Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates Characteristics
2.1.1. Digestate
2.1.2. Activated Sludge
2.1.3. External Carbon Sources
Acetic Acid
Flume Water
Molasses
2.2. Experimantal Set-Up
2.3. Analytical Methods
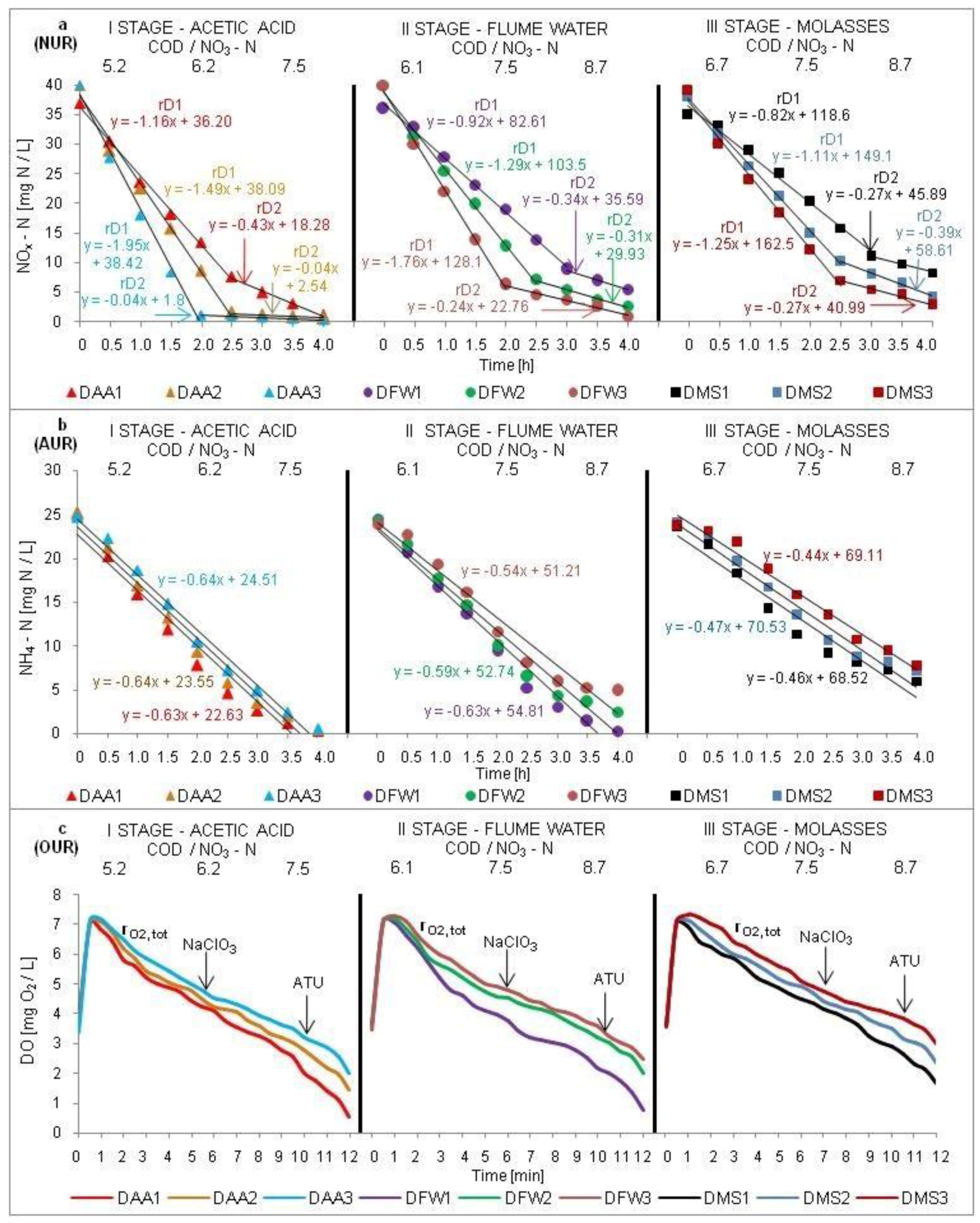
2.4. Batch Tests (NUR, AUR, OUR)
2.5. Molecular Studies
2.6. Statistics
3. Results and Discussion
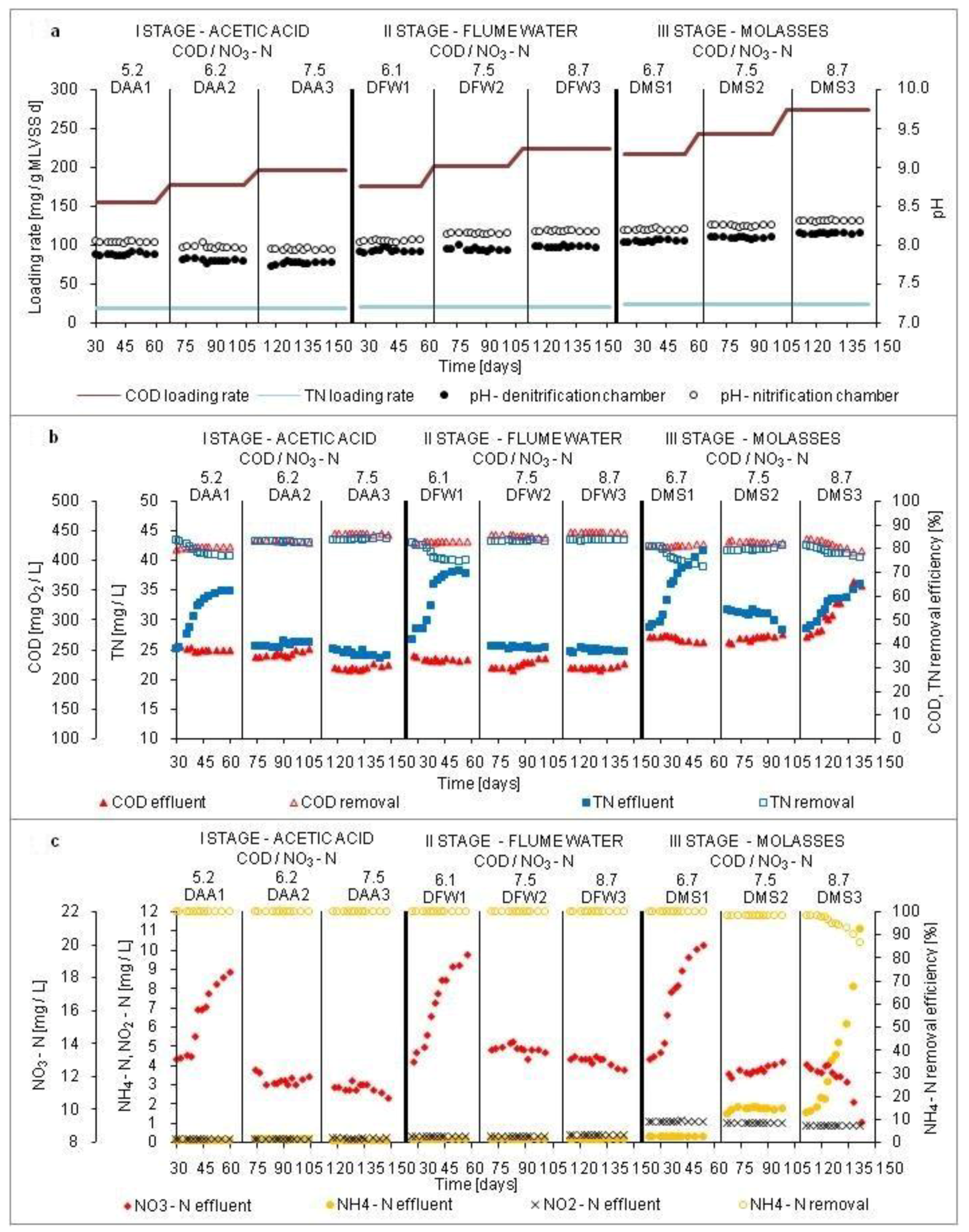
3.1. Treatment Efficiency of the Digestate Liquid Fraction
3.2. Amount and Activity of Denitrifying Bacteria
3.3. Amount and Activity of Nitrifying Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| AOB | Ammonia Oxidizing Bacteria |
| BDCOD | Biodegradable COD/biodegradable fraction |
| BOD5 | Biochemical Oxygen Demand |
| C/N | Carbon/Nitrogen |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| MLVSS | Mixed Liquor Volatile Suspended Solids |
| MLSS | Mixed Liquor Suspended Solid |
| NH4–N | Ammonia Nitrogen |
| NOB | Nitrite Oxidizing Bacteria |
| NO2–N | Nitrite Nitrogen |
| NO3–N | Nitrate Nitrogen |
| SAUR | Specific Ammonia Utilization Rate/Specific Nitrification Rate |
| SBP | Sugar Beet Pulp |
| SBR | Sequencing Batch Reactor |
| SCOD | Soluble COD |
| SNUR | Specific Nitrate Utilization Rate/Specific Denitrification Rate |
| SOUR | Specific Oxygen Utilization Rate |
| TN | Total Nitrogen |
| TP | Total Phosphorus |
| VSS | Volatile Suspended Solids |
| WWTP | Wastewater Treatment Plant |
References
- Świątczak, P.; Cydzik–Kwiatkowska, A.; Zielińska, M. Treatment of the liquid phase of digestate from a biogas plant for water reuse. Bioresour. Technol. 2019, 276, 226–235. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Šlepetys, J.; Cesevičienė, J. Towards a Full Circular Economy in Biogas Plants: Sustainable Management of Digestate for Growing Biomass Feedstocks and Use as Biofertilizer. Energies 2021, 14, 4272. [Google Scholar] [CrossRef]
- Peyrelasse, C.; Barakat, A.; Lagnet, C.; Kaparaju, P.; Monlau, F. Anaerobic Digestion of Wastewater Sludge and Alkaline–Pretreated Wheat Straw at Semi–Continuous Pilot Scale: Performances and Energy Assessment. Energies 2021, 14, 5391. [Google Scholar] [CrossRef]
- European Commission. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options; Wood Environment & Infrastructure Solutions UK Limited: London, UK, 2019. [Google Scholar]
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate mechanical separation: Efficiency profiles based on anaerobic digestion feedstock and equipment choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Khoshnevisan, B.; Lin, C.; Liu, Z.; Liu, H. Life cycle assessment of anaerobic digestion of pig manure coupled with different digestate treatment technologies. Environ. Int. 2020, 137, 105522. [Google Scholar] [CrossRef] [PubMed]
- Świątczak, P.; Cydzik–Kwiatkowska, A.; Zielińska, M. Treatment of Liquid Phase of Digestate from Agricultural Biogas Plant in a System with Aerobic Granules and Ultrafiltration. Water 2019, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Chuda, A.; Ziemiński, K. Digestate mechanical separation in industrial conditions: Efficiency profiles and fertilising potential. Waste Manag. 2021, 128, 167–178. [Google Scholar] [CrossRef]
- Bolzonella, D.; Fatone, F.; Gottardo, M.; Frison, N. Nutrients recovery from anaerobic digestate of agro–waste: Techno–economic assessment of full scale applications. J. Environ. Manag. 2018, 216, 111–119. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, P.; Li, G.; Yin, J.; Li, J.; Zhao, X. Effect of COD/N ratio on nitrogen removal in a membrane–aerated biofilm reactor. Int. Biodeterior. Biodegrad. 2016, 113, 74–79. [Google Scholar] [CrossRef]
- Gong, X.; Wang, B.; Qiao, X.; Gong, Q.; Liu, X.; Peng, Y. Performance of the anammox process treating low–strength municipal wastewater under low temperatures: Effect of undulating seasonal temperature variation. Bioresour. Technol. 2020, 312, 123590. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Webeck, E.; Li, Y.-Y. Towards more efficient nitrogen removal and phosphorus recovery from digestion effluent: Latest developments in the anammox-based process from the application perspective. Bioresour. Technol. 2020, 299, 122560. [Google Scholar] [CrossRef]
- Xu, J.; Wang, P.; Li, Y.; Niu, L.; Xing, Z. Shifts in the Microbial Community of Activated Sludge with Different COD/N Ratios or Dissolved Oxygen Levels in Tibet, China. Sustainability 2019, 11, 2284. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, L.; Xiang, F.; Zhao, L.; Qiao, Z. Activated Sludge Microbial Community and Treatment Performance of Wastewater Treatment Plants in Industrial and Municipal Zones. Int. J. Environ. Res. Public Health 2020, 17, 436. [Google Scholar] [CrossRef] [Green Version]
- Obaja, D.; Macé, S.; Costa, J.; Sans, C.; Mata–Alvarez, J. Nitrification, denitrification and biological phosphorus removal in piggery wastewater using a sequencing batch reactor. Bioresour. Technol. 2003, 87, 103–111. [Google Scholar] [CrossRef]
- Yao, R.; Yuan, Q.; Wang, K. Enrichment of Denitrifying Bacterial Community Using Nitrite as an Electron Acceptor for Nitrogen Removal from Wastewater. Water 2019, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, G.H.; Jorgensen, P.E.; Henze, M. Characterization of functional microorganism groups and substrate in activated sludge and wastewater by AUR, NUR and OUR. Water Sci. Technol. 1992, 25, 43–57. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Zhang, H.; Liu, N.; Zou, Q. Biological denitrification from mature landfill leachate using a foodwaste–derived carbon source. J. Environ. Manag. 2018, 214, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-Z.; Ma, Y.; Wang, S.-Y. Denitrification potential enhancement by addition of external carbon sources in a pre–denitrification process. J. Environ. Sci. 2007, 19, 284–289. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, C.; Cao, J.; Luo, J.; Feng, Q.; Fang, F.; Li, C.; Zhang, Q. An alternative carbon source withdrawn from anaerobic fermentation of soybean wastewater to improve the deep denitrification of tail water. Biochem. Eng. J. 2018, 132, 217–224. [Google Scholar] [CrossRef]
- Liu, F.; Tian, Y.; Ding, Y.; Li, Z. The use of fermentation liquid of wastewater primary sedimentation sludge as supplemental carbon source for denitrification based on enhanced anaerobic fermentation. Bioresour. Technol. 2016, 219, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, C.; Onnis–Hayden, A.; El–Shawabkeh, I.; Gu, A.Z. Implication of using different carbon sources for denitrification in wastewater treatments. Water Environ. Res. 2009, 81, 788–799. [Google Scholar] [CrossRef]
- Feng, X.-C.; Bao, X.; Che, L.; Wu, Q.-L. Enhance biological nitrogen and phosphorus removal in wastewater treatment process by adding food waste fermentation liquid as external carbon source. Biochem. Eng. J. 2021, 165, 107811. [Google Scholar] [CrossRef]
- Cappai, G.; Carucci, A.; Onnis, A. Use of industrial wastewaters for the optimization and control of nitrogen removal processes. Water Sci. Technol. 2004, 50, 17–24. [Google Scholar] [CrossRef]
- Rodríguez, L.; Villaseñor, J.; Fernández, F.J. Use of agro–food wastewaters for the optimisation of the denitrification process. Water Sci. Technol. 2007, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Jin, Y.; Yin, C.; Lee, J.J.; Lee, S. Hydrolyzed molasses as an external carbon source in biological nitrogen removal. Bioresour. Technol. 2005, 96, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.J.; Castro, M.C.; Villasenor, J.; Rodríguez, L. Agro–food wastewaters as external carbon source to enhance biological phosphorus removal. Chem. Eng. J. 2011, 166, 559–567. [Google Scholar] [CrossRef]
- Atimtay, A.T.; Sikdar, S.K. Security of Industrial Water Supply and Management; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Sedlak, R. Phosphorus and Nitrogen Removal from Municipal Wastewater: Principles and Practice, 2nd ed.; Routledge: Boca Raton, FL, USA, 2018; p. 256. [Google Scholar]
- Kujawa–Roeleveld, K. Estimation of Denitrification Potential with Respiration Based Techniques. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2000. [Google Scholar]
- Hartley, K. Tuning Biological Nutrient Removal Plants; IWA Publishing: London, UK, 2013. [Google Scholar]
- Sperandio, M.; Urbain, V.; Audic, J.M.; Paul, E. Use of carbon dioxide evolution rate for determining heterotrophic field and characterising denitrifying biomass. Water Sci. Technol. 1999, 39, 139–146. [Google Scholar] [CrossRef]
- Muller, A.; Wentzel, M.C.; Loewenthal, R.E.; Ekama, G.A. Heterotroph anoxic yield in anoxic aerobic activated sludge systems treating municipal wastewater. Water Res. 2003, 37, 2435–2441. [Google Scholar] [CrossRef]
- Standard ATV-DVWK-A 131P. Dimensioning of Single–Stage Activated Sludge Plants, German ATV–DVWK Rules and Standards; GFA Publishing Company of ATV–DVWK Water, Wastewater and Waste: Hennef, Germany, 2000. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Henze, M.; Gujer, W.; Mino, T.; Van Loosdrecht, M.C. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Zawilski, M.; Brzezinska, A. Variability of COD and TKN fractions of combined wastewater. Pol. J. Environ. Stud. 2009, 18, 501–505. [Google Scholar]
- Van Loosdrecht, M.C.M.; Nielsen, P.H.; Lopez–Vazquez, C.M.; Brdjanovic, D. Experimental Methods in Wastewater Treatment; IWA Publishing: London, UK, 2016. [Google Scholar]
- Ferris, M.J.; Muyzer, G.; Ward, D.M. Denaturing Gradient Gel Electrophoresis Profiles of 16S rRNA–Defined Populations Inhabiting a Hot Spring Microbial Mat Community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.R.; Xiao, Y.P.; Ren, W.W.; Liu, Z.F.; Shi, J.H.; Quan, Z.X. Abundance and composition of ammonia–oxidizing bacteria and archaea in different types of soil in the Yangtze River estuary. J. Zhejiang Univ. Sci. B 2012, 13, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Gerbl, F.W.; Weidler, G.W.; Wanek, W.; Erhardt, A.; Stan–Lotter, H. Thaumarchaeal ammonium oxidation and evidence for a nitrogen cycle in a subsurface radioactive thermal spring in the Austrian Central Alps. Front. Microbiol. 2014, 5, 225. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Lee, D.S.; Park, C.; Park, D.; Park, J.M. Effects of free cyanide on microbial communities and biological carbon and nitrogen removal performance in the industrial activated sludge process. Water Res. 2011, 45, 1267–1279. [Google Scholar] [CrossRef]
- Meng, J.; Li, J.; Li, J.; Astals, S.; Nan, J.; Deng, K.; Antwi, P.; Xu, P. The role of COD/N ratio on the start–up performance and microbial mechanism of an upflow microaerobic reactor treating piggery wastewater. J. Environ. Manag. 2018, 217, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Machat, H.; Boudokhane, C.; Roche, N.; Dhaouadi, H. Effects of C/N Ratio and DO concentration on Carbon and Nitrogen removals in a Hybrid Biological Reactor. Biochem. Eng. J. 2019, 151, 107313. [Google Scholar] [CrossRef]
- Dosta, J.; Galí, A.; Benabdallah El–Hadj, T.; Macé, S.; Mata–Alvarez, J. Operation and model description of a sequencing batch reactor treating reject water for biological nitrogen removal via nitrite. Bioresour. Technol. 2007, 98, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yan, Z.; Ou, Y.; Peng, B.; Zhang, L.; Shao, J.; Lin, Y.; Zhang, J. Effects of different carbon sources on the removal of ciprofloxacin and pollutants by activated sludge: Mechanism and biodegradation. J. Environ. Sci. 2022, 111, 240–248. [Google Scholar] [CrossRef]
- Choubert, J.-M.; Marquot, A.; Stricker, A.-E.; Racault, Y.; Gillot, S.; Heduit, A. Anoxic and aerobic values for the yield coefficient of the heterotrophic biomass: Determination at full–scale plants and consequences on simulations. Water SA 2009, 35, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Morgan-Sagastume, F.; Nielsen, J.L.; Nielsen, P.H. Substrate–dependent denitrification of abundant probe–defined denitrifying bacteria in activated sludge. FEMS Microbiol. Ecol. 2008, 66, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Peng, D.; Pan, R. Shifts in Nitrification Kinetics and Microbial Community during Bioaugmentation of Activated Sludge with Nitrifiers Enriched on Sludge Reject Water. J. Biomed. Biotechnol. 2012, 2012, 691894. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Su, C.; Wang, A.; Fan, C.; Huang, X.; Li, F.; Li, R. Comparative study of aerobic granular sludge with different carbon sources: Effluent nitrogen forms and microbial community. J. Water Process Eng. 2021, 43, 102211. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Wu, P.; Wang, H.; Deng, L.; Wang, W. Nitrification performance evaluation of activated sludge under high potassium ion stress during high–ammonia nitrogen organic wastewater treatment. J. Environ. Sci. 2022, 111, 84–92. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, D. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full–scale biological nutrient removal wastewater treatment plants. AMB Express 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.H.; Thomsen, T.R.; Nielsen, J.L. Bacterial composition of activated sludge–importance for floc and sludge properties. Water Sci. Technol. 2004, 49, 51–58. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhu, C.; Liu, S.; Wang, Q.; Wu, Z. Analysis of Nitrification Efficiency and Microbial Community in a Membrane Bioreactor Fed with Low COD/N–Ratio Wastewater. PLoS ONE 2013, 8, e63059. [Google Scholar] [CrossRef]
- Carrera, J.; Vicent, T.; Lafuente, J. Effect of influent COD/N ratio on biological nitrogen removal (BNR) from high–strength ammonium industrial wastewater. Process Biochem. 2004, 39, 2035–2041. [Google Scholar] [CrossRef]
- Mota, C.; Ridenoure, J.; Cheng, J.; Reyes, F.L. High levels of nitrifying bacteria in intermittently aerated reactors treating high ammonia wastewater. FEMS Microbiol. Ecol. 2005, 54, 391–400. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.-H. Activity enhancement of ammonia–oxidizing bacteria and nitrite–oxidizing bacteria in activated sludge process: Metabolite reduction and CO2 mitigation intensification process. Appl. Water Sci. 2019, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Dinçer, A.R.; Kargi, F. Kinetics of sequential nitrification and denitrification processes. Enzym. Microb. Technol. 2000, 27, 37–42. [Google Scholar] [CrossRef]


| Indicator | Liquid Fraction of Digestate | Flume Water | Molasses | Acetic Acid |
|---|---|---|---|---|
| COD (g O2/L) | 7.96 ± 0.55 | 6.38 ± 0.28 | 1014.00 ± 3.22 | 913.00 ± 0.00 |
| SCOD (g O2/L) | 1.97 ± 0.05 | 4.32 ± 0.16 | 650.64 ± 0.86 | 913.00 ± 0.00 |
| BDCOD (g O2/L) | 2.03 ± 0.02 | 5.13 ± 0.12 | 709.06 ± 0.94 | 912.84 ± 0.15 |
| BOD5 (g O2/L) | 1.18 ± 0.08 | 2.97 ± 0.10 | 410.00 ± 0.35 | 527.83 ± 0.26 |
| TN (g/L) | 2.32 ± 0.06 | 0.08 ± 0.00 | 25.38 ± 0.36 | – |
| NH4–N (g/L) | 1.95 ± 0.07 | 0.04 ± 0.00 | 0.53 ± 0.03 | – |
| TP (mg/L) | 23.12 ± 1.59 | 2.20 ± 0.07 | 68.40 ± 1.13 | – |
| pH | 8.07 ± 0.16 | 6.83 ± 0.09 | 7.33 ± 0.03 | 2.50 ± 0.00 |
| Stage of Study | I Stage | II Stage | III Stage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| External Carbon Source | Acetic Acid | Flume Water | Molasses | ||||||
| Indicator/Series | DAA1 | DAA2 | DAA3 | DFW1 | DFW2 | DFW3 | DMS1 | DMS2 | DMS3 |
| COD (mg O2/L) | 1268.38 ± 8.54 | 1440.98 ± 8.02 | 1592.54 ± 8.08 | 1348.78 ± 8.08 | 1519.53 ± 9.02 | 1663.01 ± 11.02 | 1423.74 ± 5.86 | 1555.49 ± 6.43 | 1699.82 ± 6.81 |
| BDCOD (mg O2/L) | 821.70 ± 8.12 | 955.32 ± 6.48 | 1126.39 ± 7.25 | 757.07 ± 6.78 | 916.49 ± 8.09 | 1037.08 ± 9.23 | 737.79 ± 7.56 | 811.95 ± 5.42 | 926.90 ± 5.95 |
| BOD5 (mg O2/L) | 475.14 ± 7.02 | 552.40 ± 7.00 | 651.32 ± 7.21 | 437.76 ± 8.19 | 529.94 ± 9.02 | 599.67 ± 9.50 | 426.62 ± 6.03 | 469.50 ± 5.69 | 535.96 ± 6.00 |
| TN (mg/L) | 149.19 ± 0.75 | 149.22 ± 0.72 | 149.30 ± 0.70 | 149.72 ± 0.90 | 148.83 ± 0.95 | 148.82 ± 0.92 | 149.81 ± 0.81 | 149.58 ± 0.88 | 149.73 ± 0.92 |
| NH4–N (mg/L) | 107.95 ± 0.68 | 107.74 ± 0.64 | 107.34 ± 0.68 | 106.84 ± 0.81 | 106.68 ± 0.85 | 106.57 ± 0.81 | 87.67 ± 0.90 | 85.06 ± 0.95 | 82.61 ± 0.85 |
| TP (mg/L) | 1.76 ± 0.02 | 1.75 ± 0.02 | 1.75 ± 0.02 | 1.68 ± 0.01 | 1.72 ± 0.02 | 1.75 ± 0.02 | 1.49 ± 0.02 | 1.47 ± 0.03 | 1.45 ± 0.03 |
| pH | 6.71 ± 0.21 | 6.72 ± 0.19 | 6.75 ± 0.28 | 7.56 ± 0.20 | 7.61 ± 0.14 | 7.65 ± 0.10 | 7.79 ± 0.17 | 7.83 ± 0.24 | 7.89 ± 0.15 |
| CODext */NO3–N | 5.20 | 6.20 | 7.50 | 6.10 | 7.50 | 8.70 | 6.70 | 7.50 | 8.70 |
| BDCODext */NO3–N | 5.10 | 6.10 | 7.50 | 4.80 | 6.00 | 7.00 | 4.70 | 5.20 | 6.10 |
| COD/TN | 8.50 | 9.70 | 10.70 | 9.00 | 10.20 | 11.20 | 9.50 | 10.40 | 11.40 |
| Target | Primer | Sequence (5′—3′) | The Composition of Reaction Mixture | PCR Program | References of Primers Seguence | |
|---|---|---|---|---|---|---|
| Bacterial 16SrDNA | 1055F | ATGGCTGTCGTCAGCT | 1 μL 10 μL 0.4 μL 0.4 μL 8.2 μL | DNA template (10 ng/μL) Real Time 2xRT–PCR Mix SYBR A (A&A Biotechnology) Primer 1055F (10 μM) Primer 1392R (10 μM) Nuclease–free water | 3 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 58 °C, 30 s at 72 °C; 65 °C → 95 °C | [39] |
| 1392R | ACGGGCGGTGTGTAC | |||||
| AOB—amoA gene | amoA–1F | GGGGTTTCTACTGGTGGT | 1 μL 10 μL 0.4 μL 1.0 μL 7.6 μL | DNA template (10 ng/μL) Real Time 2xRT-PCR Mix SYBR A (A&A Biotechnology) Primer amoA–1F (10 μM) Primer amoA–2R (10 μM) Nuclease–free water | 3 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C; 65 °C → 95 °C | [40] |
| amoA–2R | CCCCTCKGSAAAGCCTTCTTC | |||||
| NOB—nxrA gene | nxrA–RT–F | GTGGTCATGCGCGTTGAGCA | 1 μL 10 μL 0.4 μL 0.4 μL 8.2 μL | DNA template (10 ng/μL) Real Time 2xRT–PCR Mix SYBR A (A&A Biotechnology) Primer nxrA–RT–F (10 μM) Primer nxrA–RT–R (10 μM) Nuclease–free water | 3 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 60 °C, 30 s at 72 °C; 65 °C → 95 °C | [41] |
| nxrA–RT–R | TCGGGAGCGCCATCATCCAT | |||||
| Denitrifying bacteria—nirS gene | nirS 1f | TACCACCCSGARCCGCGCGT | 1 μL 10 μL 0.1 μL 0.1 μL 8.8 μL | DNA template (10 ng/μL) Real Time 2xRT–PCR Mix SYBR A (A&A Biotechnology) Primer nirS 1f (10 μM) Primer nirS 3r (10 μM) Nuclease–free water | 3 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 58 °C, 30 s at 72 °C; 65 °C → 95 °C | [42] |
| nirS 3r | GCCGCCGTCRTGVAGGAA | |||||
| Denitrifying bacteria—nirK gene | nirK 876 | ATYGGCGGVCAYGGCGA | 1 μL 10 μL 0.1 μL 0.1 μL 8.8 μL | DNA template (10 ng/μL) Real Time 2xRT–PCR Mix SYBR A (A&A Biotechnology) Primer nirK 876 (10 μM) Primer nirK 1040 (10 μM) Nuclease–free water | 3 min at 95 °C; 40 cycles of 15 s at 95 °C, 30 s at 58 °C, 30 s at 72 °C; 65 °C → 95 °C | [42] |
| nirK 1040 | GCCTCGATCAGRTTRTGGTT | |||||
| Carbon Source | Denitrification Rate SNUR | Nitrification Rate SAUR | Reactor Type | Reference |
|---|---|---|---|---|
| [mg N/g VSS h] | ||||
| octan | 3–4 | – | full–scale reactor removing N and P | [48] |
| octan | 4.7 | – | lab–scale SBR reactor | [25] |
| acetic acid | 31.1 | 27.5 | lab–scale SBR reactor | [15] |
| octan | 40 | 19 | lab–scale SBR reactor | [45] |
| wastewater from beet—sugar factory | 2.7 | 2.82 | lab–scale SBR reactor | [24] |
| wastewater from beet—sugar processing | 1.75 | – | lab–scale SBR reactor | [25] |
| hydrolyzed molasses | 3.6 | – | lab–scale SBR reactor; COD/NO3–N ratio of 5 | [26] |
| glucose | 2.4–3.1 | – | full–scale reactor removing N and P | [48] |
| glucose | 0.43 | 1.65 | lab–scale SBR reactor | [49] |
| acetic acid | 4.9–6.3 | 2.6–3.2 | lab–scale conventional activated sludge reactor | This study |
| flume water from beet—sugar factory | 4.5–6.0 | 2.3–2.9 | lab–scale conventional activated sludge reactor | This study |
| molasses | 3.7–5.0 | 1.9–2.1 | lab–scale conventional activated sludge reactor | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuda, A.; Ziemiński, K. Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process. Energies 2021, 14, 7321. https://doi.org/10.3390/en14217321
Chuda A, Ziemiński K. Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process. Energies. 2021; 14(21):7321. https://doi.org/10.3390/en14217321
Chicago/Turabian StyleChuda, Aleksandra, and Krzysztof Ziemiński. 2021. "Challenges in Treatment of Digestate Liquid Fraction from Biogas Plant. Performance of Nitrogen Removal and Microbial Activity in Activated Sludge Process" Energies 14, no. 21: 7321. https://doi.org/10.3390/en14217321






