The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance
Abstract
:1. Introduction
2. Experimental Methods
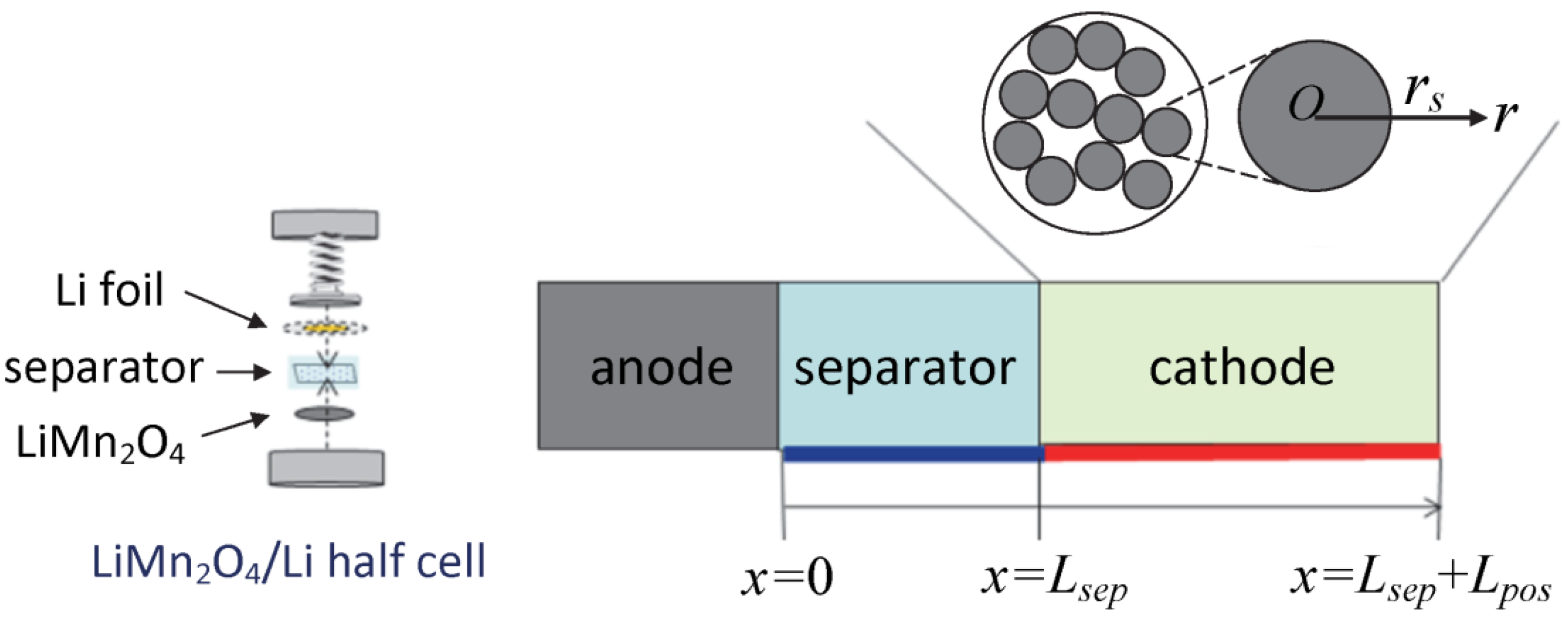
2.1. Fabrication of the LiMn2O4 Composite Electrode
2.2. Measurement of Electronic Conductivity
2.3. Impedance Measurements
2.4. Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP–OES) Measurements
3. Model Development
3.1. Electrochemical Modeling
3.1.1. Charge Conservation Equations
3.1.2. Lithium Transport Equation in the Electrolyte
3.1.3. Lithium Transport Equation in the Cathode
3.2. Side Reactions of the Cathode Material
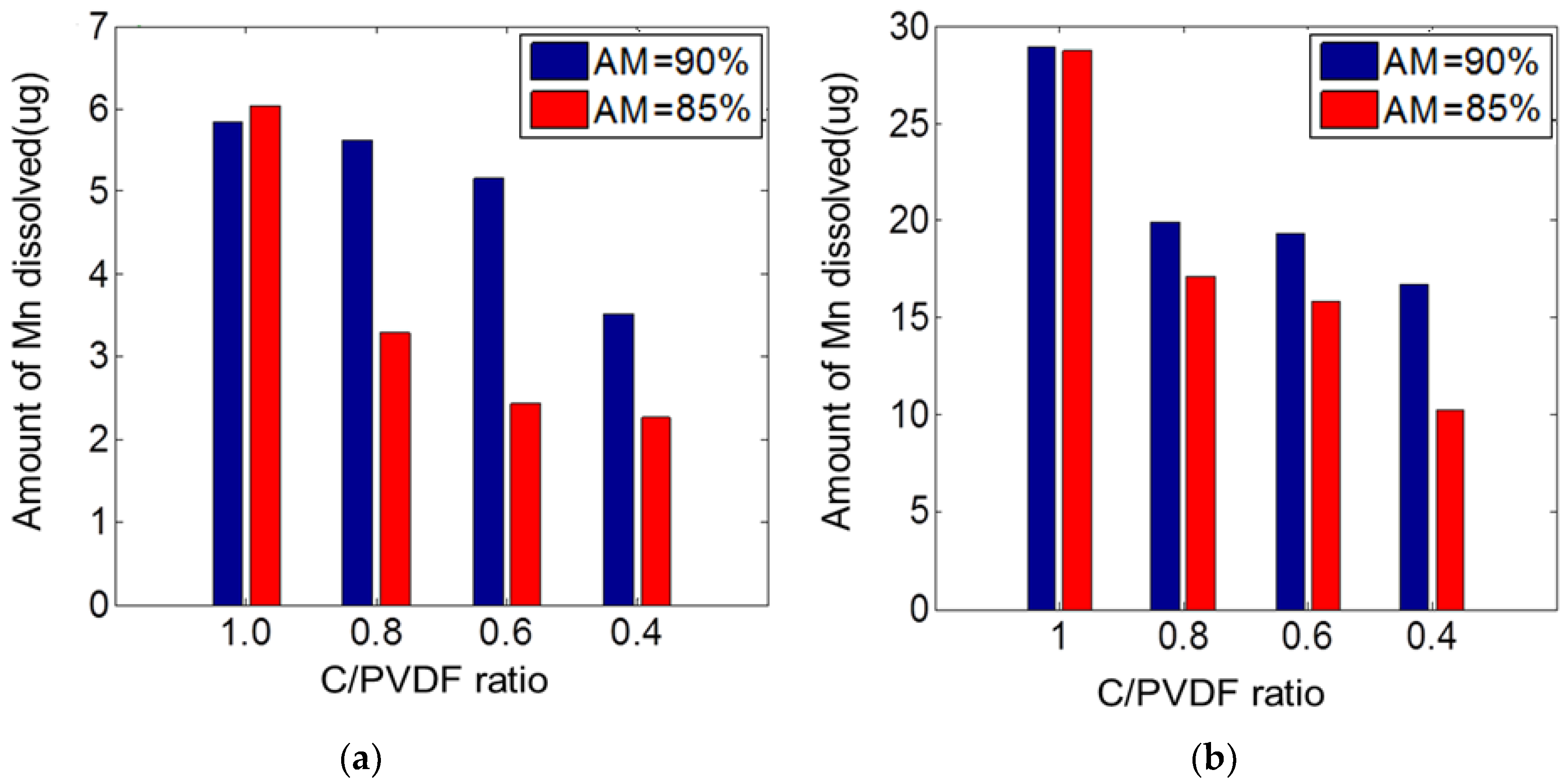
3.2.1. Manganese Dissolution
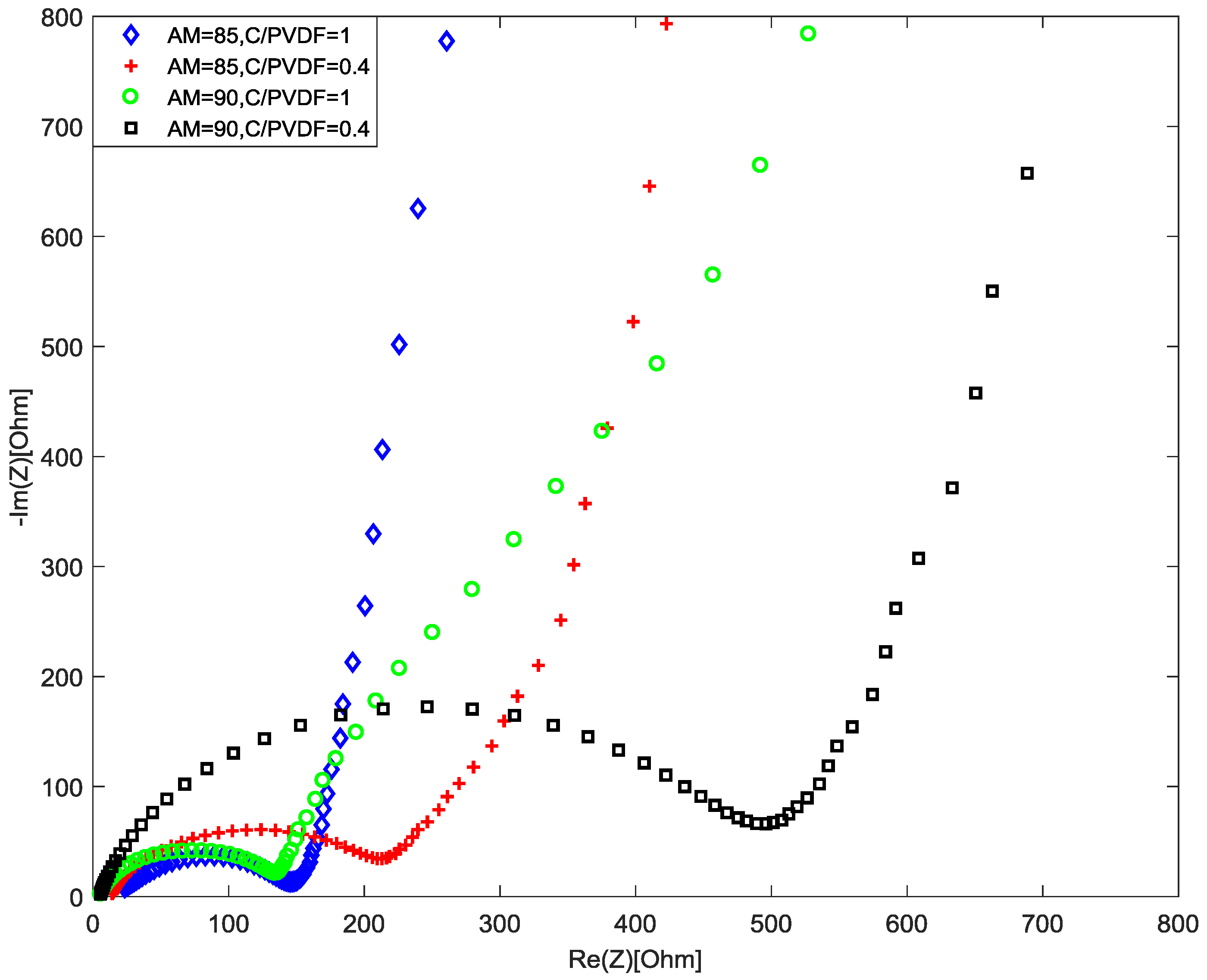
3.2.2. Charge Transfer Resistance
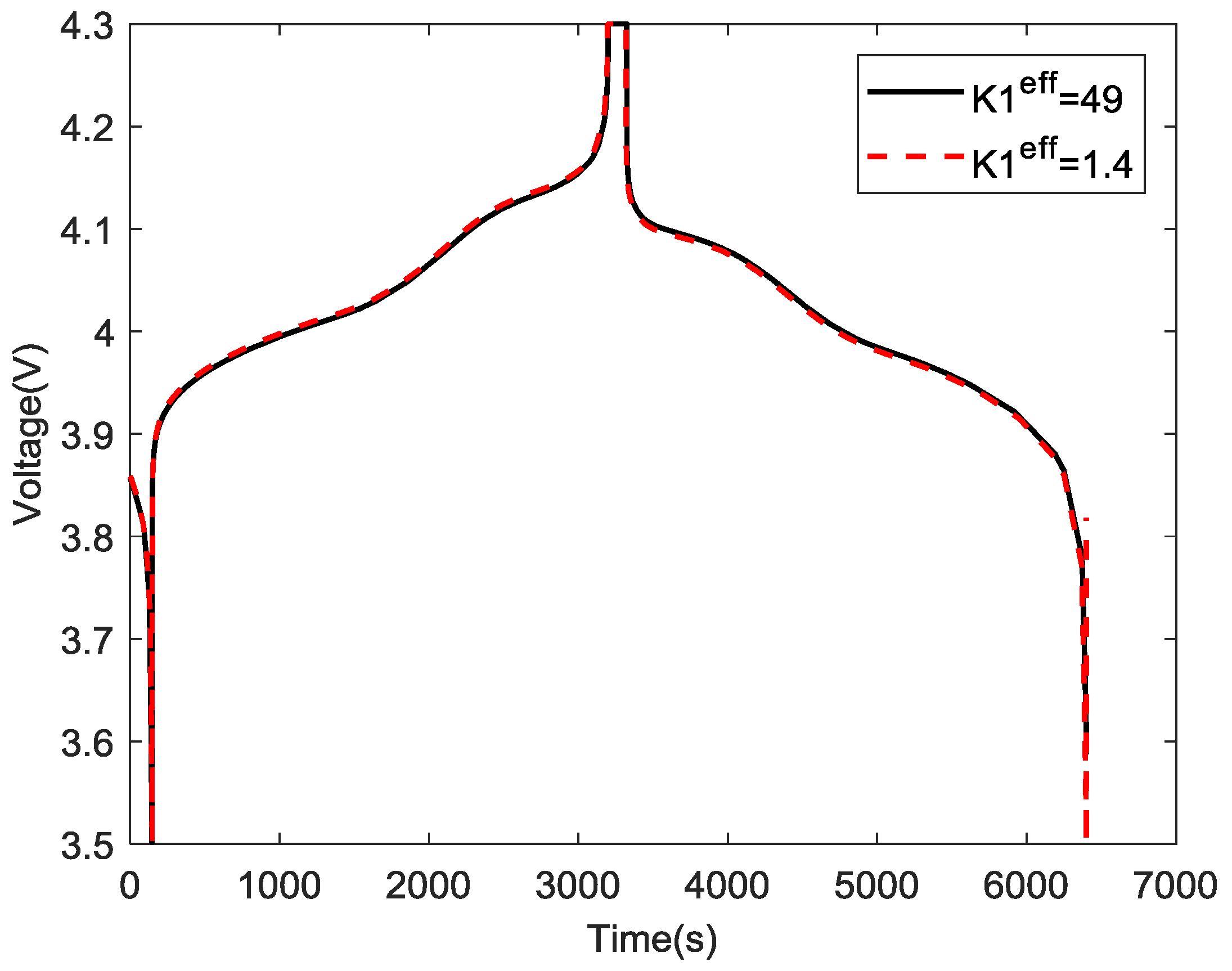
3.2.3. Effective Electronic Conductivity
4. Results and Discussions
4.1. Four-Point Probe Conductivity Measurements
4.2. Manganese Dissolution Due to Different C/PVDF Ratios
4.3. Interfacial Resistance Due to Different C/PVDF Ratios
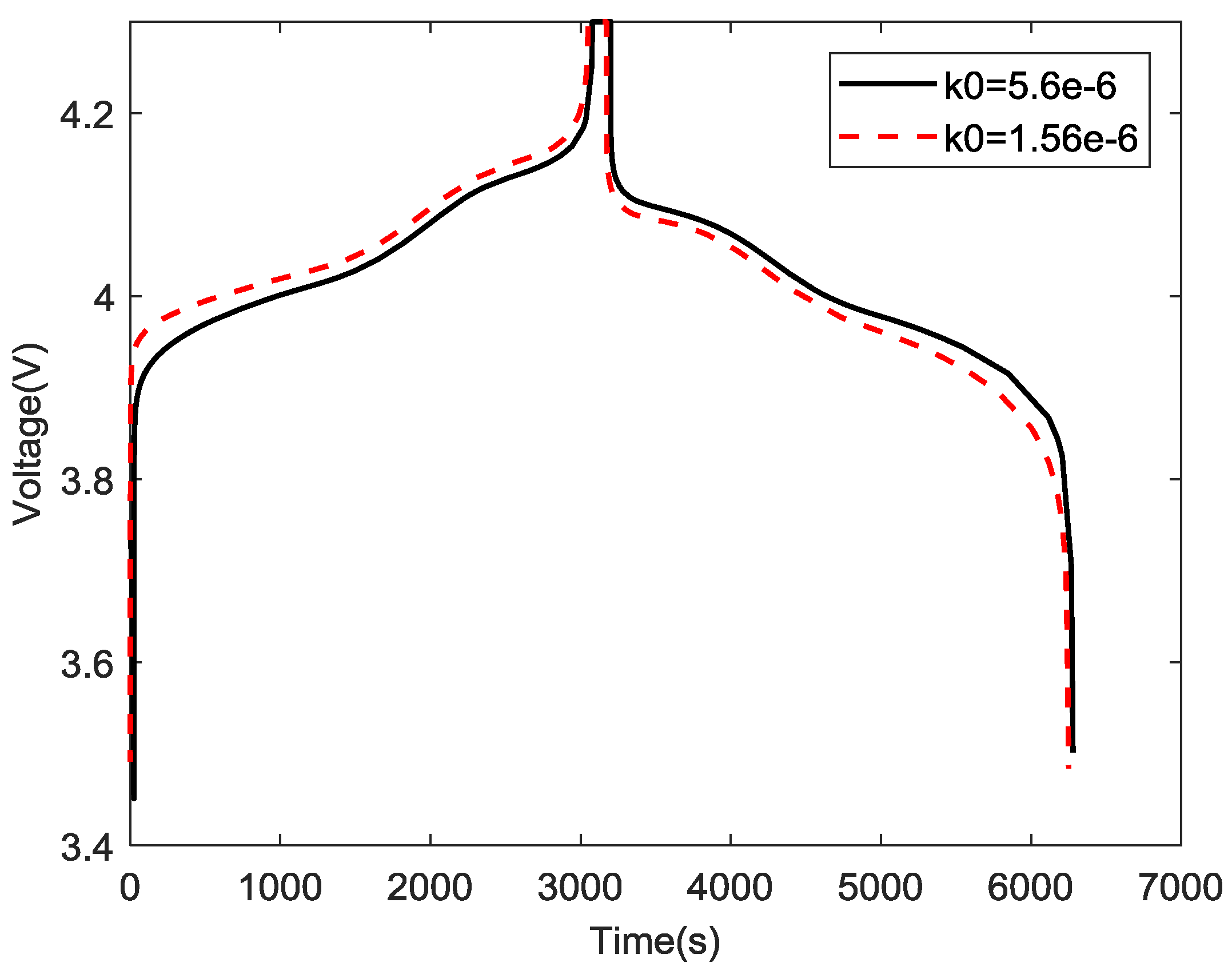
4.4. Validation of Simulation Model using Experiment Data
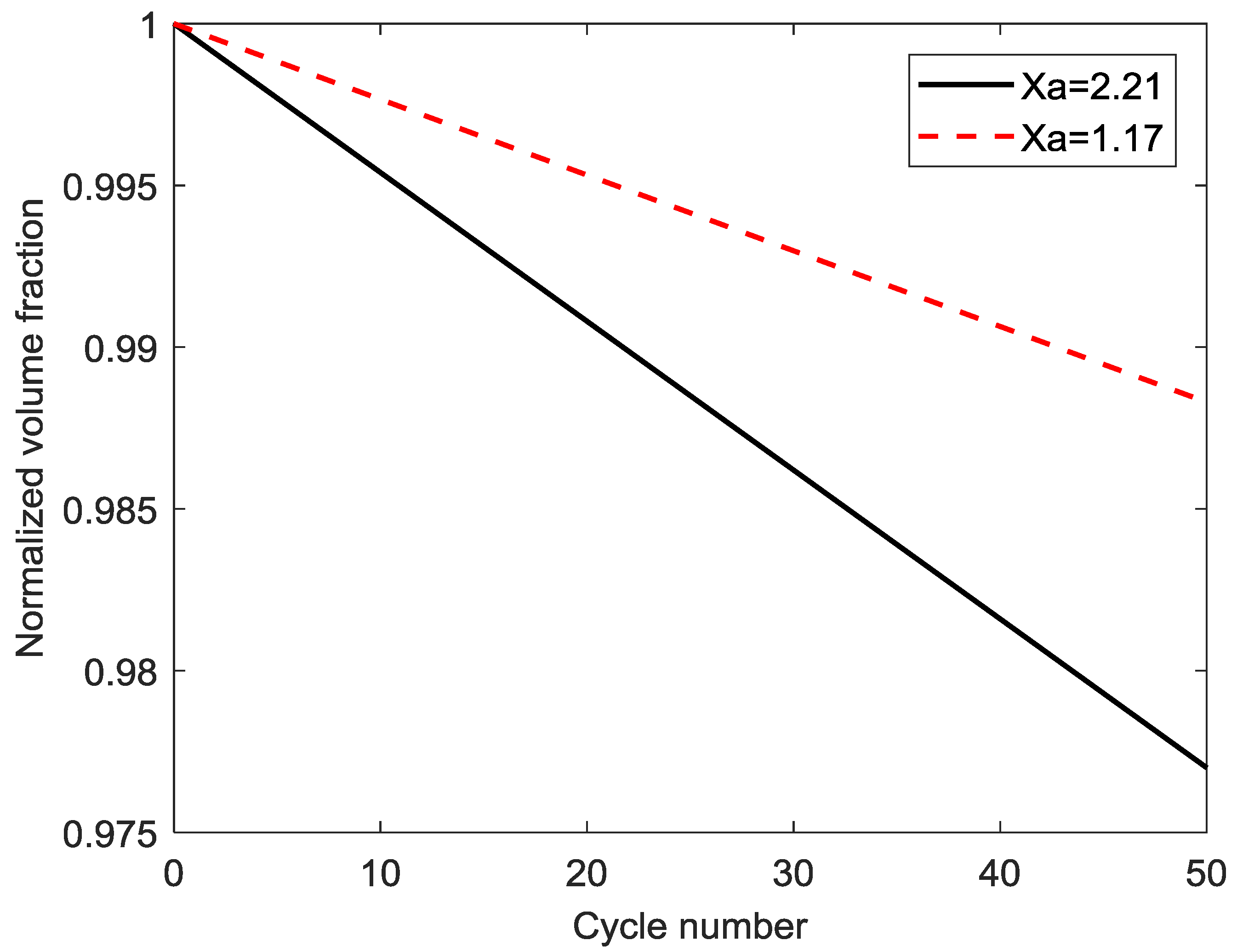
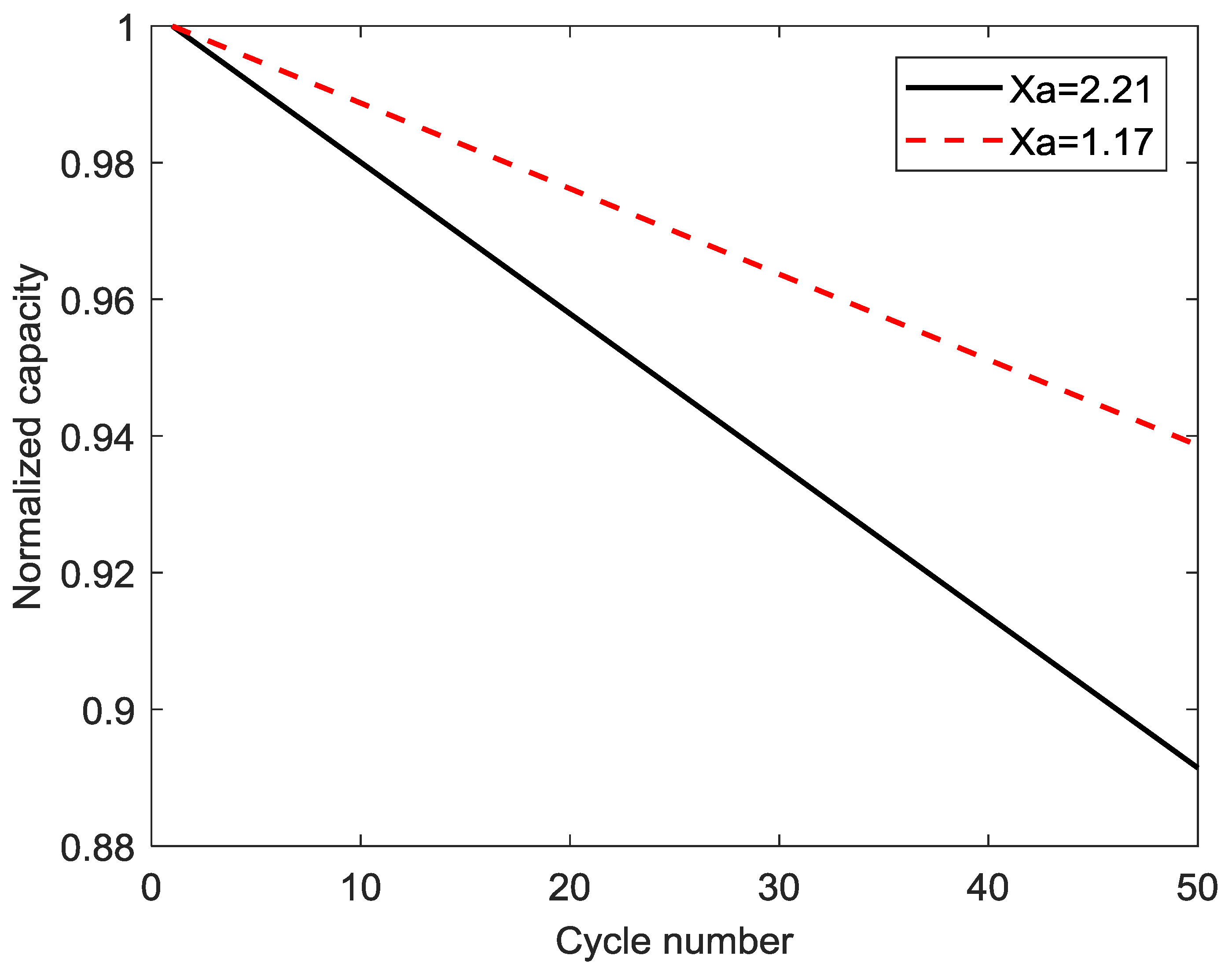
4.5. Parametric Study of Different Composition Ratios of the LiMn2O4 Composite Electrode
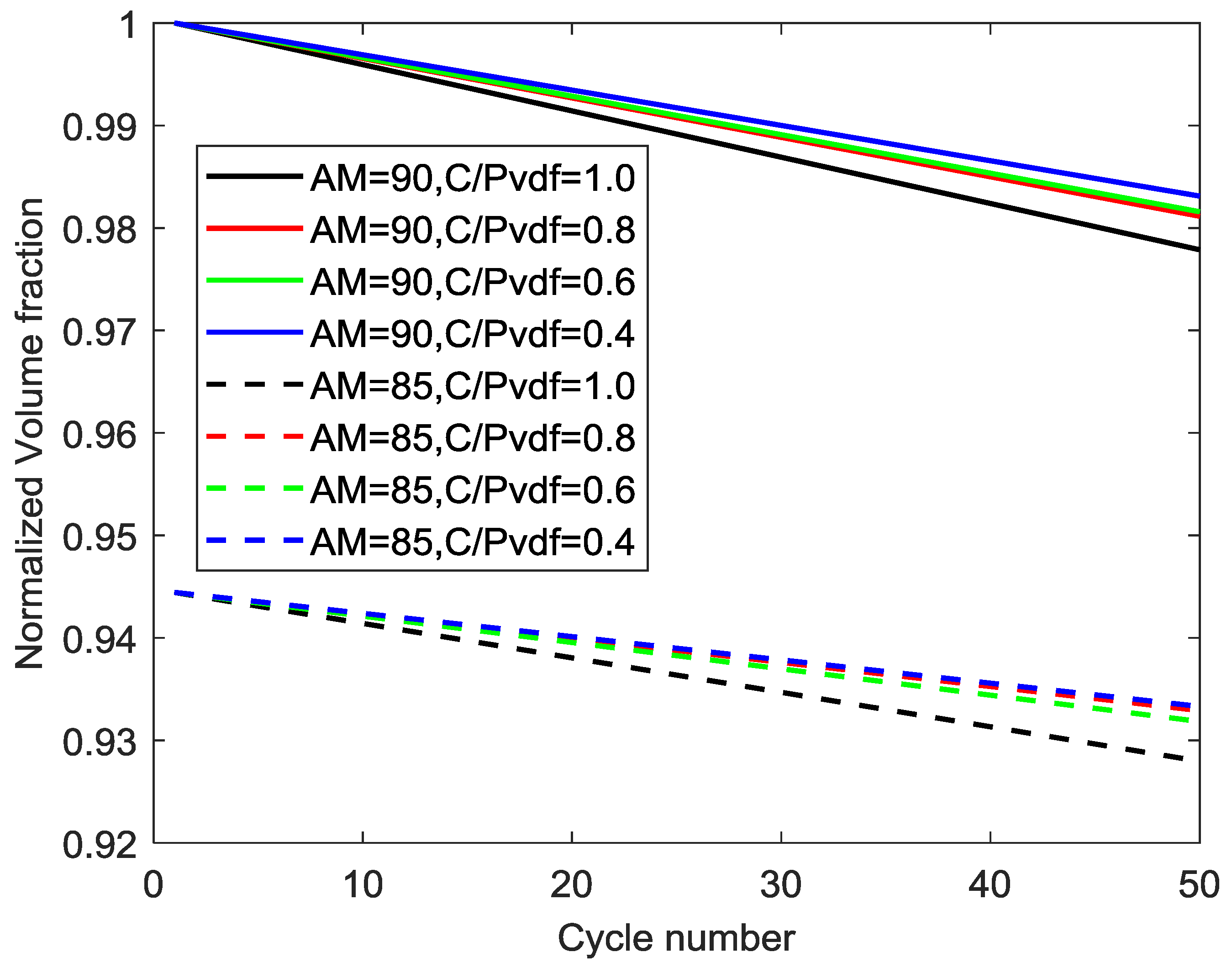
4.6. Simulation Results with Divergent Amounts of Active Material, Conductive Additives, and Polymer Binder
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Symbol
| specific surface area of positive electrode, 1/m | |
| the concentration of species i in the electrolyte, mol/m3 | |
| concentration of lithium ions in the solid phase of the cathode, mol/m3 | |
| initial concentration of lithium ions in the solid phase of the cathode, mol/m3 | |
| the maximum solid phase concentration, mol/m3 | |
| the initial maximum solid phase concentration, mol/m3 | |
| surface solid phase concentration of lithium ions in the particle electrode, mol/m3 | |
| effective diffusion coefficient of species of lithium in the electrolyte, m2/s | |
| electrolyte activity coefficient | |
| F | Faraday’s constant, C/mol |
| exchange current of lithium intercalation/deintercalation reactions at cathode, A/m2 | |
| current density due to lithium intercalation/deintercalation reaction at cathode, A/m2 | |
| current density due to reaction of lithium at lithium metal anode, A/m2 | |
| applied total current density, A/m2 | |
| particle isolation coefficient | |
| reaction rate constant of Li ion on Li metal surface, A/m0.5mol0.5 | |
| reaction-rate constant in the positive electrode, A/m2 | |
| effective electronic conductivity of the electrolyte phase, S/m | |
| ionic conductivity of the electrolyte phase, S/m | |
| length of positive electrode, m | |
| length of separator, m | |
| the amount of dissolved manganese, g | |
| initial mass of the active material, g | |
| number of electron exchanges during charge transfer reactions | |
| molar mass of lithium ion, g/mol | |
| R | universal gas constant, J/mol K |
| particle radius of active material in positive electrode, m | |
| charge transfer resistance of the electrode, Ω m2 | |
| lithium ion transference number in the electrolyte | |
| electrode thickness, mm | |
| T | room temperature, K |
| x | the intercalation level of lithium into the positive electrode |
| anodic transfer coefficient of lithium intercalation/deintercalation reactions | |
| cathodic transfer coefficient of lithium intercalation/deintercalation reactions | |
| anodic transfer coefficient at lithium metal | |
| cathodic transfer coefficient at lithium metal | |
| volume fraction of positive electrode | |
| initial volume fraction of positive electrode | |
| porosity of the region of separator and positive electrode | |
| usable volume fraction of positive electrode | |
| overpotential for lithium intercalation/deintercalation reaction, V | |
| potential of the solid phase, V | |
| potential of the electrolyte phase, V | |
| potential of lithium metal, V | |
| the ratio of the initial and dissolved masses of manganese |
References
- Chen, Y.H.; Wang, C.W.; Zhang, X.; Sastry, A.M. Porous cathode optimization for lithium cells: Ionic and electronic conductivity, capacity, and selection of materials. J. Power Sources 2010, 195, 2851–2862. [Google Scholar] [CrossRef]
- Trembacki, B.L.; Mistry, A.N.; Noble, D.R.; Ferraro, M.E.; Mukherjee, P.P.; Roberts, S.A. Mesoscale analysis of conductive binder domain morphology in lithium-ion battery electrodes. J. Electrochem. Soc. 2018, 165, E275–E736. [Google Scholar] [CrossRef]
- Inoue, G.; Kawase, M. Numerical and experimental evaluation of the relationship between porous electrode structure and effective conductivity of ions and electrons in lithium-ion batteries. J. Power Sources 2017, 342, 476–488. [Google Scholar] [CrossRef]
- Mandal, S.; Amarilla, J.M.; Ibáñez, J.; Rojo, J.M. The role of carbon black in LiMn2O4 based composites as cathodes for rechargeable lithium batteries. J. Electrochem. Soc. 2001, 148, A24–A29. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Song, X.; Battaglia, V.S. Particles and polymer binder interaction: A controlling factor in lithium-Ion electrode performance. J. Electrochem. Soc. 2012, 159, A214–A221. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, C.; Wang, L.; Luo, W.; Ma, K. Thermal-electrochemical modeling and parameter sensitivity analysis of lithium-ion battery. Chem. Eng. Trans. 2013, 33, 943–948. [Google Scholar]
- Zhang, L.; Lyu, C.; Hinds, G.; Wang, L.; Luo, W.; Zheng, J.; Ma, K. Parameter sensitivity analysis of cylindrical LiFePO4 battery performance using multi-physics modeling. J. Electrochem. Soc. 2014, 161, A762–A776. [Google Scholar] [CrossRef]
- Du, W.; Gupta, A.; Zhang, X.; Sastry, A.M.; Shyy, W. Effect of cycling rate, particle size and transport properties on lithium-ion cathode performance. Int. J. Heat Mass Transf. 2010, 53, 3552–3561. [Google Scholar] [CrossRef]
- Ghaznavi, M.; Chen, P. Sensitivity analysis of a mathematical model of lithium-sulfur cells part I: Applied discharge current and cathode conductivity. J. Power Sources 2013, 257, 4–11. [Google Scholar] [CrossRef]
- Han, S.W. Transport and Kinetic Phenomena Linked to Power Performance of Lithium-ion Batteries. Ph.D. Dissertation, University of Michigan, Ann Arbor, MI, USA, 2014. [Google Scholar]
- Hosseinzadeh, E.; Marco, J.; Jennings, P. Electrochemical-thermal modelling and optimisation of lithium-ion battery design parameters using analysis of variance. Energies 2017, 10, 1278. [Google Scholar] [CrossRef]
- Arora, P.; White, R.E.; Doyle, M. Capacity fade mechanisms and side reactions in lithium-ion batteries. J. Electrochem. Soc. 1998, 145, 3647–3667. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.J.; Xiong, D.; Dahn, J.R. A guide to li-ion coin-cell electrode making for academic researchers. J. Electrochem. Soc. 2011, 158, A51–A57. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Kim, S.; Deng, Y.; Minor, A.M.; Song, X.; Battaglia, V.S. Effects of various conductive additive and polymeric binder contents on the performance of a lithium-ion composite cathode. J. Electrochem. Soc. 2008, 155, A887–A892. [Google Scholar] [CrossRef]
- Shin, J.; Kostecki, R.; Richardson, T.; Song, X.; Striebel, K.A. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. J. Power Sources 2002, 112, 222–230. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Takahashi, M.; Wang, B. A study on capacity fading of lithium-ion battery with manganese spinel positive electrode during cycling. Electrochim. Acta 2006, 51, 3228–3234. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.; Lu, W. A comprehensive study of manganese deposition and side reactions in li-ion battery electrodes. J. Electrochem. Soc. 2017, 164, A2812–A2822. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.; Lu, W. Electronic and bonding properties of LiMn2O4 spinel with different surface orientations and doping elements and their effects on manganese dissolution. J. Electrochem. Soc. 2016, 163, A1359–A2822. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of sheet resistivities with the four-point probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Bard, A.J.; Inzelt, G.; Scholz, F. Electrochemical Dictionary, 2nd ed.; Springer: Berlin, Germany, 2008; ISBN 978-3-642-29550-8. [Google Scholar]
- Raccichini, R.; Amores, M.; Hinds, G. Critical Review of the Use of Reference Electrodes in Li- ion batteries: A Diagnostic Perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef]
- Orsini, F.; Dolle, M.; Tarascon, J.-M. Impedance Study of the Li/electrolyte interface upon cycling. Solid State Ionics 2000, 135, 213–221. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Lin, X.; Park, J.; Liu, L.; Lee, Y.K.; Sastry, A.M.; Lu, W. A comprehensive capacity fade model and analysis for li-ion batteries. J. Electrochem. Soc. 2013, 160, A1701–A1710. [Google Scholar] [CrossRef]
- Levi, M.D.; Gamolsky, K.; Aurbach, D.; Heider, U.; Oesten, R. On electrochemical impedance measurements of LixCo0.2Ni0.8O2 and LixNiO2 intercalation electrodes. Electrochim. Acta 2000, 45, 1781–1789. [Google Scholar] [CrossRef]
- Zhuang, Q.C.; Fan, X.Y.; Xu, J.M.; Wei, G.Z.; Dong, Q.F.; Sun, S.G. Impedance studies of spinel LiMn2O4 electrode/electrolyte Interfaces. Chem. Res. Chin. Univ. 2008, 24, 511–515. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Barcellona, S.; Piegari, L. Lithium ion battery models and parameter identification techniques. Energies 2017, 10, 2007. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373–A1387. [Google Scholar] [CrossRef] [Green Version]
- Raccichini, R.; Furness, L.; Dibden, J.W.; Owen, J.R.; García-Araez, N. Impedance Characterization of the Transport Properties of Electrolytes Contained within Porous Electrodes and Separators Useful for Li-S Batteries. J. Electrochem. Soc. 2018, 165, A2741–A2749. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.-M. Comparison of modeling predictions with experimental data from plastic lithium ion cells. J. Electrochem. Soc. 1996, 143, 1890–1903. [Google Scholar] [CrossRef]




| Sample Number | Active Material (g) | Carbon Black (g) | PVDF Binder (g) | Ratio of Active Materials C:PVDF |
|---|---|---|---|---|
| 1 | 1.8 | 0.1 | 0.1 | 90:5:5 (1:1) |
| 2 | 1.8 | 0.0889 | 0.1111 | 90:4.44:5.56 (0.8:1) |
| 3 | 1.8 | 0.075 | 0.125 | 90:3.75:6.25 (0.6:1) |
| 4 | 1.8 | 0.0571 | 0.1428 | 90:2.86:7.14 (0.4:1) |
| 5 | 1.7 | 0.15 | 0.15 | 85:7.5:7.5 (1:1) |
| 6 | 1.7 | 0.1333 | 0.1667 | 85:6.67:8.33 (0.8:1) |
| 7 | 1.7 | 0.1125 | 0.1875 | 85:5.63:9.38 (0.6:1) |
| 8 | 1.7 | 0.0857 | 0.2143 | 85:4.29:10.71 (0.4:1) |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| anodic and cathodic transfer coefficient of Li metal | 0.5 | initial maximum solid phase concentration | 22,730 mol/m3 |
| anodic and cathodic transfer coefficient of Li intercalation/deintercalation reactions | 0.5 | initial solid phase diffusion coefficient | 1.31 × 10−14 m2/s |
| initial volume fraction of positive electrode | 0.297 | electrolyte activity coefficient | 1 |
| porosity of electrolyte | 0.444 | F Faraday’s constant | 96,487 C equiv-1 |
| radius of Cathode particle | 5 µm | applied current density | 10.5 A/m2 |
| transference number | 0.363 | length of positive electrode | 180 × 10−6 m |
| T temperature | 298 K | length of separator | 50 × 10−6 m |
| initial voltage | 3.6 V | reaction-rate constant of Li ion on Li metal | 6.1 × 10−6 A/m2 |
| Bruggemann coefficient | 1.5 | reaction-rate constant in the positive electrode | 1 × 10−5 A/m2 |
| initial electrolyte salt concentration | 1000 mol/m3 | particle isolation coefficient | 5 |
| Sample | Slope (V/mA) | Coating Thickness (inches) | Resistance (ohm∙cm) | Conductivity (S/m) |
|---|---|---|---|---|
| 1 | 0.1958 | 0.0015 | 3.381 | 29.57 |
| 2 | 1.266 | 0.0007 | 10.20 | 9.802 |
| 3 | 0.7353 | 0.003 | 25.39 | 3.937 |
| 4 | 5.881 | 0.001 | 67.70 | 1.477 |
| 5 | 0.1767 | 0.001 | 2.034 | 49.15 |
| 6 | 0.4195 | 0.001 | 4.829 | 20.70 |
| 7 | 0.498 | 0.0012 | 6.879 | 14.53 |
| 8 | 3.664 | 0.001 | 42.18 | 2.370 |
| Sample | Rohm (Ohm) | Rct (Ohm) | Cdl (F) | Impedance Model |
|---|---|---|---|---|
| AM = 85, C/PVDF = 1 | 7.163 | 120.9 | 1.62 × 10−6 | 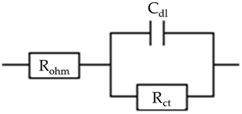 |
| AM = 85, C/PVDF = 0.4 | 28.91 | 129.4 | 9.72 × 10−6 | |
| AM = 90, C/PVDF = 1 | 17.13 | 190.5 | 1.87 × 10−6 | |
| AM = 90, C/PVDF = 0.4 | 6.395 | 440.0 | 1.49 × 10−6 |
| Sample | Xa | |||
|---|---|---|---|---|
| 1 | 0.297 | 5.600 × 10−6 | 29.57 | 2.212987283 |
| 2 | 0.297 | 4.246 × 10−6 | 9.802 | 1.885565102 |
| 3 | 0.297 | 2.892 × 10−6 | 3.937 | 1.841767796 |
| 4 | 0.297 | 1.539 × 10−6 | 1.477 | 1.689016773 |
| 5 | 0.2805 | 5.234 × 10−6 | 49.15 | 1.744319236 |
| 6 | 0.2805 | 4.673 × 10−6 | 20.7 | 1.221354539 |
| 7 | 0.2805 | 4.113 × 10−6 | 14.53 | 1.334272528 |
| 8 | 0.2805 | 3.553 × 10−6 | 2.37 | 1.178505667 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.K. The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance. Energies 2019, 12, 658. https://doi.org/10.3390/en12040658
Lee YK. The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance. Energies. 2019; 12(4):658. https://doi.org/10.3390/en12040658
Chicago/Turabian StyleLee, Yoon Koo. 2019. "The Effect of Active Material, Conductive Additives, and Binder in a Cathode Composite Electrode on Battery Performance" Energies 12, no. 4: 658. https://doi.org/10.3390/en12040658





