Effect of Drying Control Agent on Physicochemical and Thermal Properties of Silica Aerogel Derived via Ambient Pressure Drying Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
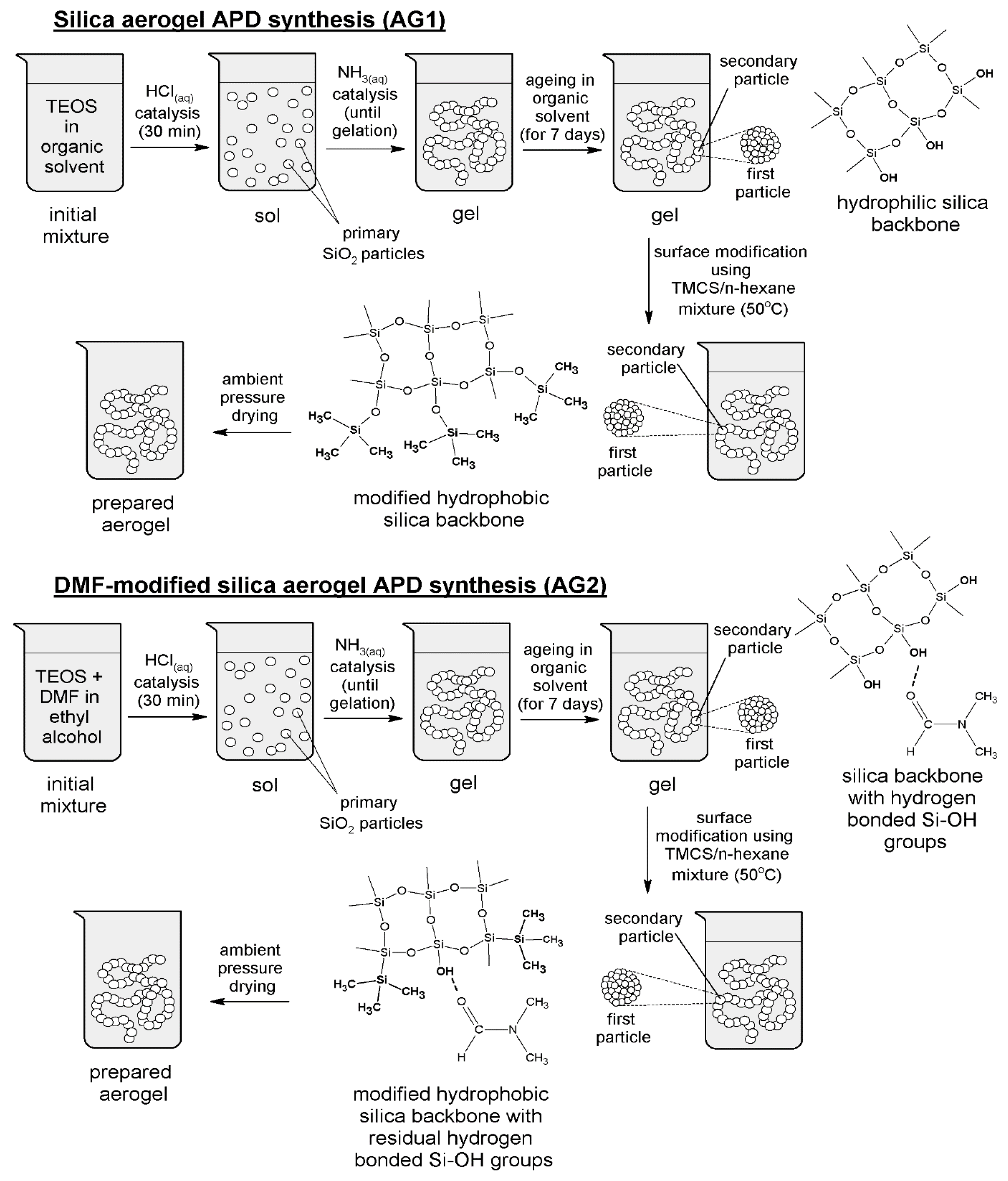
2.2. Samples Preparation Procedure
- (1)
- A mixture of TEOS in ethyl alcohol was prepared, and an appropriate amount of hydrochloric acid (at a concentration of 0.1 mol/L) was added to achieve pH ≈ 2. In the case of AG2 sample, DMF was introduced simultaneously with TEOS and ethanol. The obtained mixtures were sealed and vigorously stirred for 30 min. Next, the ammonium hydroxide solution (0.5 mol/L) was added dropwise into initial mixtures to adjust pH ≈ 6 and stirred for a few minutes until gelation occurred. The volume ratios of the components used during samples preparation equaled: TEOS: EtOH = 1:1 (AG1) and TEOS: EtOH: DMF = 1:1:0.5 (AG2).
- (2)
- Obtained silica gels were aged in ethyl alcohol for seven days to strengthen the gel network and exchange the pore fluids with a volatile liquid.
- (3)
- Obtained silica alcogels were treated with a mixture of TMCS and n-hexane (1:5 volume ratio) at 50 °C for two days for surface modification. After the silylation process, the prepared samples were dried under ambient pressure to remove all liquids from the porous structure.
2.3. Material Characterization
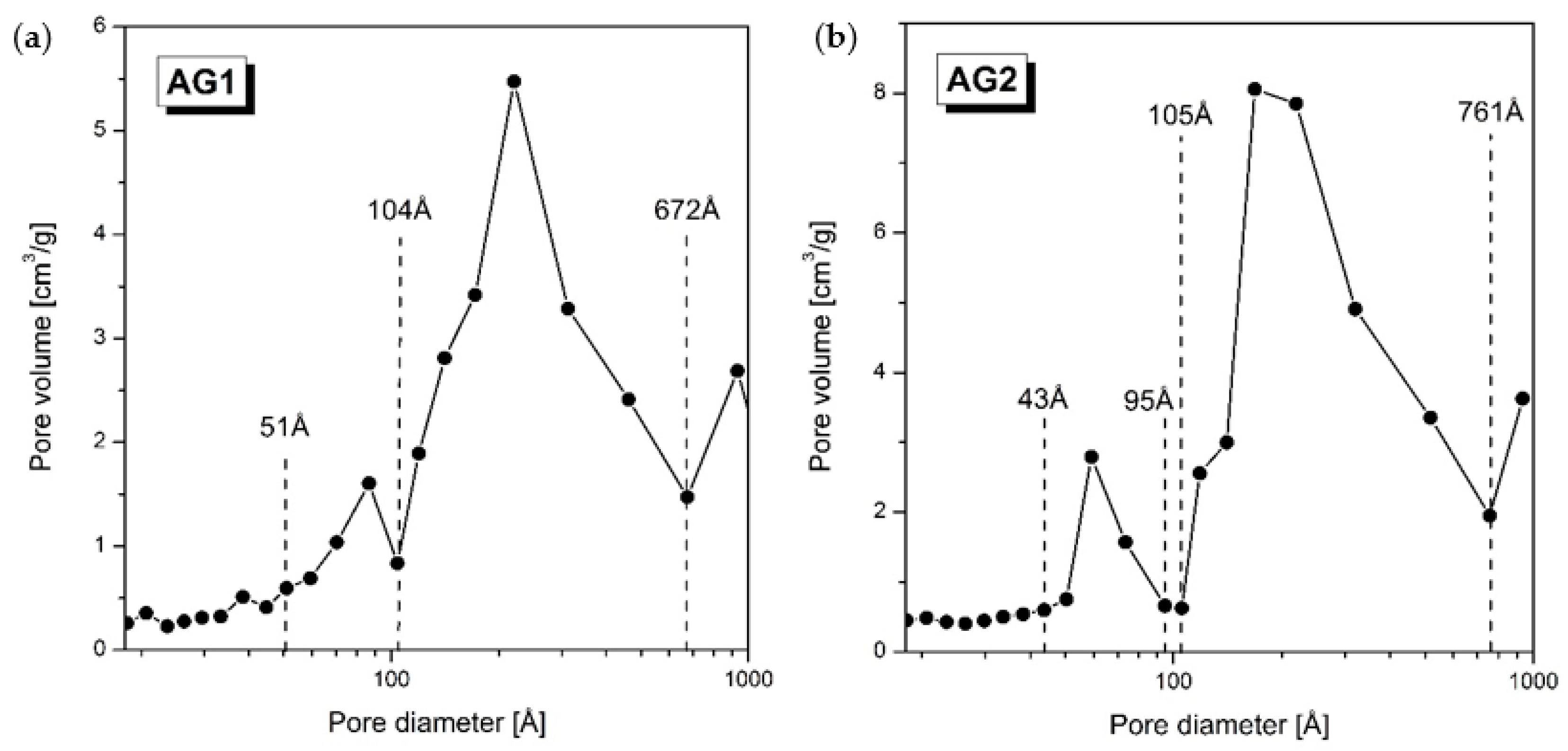
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Duan, Y.; Jana, S.C.; Lama, B.; Espe, M.P. Hydrophobic silica aerogels by silylation. J. Non-Cryst. Solids 2016, 437, 26–33. [Google Scholar] [CrossRef]
- Fedyukhin, A.V.; Strogonov, K.V.; Soloveva, O.V.; Solovev, S.A.; Akhmetova, I.G.; Berardi, U.; Zaitsev, M.D.; Grigorev, D.V. Aerogel Product Applications for High-Temperature Thermal Insulation. Energies 2022, 15, 7792. [Google Scholar] [CrossRef]
- Miros, A.; Psiuk, B.; Szpikowska-Sroka, B. Aerogel insulation materials for industrial installation: Properties and structure of new factory-made products. J. Sol-Gel Sci. Technol. 2017, 84, 496–506. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wei, Y.; Zhang, X. Fast and one-pot synthesis of silica aerogels via a quasi-solvent-exchange-free ambient pressure drying process. Micropor. Mesopor. Mater. 2015, 218, 192–198. [Google Scholar] [CrossRef]
- Pedroso, M.; Gomes, M.d.G.; Silvestre, J.D.; Hawreen, A.; Flores-Colen, I. Thermophysical Parameters and Hygrothermal Simulation of Aerogel-Based Fibre-Enhanced Thermal Insulating Renders Applied on Exterior Walls. Energies 2023, 16, 3048. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Poco, J.F. Thin aerogel films for optical, thermal, acoustic and electronic applications. J. Non-Cryst. Solids 1995, 188, 46–53. [Google Scholar] [CrossRef]
- Liu, H.; Sha, W.; Cooper, A.T.; Fan, M. Preparation and characterization of a novel silica aerogel as adsorbent for toxic organic compounds. Colloids Surf. A Physicochem. Eng. 2009, 347, 38–44. [Google Scholar] [CrossRef]
- Wang, C.T.; Wu, C.L.; Chen, I.C.; Huang, Y.H. Humidity sensors based on silica nanoparticle aerogel thin films. Sens. Actuators B Chem. 2005, 107, 402–410. [Google Scholar] [CrossRef]
- Amiri, T.U.; Moghaddas, J.; Khajeh, S.R. Silica aerogel-supported copper catalyst prepared via ambient pressure drying process. J. Sol-Gel Sci. Technol. 2016, 77, 627–635. [Google Scholar] [CrossRef]
- Guenther, U.; Smirnova, I.; Neubert, R.H.H. Hydrophilic silica aerogels as dermal drug delivery systems—Dithranol as a model drug. Eur. J. Pharm. Biopharm. 2008, 69, 935–942. [Google Scholar] [CrossRef]
- Bheekhun, N.; Talib, A.R.A.; Hassan, M.R. Aerogels in Aerospace: An Overview. Adv. Mater. Sci. Eng. 2013, 406065, 18. [Google Scholar] [CrossRef]
- Poelz, G. Preparation of silica aerogel for Cherenkov counters. Nucl. Instrum. Methods Phys. Res. 1982, 195, 491–503. [Google Scholar] [CrossRef]
- Reim, M.; Körner, W.; Manara, J.; Korder, S.; Arduini-Schuster, M.; Ebert, H.P.; Fricke, J. Silica aerogel granulate material for thermal insulation and daylighting. Sol. Energy 2005, 79, 131–139. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Smith, D.M.; Maskara, A.; Boes, U. Aerogel-based thermal insulation. J. Non-Cryst. Solids 1998, 225, 254–259. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Chen, Z.; Tang, C.; Ren, X.; Hu, H.; Fang, Q. Measurement of the Kinetics and Thermodynamics of the Thermal Degradation for a Flame Retardant Polyurethane-Based Aerogel. Energies 2022, 15, 6982. [Google Scholar] [CrossRef]
- Kuang-Sheng, L.; Xiao-Feng, Z.; Chia-Hsing, H.; Shin-Ku, L. The Application of Silica-Based Aerogel Board on the Fire Resistance and Thermal Insulation Performance Enhancement of Existing External Wall System Retrofit. Energies 2021, 14, 4518. [Google Scholar]
- Golder, S.; Narayanan, R.; Hossain, M.R.; Rofiqul Islam, M. Experimental and CFD Investigation on the Application for Aerogel Insulation in Builginds. Energies 2021, 14, 3310. [Google Scholar] [CrossRef]
- Lakatos, Á. Novel Thermal Insulation Materials for Buildings. Energies 2022, 15, 6713. [Google Scholar] [CrossRef]
- Michael, M.; Favoino, F.; Jin, Q.; Luna-Navarro, A.; Overend, M. A Systematic Review and Classification of Glazing Technologies for Building Façades. Energies 2023, 16, 5357. [Google Scholar]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of Silica Aerogel by Supercritical Drying Method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, G.; Cheng, X.; Zhang, Y. Rapid synthesis of amine cross-linked epoxy and methyl co-modified silica aerogels by ambient pressure drying. J. Non-Cryst. Solids 2012, 358, 2612–2615. [Google Scholar] [CrossRef]
- Shahzamani, M.; Bagheri, R.; Masoomi, M.; Haghgoo, M.; Dourani, A. Effect of drying method on the structure and porous texture of silica-polybutadiene hybrid gels: Supercritical vs. ambient pressure drying. J. Non-Cryst. Solids 2017, 460, 119–124. [Google Scholar] [CrossRef]
- Perissinotto, A.P.; Awano, C.M.; de Vicente, F.S.; Donatti, D.A.; Mesquita, A.; da Silva, L.F.; Vollet, D.R. Structure and diffuse-boundary in hydrophobic and sodium dodecyl sulfate-modified silica aerogels. Micropor. Mesopor. Mater. 2016, 223, 196–202. [Google Scholar] [CrossRef]
- Wu, G.; Yu, Y.; Cheng, X.; Zhang, Y. Preparation and Surface modification mechanism of silica aerogels via ambient pressure drying. Mater Chem. Phys. 2011, 129, 308–314. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Liu, J. Synthesis and characterization of silica aerogels by a novel fast ambient pressure drying process. Mater. Lett. 2006, 60, 3718–3722. [Google Scholar] [CrossRef]
- Prakash, S.S.; Brinker, C.J.; Hurd, A.J. Silica aerogel films at ambient pressure. J. Non-Cryst. Solids 1995, 190, 264–275. [Google Scholar] [CrossRef]
- Mazraeh-shahi, Z.T.; Shoushtari, A.M.; Bahramian, A.R.; Abdouss, M. Synthesis, pore structure and properties of polyurethane/silica hybrid aerogels dried at ambient pressure. J. Ind. Eng. Chem. 2015, 21, 797–804. [Google Scholar] [CrossRef]
- He, S.; Huang, D.; Bi, H.; Li, Z.; Yang, H.; Cheng, X. Synthesis and characterization of silica aerogels dried under ambient pressure bed on water glass. J. Non-Cryst. Solids 2015, 410, 58–64. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Strauchmann, W.; Ziółkowski, P.; Jakubowska, P. Synthesis and characterization of carbon fiber/silica aerogel nanocomposites. J. Non-Cryst. Solids 2015, 416, 1–3. [Google Scholar] [CrossRef]
- García-Torres, B.A.; Aguilar-Elguezabal, A.; Román-Aguirre, M.; Álvarez-Contreras, L. Synthesis of silica aerogels microspheres prepared by ink jet printing and dried at ambient pressure without surface hydrophobization. Mater. Chem. Phys. 2016, 172, 32–38. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H.; Zhang, H. Tailoring thermal properties of ambient pressure dried MTMS/TEOS co-precursor aerogels. Mater Lett. 2016, 171, 91–94. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Barełkowski, M.; Niemier, S.; Jakubowska, P. Synthesis and characterization of silica aerogel/carbon microfibers nanocomposites dried in supercritical and ambient pressure conditions. J. Sol-Gel Sci. Technol. 2015, 76, 227–232. [Google Scholar] [CrossRef]
- Ślosarczyk, A. Synthesis and characterization of silica aerogel-based nanocomposites with carbon fibers and carbon nanotubes in hybrid system. J. Sol-Gel Sci. Technol. 2017, 84, 16–22. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Klapiszewski, Ł.; Buchwald, T.; Krawczyk, P.; Kolanowski, Ł.; Lota, G. Carbon Fiber and Nickel Coated Carbon Fiber–Silica Aerogel Nanocomposite as Low Frequency Microwave Absorbing Materials. Materials 2020, 13, 400. [Google Scholar] [CrossRef]
- Rao, A.V.; Sakhare, H.M.; Tamhankar, A.K.; Shinde, M.L.; Gadave, D.B.; Wagh, P.B. Influence of N,N-dimethylformamide additive on the physical properties of citric acid catalyzed TEOS silica aerogels. Mater. Chem. Phys. 1999, 60, 268–273. [Google Scholar]
- Parvathy Rao, A.; Venkateswara Rao, A. Study the Influence of Drying Control Chemical Additives on the Physical and Optical Properties of Nanocrystalline Cadmium Sulfide–Doped Tetraethylorthosilicate Silica Xerogels. J. Mat. Syn. Process. 2002, 10, 1. [Google Scholar]
- Nah, H.-Y.; Parale, G.; Jung, H.-N.-R.; Lee, K.-Y.; Lim, C.-H.; Ku, Y.; Park, H.-H. Role of oxalic acid in structural formation of sodium silicate-based silica aerogel by ambient pressure drying. J. Sol-Gel Sci. Technol. 2018, 85, 302–310. [Google Scholar] [CrossRef]
- Ochoa, M.; Lamy-Mendes, A.; Maia, A.; Portugal, A.; Durães, L. Influence of Structure-Directing Additives on the Properties of Poly(methylsilsesquioxane) Aerogel-Like Materials. Gels 2019, 5, 6. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Alves, P.; Santos, P.; Durães, L. Progress in silica aerogel-containing materials for buildings’ thermal Insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar] [CrossRef]
- Ślosarczyk, A. Recent Advances in Research on the Synthetic Fiber Based Silica Aerogel Nanocomposites. Nanomaterials 2017, 7, 44. [Google Scholar] [CrossRef]
- Buratti, C.; Belloni, E.; Merli, F.; Zinzi, M. Aerogel glazing systems for building applications: A review. Energy Build. 2021, 231, 110587. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel based thermal insulating cementitious composites: A review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Garbalińska, H.; Strzałkowski, J. Lightweight alkali-activated composites containing sintered fly ash aggregate and various amounts of silica aerogel. J. Build. Eng. 2023, 74, 106879. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Vashchuk, A.; Klapiszewski, Ł. Research Development in Silica Aerogel Incorporated Cementitious Composites—A Review. Polymers 2022, 14, 1456. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Z.; Shi, X.; Yang, H.; Gong, L.; Cheng, X. Rapid synthesis of sodium silicate based hydrophobic silica aerogel granules with large surface area. Adv. Powder Technol. 2015, 26, 537–541. [Google Scholar] [CrossRef]
- Liu, M.L.; Yang, D.A.; Qu, Y.F. Effect of different chemical additives and heat-treatment on ambient pressure dried silica aerogels. J. Exp. Nanosci. 2010, 5, 83–91. [Google Scholar] [CrossRef]
- Chiappim, J.W.; Awano, C.M.; Donatti, D.A.; de Vicente, F.S.; Vollet, D.R. Structure and Hydrophobic Ambient-Pressure-Dried Aerogels Prepared by Sonohydrolysis of Tetraethoxysilane with Additions of N,N-Dimethylformamide. Langmuir 2014, 30, 1151–1159. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Kawai, H.; Sumiyoshi, T.; Yokogawa, H. Hydrophobic silica aerogel production at KEK. Nucl. Instrum. Methods Phys. Res. A 2012, 668, 64–70. [Google Scholar] [CrossRef]
- Tabata, M.; Kawai, H. Progress in Development of Silica Aerogel for Particle- and Nuclear-Physics Experiments at J-PARC. JPS Conf. Proc. 2005, 8, 022004. [Google Scholar]
- Yu, H.; Liang, X.; Wang, J.; Wang, M.; Wang, M.; Yang, S. Preparation and characterization of hydrophobic silica aerogel sphere products by co-precursor method. Solid State Sci. 2015, 48, 155–162. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol-gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Szpikowska-Sroka, B.; Pawlik, N.; Goryczka, T.; Pisarski, W.A. Influence of silicate sol-gel host matrices and catalyst agents on the luminescent properties of Eu3+/Gd3+ under different excitation wavelength. RSC Adv. 2015, 5, 98773–98782. [Google Scholar] [CrossRef]
- Matias, T.; Marques, J.; Quina, M.J.; Gando-Ferreira, L.; Valente, A.J.M.; Portugal, A.; Durães, L. Silica-based aerogels as adsorbents for phenol-derivative compounds. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 260–269. [Google Scholar] [CrossRef]
- Smitha, S.; Shajesh, P.; Aravind, P.R.; Kumar, S.R.; Pillai, P.K.; Warrier, K.G.K. Effect of aging time and concentration of aging solution on the porosity characteristics of subcritically dried silica aerogels. Micropor. Mesopor. Mater. 2006, 91, 286–292. [Google Scholar] [CrossRef]
- Zhou, B.; Shen, J.; Wu, Y.; Wu, G.; Ni, X. Hydrophobic silica aerogels derived from polyethoxydisiloxane and perfluoroalkylsilane. Mater. Sci. Eng. C 2007, 27, 1291–1294. [Google Scholar] [CrossRef]
- Malik, A.S.; Dabbs, D.M.; Katz, H.E.; Aksay, I.A. Silica Monoliths Templated on L3 Liquid Crystals. Langmuir 2006, 22, 325–331. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol-gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Riegel, B.; Hartmann, I.; Kiefer, W.; Groβ, J.; Fricke, J. Raman spectroscopy on silica aerogels. J. Non-Cryst. Solids 1997, 211, 294–298. [Google Scholar] [CrossRef]
- Snyder, R.G.; Hsu, S.L.; Krimm, S. Vibrational spectra in the C-H stretching region and the structure of the polymethylene chain. Spectrochim. Acta A 1978, 34, 395–406. [Google Scholar] [CrossRef]
- Amonkosolpan, J.; Wolverson, D.; Goller, B.; Polisski, S.; Kovalev, D.; Rollings, M.; Grogan, M.D.W.; Birks, T.A. Porous silicon nanocrystals in a silica aerogel matrix. Nanoscale Res. Lett. 2012, 7, 397–402. [Google Scholar] [CrossRef]
- Bae, S.C.; Lee, H.; Lin, Z.; Granick, S. Chemical Imaging in a Surface Forces Apparatus: Confocal Raman Spectroscopy of Confined Poly(dimethylsiloxane). Langmuir 2005, 21, 5685–5688. [Google Scholar] [CrossRef] [PubMed]
- Nazriati, N.; Setyawan, H.; Affandi, S.; Yuwana, M.; Winardi, S. Using bagasse ash as a silica source when preparing silica aerogels via ambient pressure drying. J. Non-Cryst. Solids 2014, 400, 6–11. [Google Scholar] [CrossRef]
- Casula, M.F.; Corrias, A.; Paschina, G. FeCo-SiO2 nanocomposite aerogels by high temperature supercritical drying. J. Mater. Chem. 2002, 12, 1505–1510. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Patsis, A.P. Infrared-reflectance spectra of heat-treated, sol-gel-derived silica. Phys. Rev. B 1993, 48, 12499–12505. [Google Scholar] [CrossRef]
- Chen, J.Y.; Pan, F.M.; Chang, L.; Chao, A.T.; Chao, K.J. Thermal stability of trimethylsilylated mesoporous silica thin films as the ultralow—K dielectric for cooper interconnects. J. Vac. Sci. Technol. B 2005, 23, 2034–2040. [Google Scholar] [CrossRef]
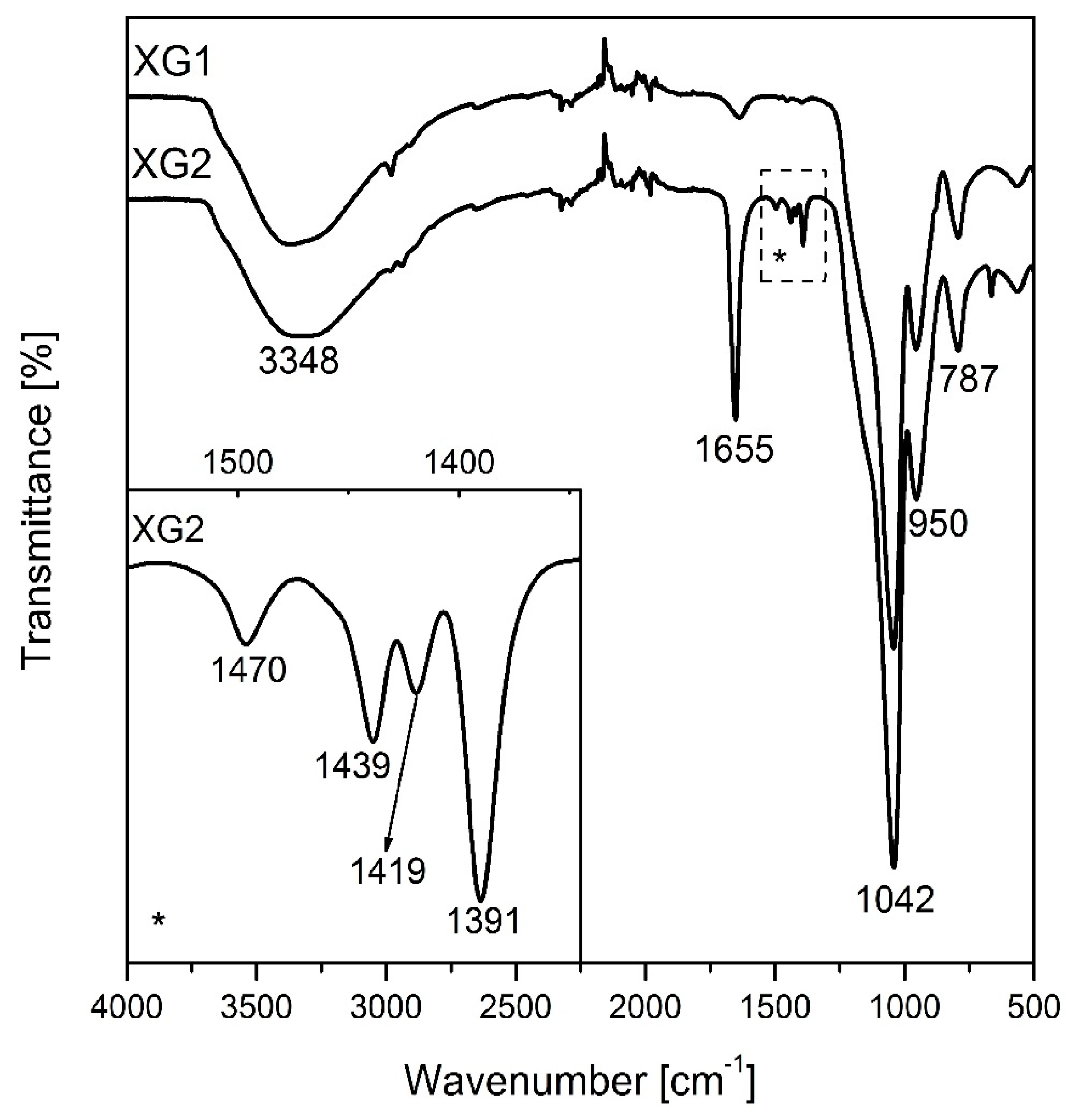


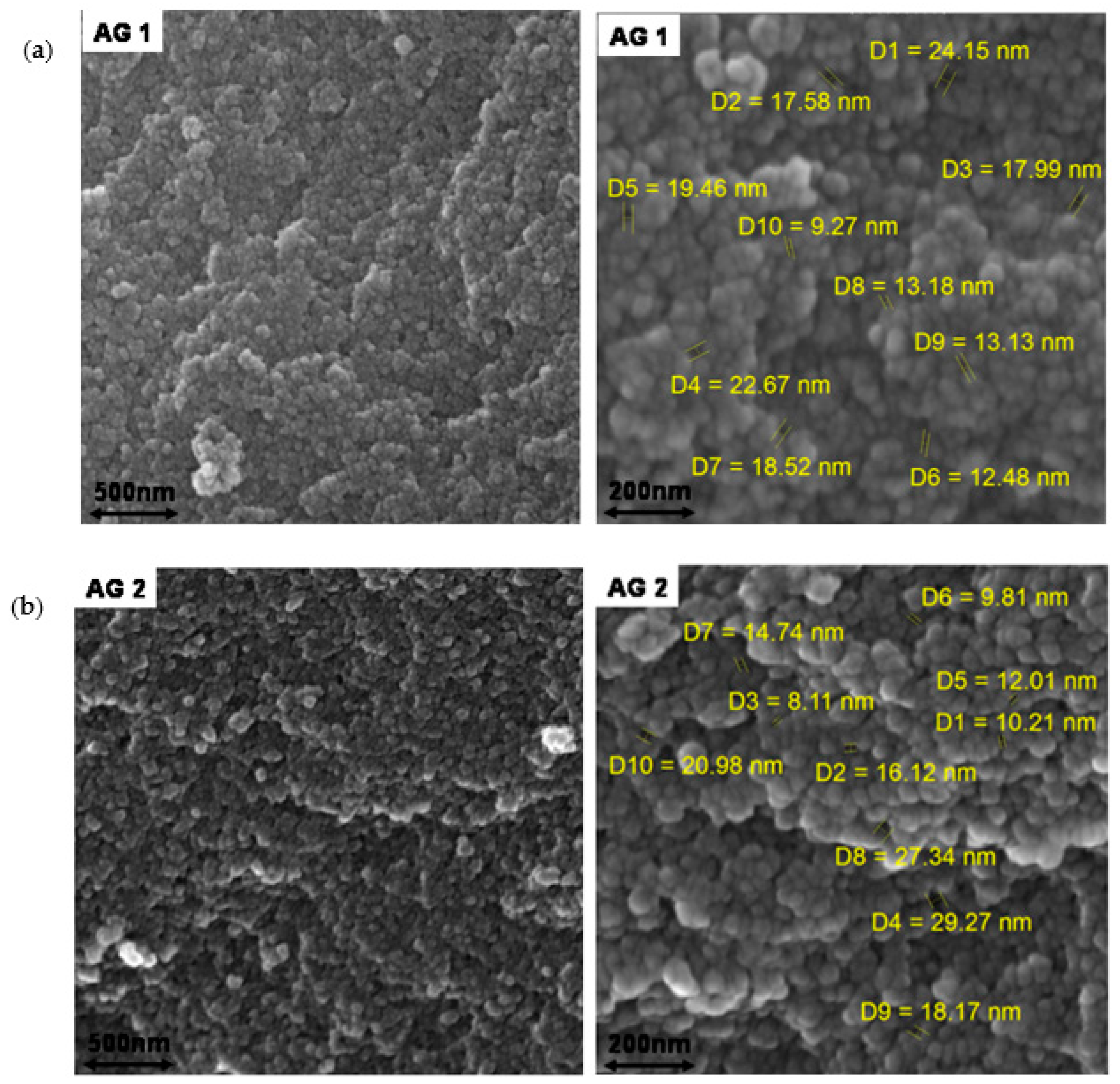
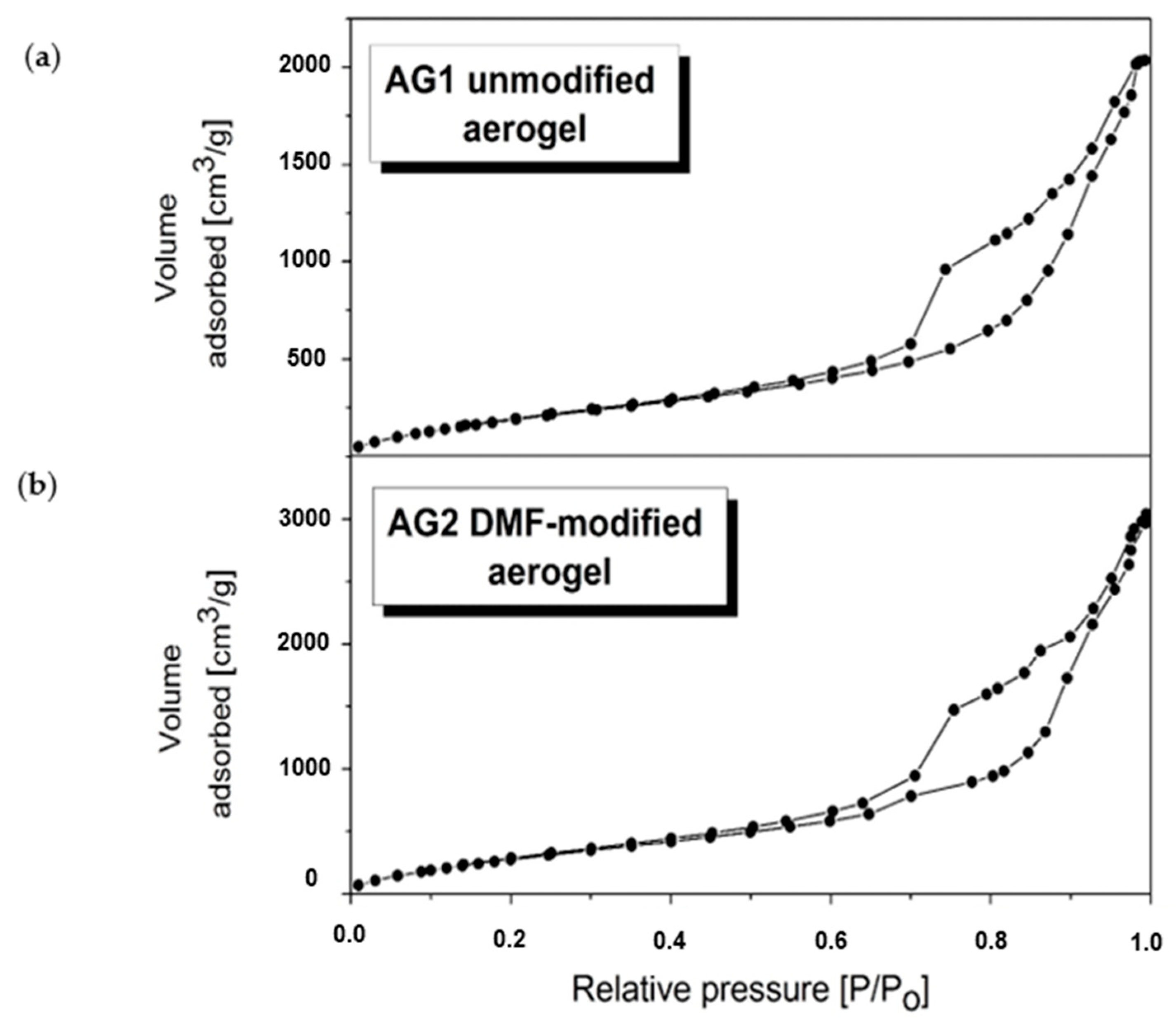

| Silica Source | Used Catalyst(s) | Drying Method | Surface Modification | DMF:Si Molar Ratio | BET Surface Area (m2/g) | Ref. |
|---|---|---|---|---|---|---|
| Si(OC2H5)4 (TEOS) | HCl NH3∙H2O | APD | TMCS in n-hexane | 0.50 | 1231 | Present work |
| Na2O:3.33 SiO2 | HCl | APD | TMCS in n-hexane | 0 0.30 0.45 0.90 1.20 | 469 551 680 583 565 | [30] |
| Si(OC2H5)4 (TEOS) | C6H8O7∙H2O (citric acid) | SCD | 0 0.2 0.4 0.6 0.8 1.0 | 875 860 830 770 655 480 | [37] | |
| Na2O:3.55 SiO2 | NH3∙H2O | APD | TMCS in n-hexane | 0 0.31 0.62 0.93 1.24 1.55 | 740 817 728 627 456 786 | [47] |
| Si(OC2H5)4 (TEOS) | HCl, NH3∙H2O | APD | TMCS in isopropyl alcohol (IPA) | 0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 | 878 939 927 938 939 894 930 908 935 | [49] |
| Density (g/cm3) | Surface Area (m2/g) | Average Pores Diameter (nm) | Micropores Volume (cm3/g) | Mass Changes at 2% Mass Loss | Temperature 4% Mass Loss | (°C): 10% Mass Loss | |
|---|---|---|---|---|---|---|---|
| AG1 | 0.115 | 828 | 13.2 | 2.7 | 90 | 390 | 800 |
| AG2 | 0.114 | 1231 | 13.2 | 4.1 | 310 | 350 | 630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, N.; Szpikowska-Sroka, B.; Miros, A.; Psiuk, B.; Ślosarczyk, A. Effect of Drying Control Agent on Physicochemical and Thermal Properties of Silica Aerogel Derived via Ambient Pressure Drying Process. Energies 2023, 16, 6244. https://doi.org/10.3390/en16176244
Pawlik N, Szpikowska-Sroka B, Miros A, Psiuk B, Ślosarczyk A. Effect of Drying Control Agent on Physicochemical and Thermal Properties of Silica Aerogel Derived via Ambient Pressure Drying Process. Energies. 2023; 16(17):6244. https://doi.org/10.3390/en16176244
Chicago/Turabian StylePawlik, Natalia, Barbara Szpikowska-Sroka, Artur Miros, Bronisław Psiuk, and Agnieszka Ślosarczyk. 2023. "Effect of Drying Control Agent on Physicochemical and Thermal Properties of Silica Aerogel Derived via Ambient Pressure Drying Process" Energies 16, no. 17: 6244. https://doi.org/10.3390/en16176244





