kWh when rapeseed oil ethyl ester was used instead of fossil diesel fuel. Finally, these authors found that the rapeseed oil ethyl ester was more rapidly biodegradable in a water environment than rapeseed oil methyl ester and especially when blended with fossil diesel fuel.
However, the utilization of ethanol also presents inconveniences. Effectively, as it is indicated in the literature [
16], the base-catalyzed formation of ethyl ester is difficult compared to the formation of methyl esters. Specifically the formation of a stable emulsion during ethanolysis is a problem. Methanol and ethanol are not miscible with triglycerides at room temperature, and the reaction mixture is usually mechanically stirred to enhance mass transfer. During the course of reaction, emulsions are usually formed. In the case of methanolysis, these emulsions break down quickly and easily to form a lower glycerol rich layer and upper methyl ester rich layer. In ethanolysis, these emulsions are more stable and severely complicate the separation and purification of esters.
With these considerations, and as a continuation of previous works [
17,
18,
19], we carried out a study on the transesterification process of four different vegetable oils (sunflower, rapeseed, olive oil and used frying oil) utilizing ethanol, in order to characterize the ethyl esters obtained for their applications as fuels in internal combustion engines.
2. Experimental Section
2.1. Materials
The ethanol (anhydrous, purity ≥ 99.8%), NaOH (anhydrous, purity ≥ 98%) and analytical reagents (e.g., standards for GC analysis) were of high grade and were supplied from Sigma Chemical Co. Commercial grade olive oil was purchased from a local grocery store. Sunflower and rapeseed oil were obtained from Elin biofuels S.A, while the waste vegetable oil used in this work was a mixture of olive oil and sunflower oil collected from local fast food restaurants. The three vegetable oils (sunflower oil, olive oil and rapeseed oil) were used as received without further purification. Waste frying oil was filtered under vacuum, after being dehydrated overnight using anhydrous sodium sulphate and finally again filtered under vacuum, prior to use.
2.2. Transesterification Procedure
The transesterification reactions were carried out in a 500 mL glass spherical reactor, provided with a thermostat, mechanical stirring, sampling outlet, and condensation system. The procedure followed is described next. The reactor was preheated to 75 °C, to eliminate moisture, and then 250 g of each vegetable oil was added. When the reactor reached the temperature established for the reaction, the ethanol and the catalyst were added, in the amounts established for each experiment, and the stirring system was connected, taking this moment as time zero of the reaction. Each mixture was vigorously stirred and refluxed for the required reaction time. After the ethanolysis reaction finished, the excess ethanol was distilled off under vacuum (absolute pressure of 150 mmHg). The transesterification product was allowed to stand in a separating funnel for glycerol separation. Due to the strong emulsions formed in the case of the ethanolysis products, glycerol was not separated only by gravity, and in order to separate it from the ethyl ester phase, approximately 10 g of pure glycerol was added to the transesterification product, the separating funnel was shaken vigorously and the product was allowed to stand. The glycerol layer separated from the ester layer within an hour. The addition of pure glycerol to the mixtures, removes the residual catalyst and the soaps which have been formed during the transesterification reaction, thus creating a difference in the density between the two phases, and in this way making it easier for their separation by gravity. After separation of the two layers, crude methyl esters were washed several times (up to 10) with 50 cm3 of hot distilled water (50 °C) in a separatory funnel until neutral pH. Finally, the water present was eliminated by heating at 110 °C.
2.3. Analytical Methods
Different properties of the raw materials were determined: density at 15 °C (EN ISO 12185), kinematic viscosity at 40 °C (EN ISO 3104), flash point (ISO 3679), iodine number (EN 14111), acid value (EN 14104), saponification value (AOCS CD3 1993), sulfur content (EN ISO 20846), water content (EN ISO 12937), pour point (ISO 3016) and carbon residue (EN ISO 10370). The average molecular weight of vegetable oils is calculated by MW = 56.1 × 1000 × 3/(SV–AV), where AV (m
KOH/m
oil, mg/g) and SV is the saponification value (m
KOH/m
oil, mg/g) [
20]. It should be noted that the significance of AV in the above formula is negligible, particularly when dealing with oils which have an acid value of less than 1 mg KOH/g.
The biodiesel quality was evaluated according to the European biodiesel standard EN 14214. The following properties were determined: density at 15 °C (EN ISO 12185), viscosity at 40 °C (EN ISO 3104), flash point (EN 22719), cloud point (EN 23015), pour point (ISO 3016), CFPP (EN 116), water content (EN ISO 12937), sulfur content (EN ISO 20846), ash content (EN ISO 6245), carbon residue (EN ISO 10370), oxidation stability (EN 14112), ester content (EN 14103), mono-, di-, and triglycerides content (EN 14105), free and total glycerol content (EN 14105/14106), acid number (ISO 7537), and calorific value (ISO 1928). Regarding chromatographic analysis, an HP 5890 gas chromatograph equipped with a flame ionization detector and a HP-INNOWAX (30 m × 0.15 mm × 0.2 μm) capillary column was used. Four μL of the upper oil layer were dissolved in 300 μL of n-hexane and 100 μL of the internal standard solutions for GC analysis. Samples (1 μL) were injected by a sampler at an oven temperature of 220 °C. After an isothermal period of 4 min, the GC oven was heated at 10 °C/min to 230 °C, and held for 7.5 min. Nitrogen was used as carrier gas at a flow rate of 2 mL/min measured at 20 °C and as detector make up gas at a flow rate of 30 mL/min. The inlet pressure was 96.4 kPa. The split ratio was 10:1. The injector temperature and detector temperatures were 300 °C and 320 °C, respectively.
The FAEE yield in each experiment was calculated by the following expression:
where both
mactual [g] and
mtheoretical [g] are the masses of ethyl ester;
Cester [g/mL] is the mass concentration of ethyl ester which was acquired by GC;
n is the diluted multiple of ethyl ester; ρ
oil [g/mL] is the density of the vegetable oil.
3. Results and Discussion
The major physicochemical properties of raw materials are given in
Table 1. The kinematic viscosity of the oils varied between 29.4 – 40.2 cSt at 40 °C, while the sulfur content lay within the range of 0.23 – 5 mg/kg. The saponification value was found to be 170.4 – 196.2 mg KOH/g. The feedstock acid values obtained in this study differed significantly ranging from 0.25 to 1.6 mg KOH/g oil. Several studies have shown that the acid value of the feedstock for alkaline transesterification should be less than 1 mg KOH/g and that all raw materials should be anhydrous (water content < 0.3%) [
21,
22]. Thus, in the light of the previous discussion on the requirements for the feedstock acid values, it could be concluded that rapeseed oil and mainly used frying oil had values above the recommended 1 mg KOH/g. With regard to the water content, which was in the range of 347 to 995 mg/kg, all feedstocks satisfied the above recommended limit for water content. Although the iodine number is not included in the properties that influence the transesterification reaction, it should be considered in determining the oil of choice. The specified limit for this parameter is 120 according to the FAME standard EN 14214. The iodine value of sunflower oil is greater than that of the other vegetable oils, and the maximum value set by the European standard. This result was expected, since it was based on the greater degree of unsaturation of this oil. Unsaturation in the fatty acid chain is the main source of instability in vegetable oils [
23]. If two or more double bonds are present, there is a mutually activating effect. The metals and elastomers in contact with vegetable oils during storage can also impact stability. Oxidation leads to the formation of hydroperoxides, which can attack elastomers or polymerize to form insoluble gums [
24].
Table 1.
Physicochemical properties of vegetable oils.
Table 1.
Physicochemical properties of vegetable oils.
| Property | Sunflower oil | Rapeseed oil | Olive oil | Used frying oil |
|---|
| Kinematic viscosity (cSt, 40 °C) | 32.6 | 33.07 | 29.4 | 40.2 |
| Density (kg/m3, 15 °C) | 921.7 | 918.6 | 908.2 | 926 |
| Flash point (°C) | 272 | 246 | 268 | 286 |
| Iodine number (cg I/g oil) | 132 | 108 | 100 | 108 |
| Acid value (mg KOH/g) | 0.33 | 1.02 | 0.25 | 2.1 |
| Saponification value (mg KOH/g) | 192.1 | 170.4 | 196.2 | 193.2 |
| Water content (mg/kg ) | 347 | 512 | 274 | 1200 |
| Sulfur content (mg/kg) | 0.23 | 3 | 2.6 | 5 |
| Pour point (°C) | -14 | -19 | -16 | -15 |
| Carbon residue (% wt/wt) | 0.03 | 0.05 | 0.09 | 0.18 |
| Average molecular weight (g/mol) | 876 | 992 | 857 | 882 |
Four types of ethyl esters were prepared by using a two-stage transesterification reaction. In both stages, the influence of various reaction variables on the conversion of sunflower oil was examined in order to assess the optimal reaction conditions.
3.1. Transesterification in One Stage
In the first stage, the operation variables employed were ethanol/oil molar ratio (6:1 – 14:1), catalyst concentration (0.25 – 1.5% wt/wt), and temperature (35, 80 and 90 °C). Oil mass, reaction time, and alcohol type were fixed as common parameters in all experiments.
3.1.1. Effect of mass ratio of catalyst to oil on esters yield
Figure 1 shows the influence of sodium hydroxide concentration on the evolution of ester yields with time. The best results were reached with a concentration of 1.0%. For higher values the yields were lower. This fact, as has it been indicated, seems to be related to the free acidity of the oil. When there is a large free fatty acid content, the addition of more sodium hydroxide, or any other alkaline catalyst, compensates this acidity and avoids catalyst deactivation [
5,
26]. The addition of an excessive amount of catalyst, however, gives rise to the formation of an emulsion, which increases the viscosity and leads to the formation of gels. These hinder the glycerol separation and, hence, reduce the apparent ester yield. The result of these two opposing effects is an optimal catalyst concentration that, in this case, is 1.0% NaOH. In consequence, further increases in the catalyst concentration did not increase the conversion and lead to extra costs because it was necessary to remove it from the reaction medium at the end. These results were qualitatively similar to those obtained by other authors in the ethanolysis of rapeseed oil [
25]. In the case of the alkaline catalysis, the literature presents many works relating to these processes [
4,
17,
18,
19,
26,
27]. In each case, the more suitable catalyst depends on the type of oil utilized, and the best-suited concentrations are between 0.5 and 1.0 wt.%.
Figure 1.
First stage transesterification. Effect of the mass ratio of NaOH to oil on ethyl ester yield. Ethanol/oil molar ratio, 12:1; reaction temperature, 80 °C.
Figure 1.
First stage transesterification. Effect of the mass ratio of NaOH to oil on ethyl ester yield. Ethanol/oil molar ratio, 12:1; reaction temperature, 80 °C.
3.1.2. Effect of reaction temperature on esters yield
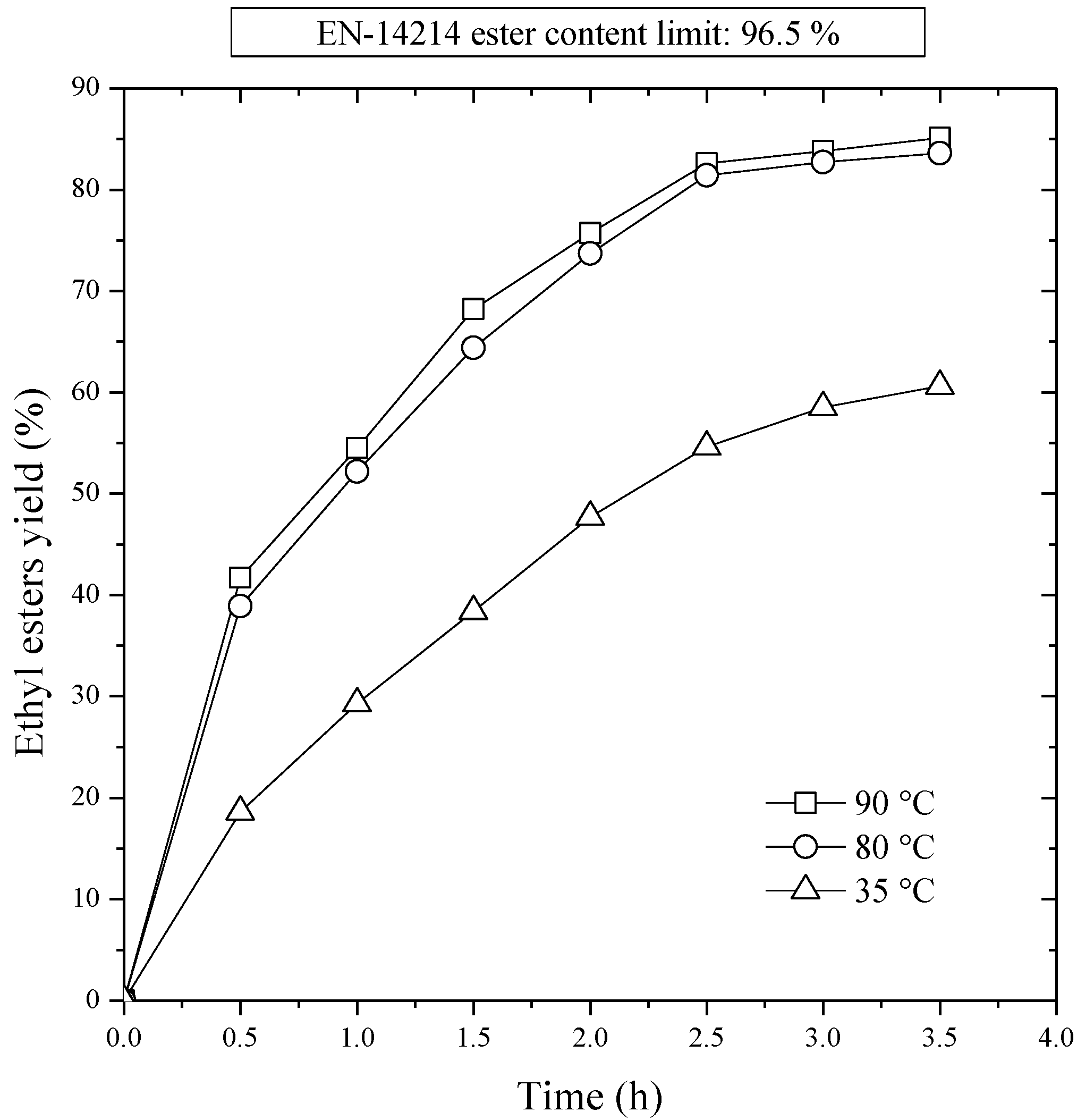
Reaction temperature can influence the reaction rate and the ethyl esters yield because the intrinsic rate constants are strongly dependent on temperature. In all experiments, an ethanol/sunflower oil molar ratio of 12:1, and a catalyst concentration of 1% wt/wt were used. The reaction temperature was varied between 35 and 90 °C.
Figure 2 shows the effect of the reaction temperature on the biodiesel yield. It indicates that the reaction rate was higher at high temperature than at low temperature. The ethyl esters yield was only 54.6% at 35 °C after 2.5 h of reaction, and it reached to 81.4% at 80 °C at the same reaction period. Therefore, the final ethyl ester concentration was almost reached in 2.5 h at 80 °C. After this initial period, there was a second period in which the composition evolved slowly. The yields obtained in the 90 and 80 °C experiment were very similar, and the one in the 35 °C run was clearly less.
Figure 2.
First stage transesterification. Effect of reaction temperature on ethyl ester yield. NaOH/oil mass ratio, 1.0%; ethanol/oil molar ratio, 12:1.
Figure 2.
First stage transesterification. Effect of reaction temperature on ethyl ester yield. NaOH/oil mass ratio, 1.0%; ethanol/oil molar ratio, 12:1.
3.1.3. Effect of the molar ratio of ethanol to oil on biodiesel yield
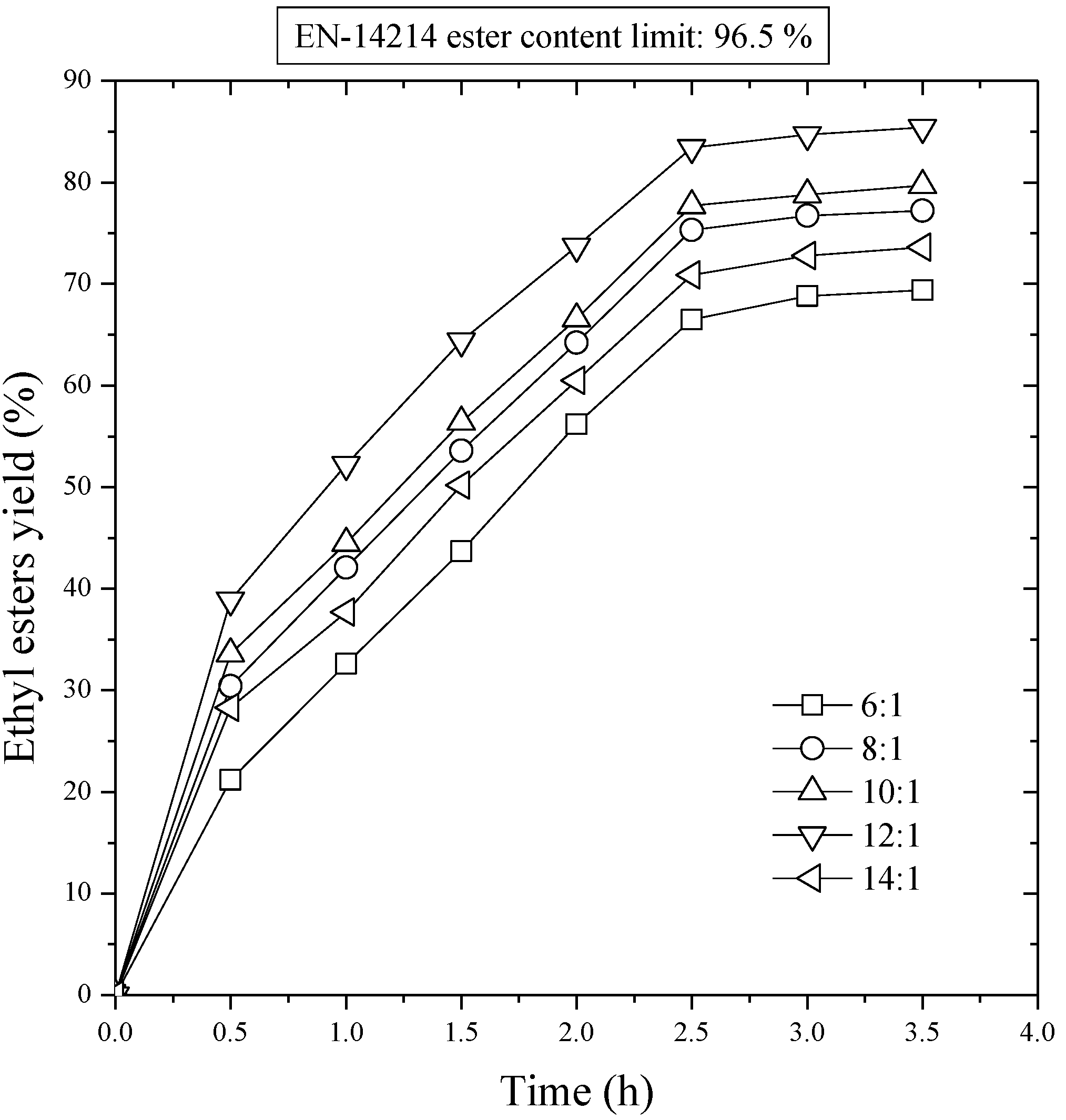
Five experiments were carried out varying the ethanol/oil molar ratio between 6:1 and 14:1. According to the results of the previous sections, a catalyst concentration of 1% wt/wt, was used. The temperature was fixed at 80 °C.
Figure 3 shows the evolution of ester yield with the reaction time. As can be observed, with a 6:1 molar ratio, the conversion to esters was near 69.4% wt/wt after 3.5 h. The ester yield increased as the molar ratio increased, with the best results (85.4%) being for a molar ratio 12:1. Nevertheless, a later increase of molar ratio to 14:1 did not result in an increase in the yield, since a lower value is obtained (79.7%). This was because for higher molar ratios the separation of the glycerol was difficult, as the ethanol excess hinders the decantation by gravity so that the apparent yield of esters decreases since part of the glycerol remains in the biodiesel phase. These results are similar to those obtained by Feuge and Gros in the ethanolysis of peanut oil [
28], and Freedman
et al. [
26], and Schwad
et al. [
4] in the ethanolysis of sunflower oil. The excess of alcohol seems to favor conversion of di- to monoglycerides, but there is also a slight recombination of esters and glycerol to monoglycerides since their concentration keeps increasing during the course of the reaction, in contrast with reactions conducted with low molar ratios [
25]. Krisnamgkura and Simamaharnnop [
29] have observed that when glycerol remains in solution it helps to drive the equilibrium back to the left, lowering the esters yield. In consequence, the alcohol/oil molar ratio is one of the most important variables affecting the esters yield, and although the stoichiometric ratio for transesterification requires three moles of alcohol and one mol of triglyceride, an excess of alcohol is used in practice. Hence, the alcohol molar/oil ratio is a variable that must be always optimized.
Figure 3.
First stage transesterification. Effect of the molar ratio of ethanol to sunflower oil on ethyl ester yield. NaOH/oil mass ratio, 1.0%; reaction temperature, 80 °C.
Figure 3.
First stage transesterification. Effect of the molar ratio of ethanol to sunflower oil on ethyl ester yield. NaOH/oil mass ratio, 1.0%; reaction temperature, 80 °C.
3.2. Transesterification in Two Stages
In one-stage transesterification the maximum yield of sunflower ethyl esters was 81.4% wt/wt. According to the EN 14214 standard the yield of ethyl esters should be 96.5%. Hence, after the transesterification in one stage, the biodiesel must contain unreacted vegetable oil in the form of glycerides. In accordance with the literature, in the final equilibrium of the transesterification reaction there are significant amounts of triglycerides, diglycerides, and monoglycerides [
25]. In this situation, the equilibrium can be shifted to the right by carrying out a multistage transesterification process. This idea is the basis of the industrial process, which is carried out in a two-stage reaction with separation of the glycerol after each stage. In our case, as it has been indicated, we started from oil with 81.4% wt/wt ethyl esters, where the glycerol formed in the first stage was withdrawn. This reaction mixture contained ethyl esters and mono-, di-, and triglycerides. The process continued aggregating ethanol and catalyst. The procedure used agrees with the previously described results. The variables studied were ethanol/oil molar ratio of the second stage (4:1, 6:1, and 8:1) and catalyst concentration of the second stage (0.25 – 1%). The temperature (80 °C) remained fixed.
Figure 4 shows the influence of catalyst concentration on the evolution of ester yield with time in the second transesterification stage. As it can be observed, the second stage gave rise to an increment in the yield of ethyl esters in relation to the equilibrium value of the first stage. The curves are similar to those of the first stage. In fact, there is a sharp increase in the first minutes, and after that the curves are asymptotic with time. The esters yield was increased firstly with the increase of catalyst from 0.25 to 0.75%. But, with further increase in the catalyst the ester yield decreased, which was possibly due to the occurrence of saponification reactions. The maximum esters yield was 98.2% wt/wt after a reaction time of 1.5 h and the concentration of the catalyst was 0.75%. However, the EN 14214 limit of 96.5%, which has been set as the acceptable limit for the ethyl esters yield, was achieved at the same catalyst concentration and a reaction period of 30 min. Therefore, the addition of ethanol, once the glycerol of the first stage was withdrawn, improved the yield and displaced the reaction equilibrium to the right.
Figure 4.
Second stage transesterification. Effect of the mass ratio of NaOH to sunflower oil on ethyl ester yield. Ethanol/oil molar ratio, 6:1; reaction temperature, 80 °C.
Figure 4.
Second stage transesterification. Effect of the mass ratio of NaOH to sunflower oil on ethyl ester yield. Ethanol/oil molar ratio, 6:1; reaction temperature, 80 °C.
Figure 5 presents the influence of ethanol/oil molar ratio on the evolution of esters yield with time in the second transesterification stage. According to the experimental results when the ethanol amount was increased, the conversion was also increased considerably. At 80 °C, a conversion of 88.9% wt/wt was reached in 30 min for an ethanol/oil molar ratio of 4:1, while for the same reaction period, increasing the molar ratio to 6:1 resulted in a 96.6 wt.% yield of sunflower oil ethyl esters, which satisfies the European specification in terms of total ester content. However, with further increase in the molar ratio to 8:1 there was a little improvement in the conversion (97% wt/wt). Thus, it can be concluded that an excess ethanol feed is effective in elevating in conversion only to certain extent.
Figure 5.
Second stage transesterification. Effect of the molar ratio of ethanol to sunflower oil on ethyl ester yield. NaOH/oil mass ratio, 0.75%; reaction temperature, 80 °C.
Figure 5.
Second stage transesterification. Effect of the molar ratio of ethanol to sunflower oil on ethyl ester yield. NaOH/oil mass ratio, 0.75%; reaction temperature, 80 °C.
Figure 6.
Two-stage transesterification of rapeseed oil, olive oil and used frying oil under optimum reaction conditions.
Figure 6.
Two-stage transesterification of rapeseed oil, olive oil and used frying oil under optimum reaction conditions.
Figure 6 illustrates the conversion of the rest vegetable oils in both transesterification stages. It ought to be taken into account that the conversion procedure of vegetable oils to ethyl esters took place under the conditions which have already been outlined above. According to the experimental results, the yield rates which coincided with sunflower oil were also apparent in the rapeseed oil as well as in the olive oil. Particularly, the esters yield for the 1
st stage transesterification of rapeseed oil ethyl esters reached 81.4%, whereas, for the olive oil ethyl esters it was 82.6%. In the second transesterification stage of both vegetable oils the yields increase even more, as far as the content of final reaction products in esters is concerned satisfying the acceptable limit of 96.5%, set by European specifications. However, in the case of used frying oil, the yields in both transesterification stages seem to be less in comparison with those of the rest of the vegetable oils. This can be derived from the fact that there is high concentration of free fatty acids in used frying oil, which probably leads to the partial deactivation of the catalyst. This deactivation could be attributed to the parallel reaction between free fatty acids and the sodium hydroxide, where the free fatty acids in the raw oil predominantly react with the alkaline to form sodium based soap and water.
3.3. FAEE Properties
The four types of ethyl esters were studied in terms of their physicochemical properties so as to study whether their quality parameters where indeed within the European standard EN 14214. The results of analysis are given in
Table 2. It is observed that three out of four types of ethyl esters do satisfy the European norm of viscosity. The ester which was different from the other three was the used frying ethyl ester, which appeared to be above the highest limit (5 cSt) of the European specification. On the other hand, each and every type of ethyl ester had a density within the range of 860 – 900 kg/m
3. The results dealing with the gross calorific value showed that the ethyl esters of used frying oil have the lowest value, whereas the highest was yielded by the sunflower oil ethyl esters. It is important to mention that in the standard EN 14214, specifications concerning the HHV of a biodiesel do not exist, but considering that the HHV of the diesel fuel was about 46 MJ/kg, the ethyl esters contained approximately 10% less energy. The sulfur content was proven to be almost at zero for the four ethyl ester samples. Yet, the frying oil ethyl esters proved to be unsatisfactory in terms of EN-14214 specifications set to measure the content of water, as they surpassed 500 mg/kg. However, the other types seemed to be within the specification. The flash point for all samples was proved to be higher than 101 °C which has been set as a lowest limit by the European specification. The acid value of the esters ranged within the limits specified by the standard (0.5 mg KOH/g). Also, the microcarbon residue is in accordance with the maximum required limits given in the EN 14214 (maximum 0.30% wt/wt). All types of the ethyl esters which were produced in this study did not meet the European norm of oxidation stability (minimum Rancimat induction period of six hours). Nevertheless, according to the experimental results dealing with vegetable oils which were reach in linoleic acid such as sunflower and used frying oil rendered ethyl esters with particularly poor oxidation stability. Whereas, non polysaturated ethyl esters such as those of olive oil illustrated improved stability.
In the standard EN 14214 the CFPP value is not specified, since it is different in each country. In the Mediterranean countries, the required limits for the CFPP of automotive diesel are announced as “Winter Grade” and “Summer Grade”. The required limit for “Winter Grade” is −10 °C, whereas for “Summer Grade” it is 5 °C [
30]. The values of CFPP indicated in
Table 3 ranged between −2 and 3 °C; in consequence the four types of ethyl esters produced can be used as substitutes for“Summer Grade” automotive diesel. A possible solution for the use as “Winter Grade” would be the use of CFPP depressants. Finally, the values of cloud and pour points in
Table 1 are very high. For example, the cloud and pour points of diesel fuel were −2 and −16 °C, respectively. In general, as it can be observed, these parameters follow a parallel evolution with the CFPP. Therefore, the comments in relation to the CFPP also are applicable to the cloud and pour points.
Table 2.
Properties of fatty acid ethyl esters.
Table 2.
Properties of fatty acid ethyl esters.
| Property | Sunflower oil methyl esters | Rapeseed oil methyl esters | Olive oil methyl esters | Used frying oil methyl esters | EN 14214 limits |
|---|
| Density (kg/m3, 15 °C) | 882.7 | 881.2 | 881.5 | 888.5 | 860–900 |
| Kinematic viscosity (cSt, 40 °C) | 4.63 | 4.84 | 4. | 5.81 | 3.50–5.00 |
| Flash point (°C) | 178 | 181 | 182 | 188 | 120 min |
| Sulfur content (mg/kg) | 3.7 | 3.5 | 2.8 | 4.8 | 10 max |
| Cloud point (°C) | 2 | 1 | 7 | 9 | - |
| Pour point (°C) | −6 | −8 | −5 | −1 | - |
| CFPP (°C) | −3 | −4 | −2 | 3 | +5 max |
| Oxidation stability (h, 110 °C) | 0.8 | 2.0 | 3.3 | 0.33 | 6 h min |
| Ash content (% wt/wt) | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 max |
| Water content (mg/kg ) | 154 | 189 | 208 | 376 | 500 max |
| Carbon residue (% wt/wt) | 0.0164 | 0.0110 | 0.0028 | 0.1549 | 0.3 max |
| Gross calorific value (MJ/kg) | 38.6 | 38.3 | 38.2 | 37.8 | - |
| Acid value (mg KOH/g) | 0.15 | 0.35 | 0.19 | 0.46 | 0.5 max |
| Phosphorus content (mg/kg) | 4 | 6 | 3 | 4 | 10 max |
| Ester content (% wt/wt) | 96.7 | 97.2 | 97.8 | 93.2 | 96.5 min |
| Monoglyceride content (% wt/wt) | 0.478 | 0.512 | 0.644 | 1.367 | 0.80 max |
| Diglyceride content (% wt/wt) | 0.152 | 0.095 | 0.130 | 2.228 | 0.20 max |
| Triglyceride content (%wt/wt) | 0.087 | 0.275 | 0.116 | 3.132 | 0.20 max |
| Free glycerol content (% wt/wt) | 0.017 | 0.005 | 0.014 | 0.148 | 0.02 max |
| Total glycerol (% wt/wt) | 0.148 | 0.180 | 0.12 | 0.254 | 0.25 max |
Mono- and diglycerides as well as triglycerides are referred to as bound glycerol. They are present in the feedstock oil and can remain in the final product in small quantities. A high excess of alcohol in the transesterification reaction should ensure that all triglycerides (the major component of vegetable oil) are reacted. A higher content of glycerides in the ester, especially triglycerides, may cause formation of deposits at the injection nozzles and at the valves [
31,
32]. The GC analysis of the produced ethyl ester of sunflower, rapeseed, and olive oil showed that the triglycerides of the parent oils reacted at a satisfactory yield to mono- and diglycerides. Their values were found to agree with the specified EN 14214 limits. However, the content of individual glycerides (monoglycerides, diglycerides and triglycerides) found in used frying oil ethyl ester, were not within the three European specifications, which imply that the transesterification reaction was incomplete.
Regarding the free and total glycerol contents, the measured values for sunflower, rapeseed and olive oil ethyl ester were found below the specification limits, while in the case of the used frying oil ethyl ester, the values were found to be higher than their parameter limits.
Table 3.
Fatty acid ethyl ester composition.
Table 3.
Fatty acid ethyl ester composition.
| Fatty acid | Chemical Structure | Sunflower oil ethyl esters | Rapeseed oil ethyl esters | Olive oil ethyl esters | Used frying oil ethyl esters |
|---|
| Lauric | CH3(CH2)10COOH | 0.00 | 0.00 | 0.00 | 1.98 |
| Palmitic | CH3(CH2)14COOH | 6.20 | 4.90 | 11.60 | 15.65 |
| Palmitoleic | CH3(CH2)5CH=CH(CH2)7COOH | 0.10 | 0.00 | 0.90 | 0.31 |
| Stearic | CH3(CH2)16COOH | 3.70 | 1.60 | 3.10 | 3.10 |
| Oleic | CH3(CH2)7CH=CH(CH2)7COOH | 25.20 | 33.00 | 74.98 | 29.57 |
| Linoleic | CH3(CH2)3(CH2CH=CH)2(CH2)7COOH | 63.10 | 20.40 | 7.80 | 41.53 |
| Linolenic | CH3(CH2CH=CH)3(CH2)7COOH | 0.30 | 7.90 | 0.60 | 1.04 |
| Eicosenoic | CH3(CH2)8CH=CH(CH2)8COOH | 0.20 | 9.30 | 0.01 | 0.11 |
| Behenic | CH3(CH2)20COOH | 0.70 | 0.00 | 0.10 | 0.24 |
| Erucic | CH3(CH2)7CH=CH(CH2)11COOH | 0.10 | 23.0 | 0.00 | 0.02 |
| Lignoceric | CH3(CH2)22COOH | 0.20 | 0.00 | 0.50 | 0.30 |
The main fatty acid composition of the four types of ethyl esters is shown in
Table 3. As it can be seen, the fatty acid profile between the individual types of ethyl esters was different. The dominant fatty acids were palmitic, oleic linoleic and erucic. More specifically, the major fatty acids of sunflower oil ethyl ester were oleic and linoleic, while the rapeseed oil biodiesel consisted mainly of ethyl ester of oleic, linoleic and erucic acids. Olive oil ethyl ester was composed primarily of oleic acid; whereas, the used frying oil ethyl ester was found to have high contents of oleic and linoleic fatty acids.