Fabrication and Performance Evaluation of Six-Cell Two-Dimensional Configuration Solid Oxide Fuel Cell Stack Based on Planar 6 × 6 cm Anode-Supported Cells
Abstract
:1. Introduction
2. Materials and Methods
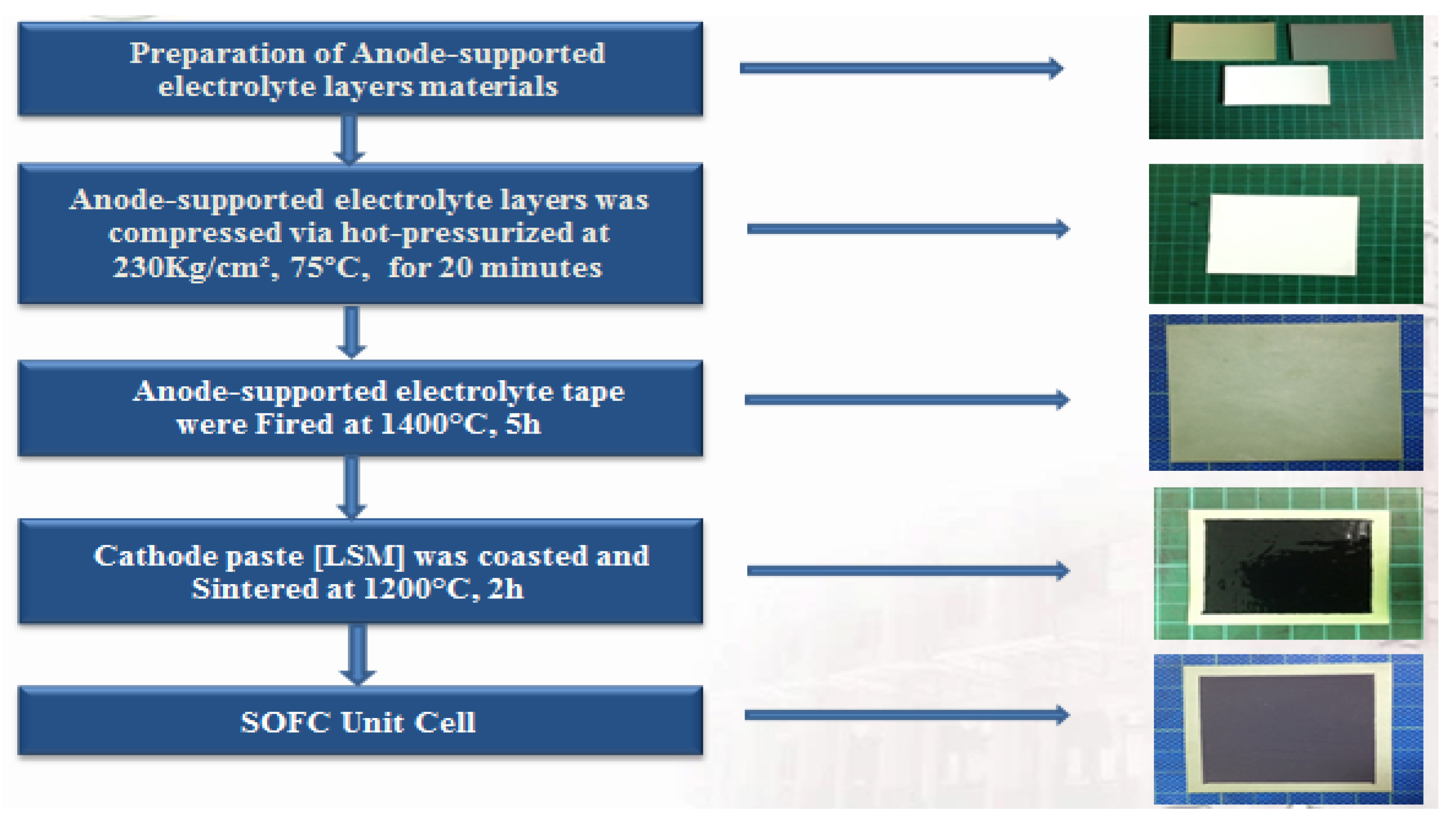
2.1. Anode-Supported Cell Fabrication
2.2. Manifold Design
2.3. Interconnected Design
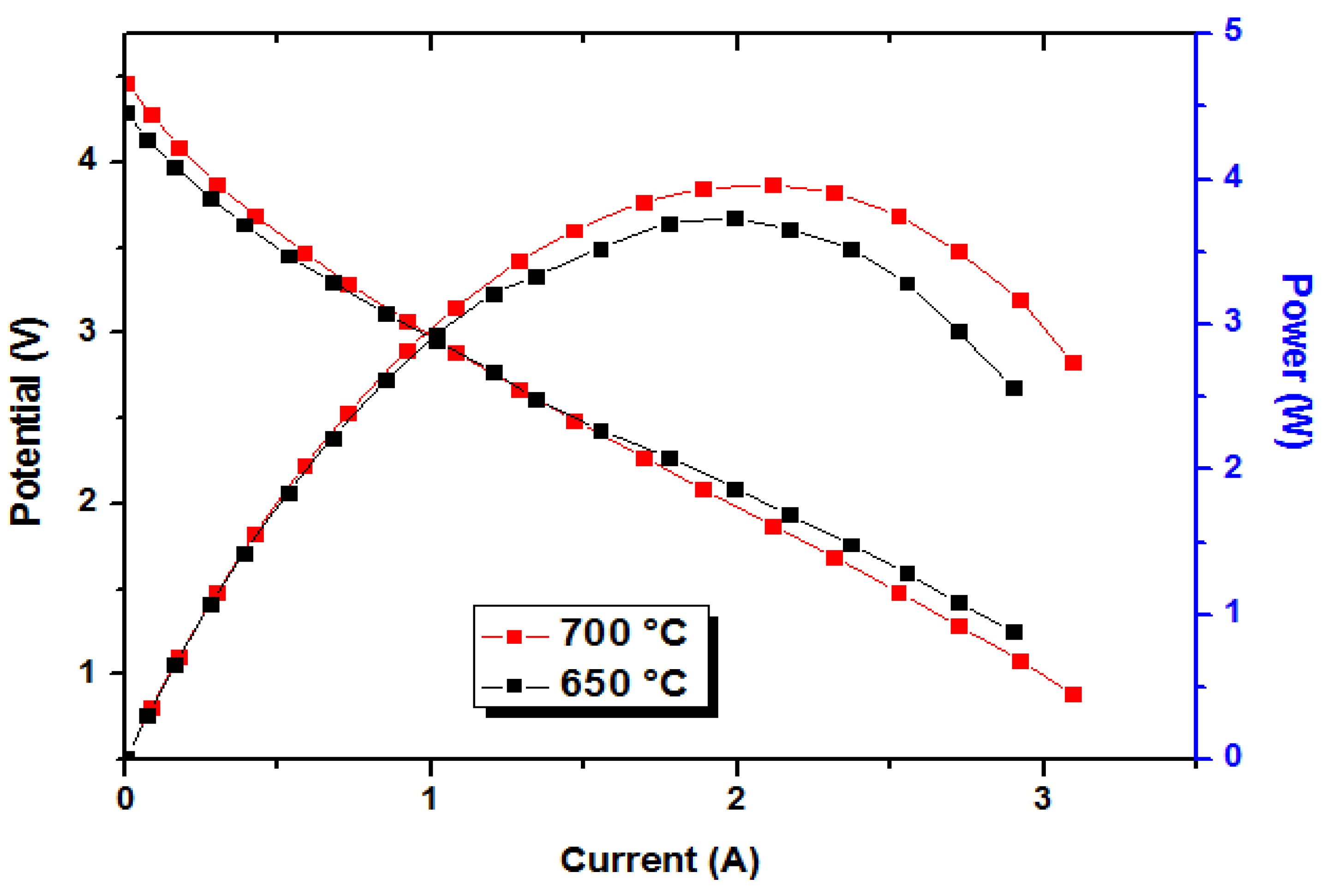
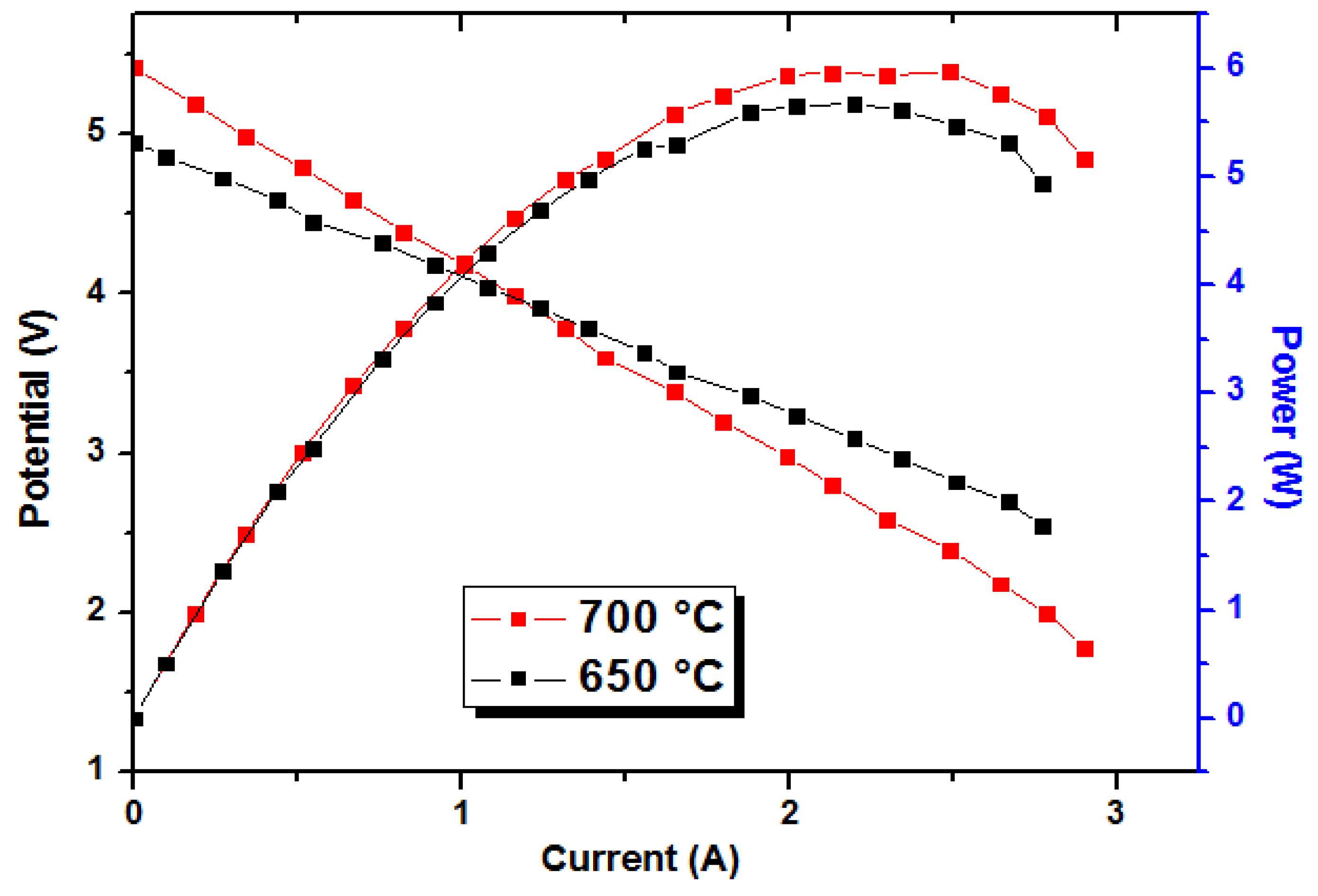
2.4. Cell Performance Testing
3. Results and Discussion
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, Q.M. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar]
- Andu, J.M.; Segura, F. Fuel cells: History and updating. A walk along two centuries. Renew. Sustain. Energy Rev. 2009, 13, 2309–2322. [Google Scholar]
- Nguyen, X.V.; Chang, C.T.; Jung, G.B.; Chan, S.H.; Yeh, C.C.; Yu, J.W.; Lee, C.Y. Improvement on the design and fabrication of planar SOFCs with anode–supported cells based on modified button cells. Renew. Energy 2018, 129, 806–813. [Google Scholar] [CrossRef]
- Chung, B.; Chervin, C.; Haslam, J.; Pham, A.; Glass, R. Development and characterization of a high performance thin-film planar SOFC stack. J. Electrochem. Soc. 2005, 152, 265–269. [Google Scholar] [CrossRef]
- Mizutani, Y.; Hisada, K.; Ukai, K.; Sumi, H.; Yokoyama, M.; Nakamura, Y.; Yamamoto, O. From rare earth doped zirconia to 1 kW solid oxide fuel cell system. J. Alloy. Compd. 2006, 408–412, 518–524. [Google Scholar] [CrossRef]
- Shibata, M.; Murakami, N.; Akbay, T.; Eto, H.; Hosoi, K.; Nakajima, H.; Kano, J.; Nishiwaki, F.; Inagaki, T.; Yamasaki, S. Development of intermediate−temperature SOFC modules and systems. ECS Trans. 2007, 7, 77–83. [Google Scholar]
- Yoshida, H.; Yakabe, H.; Ogasawara, K.; Sakurai, T. Development of envelope-type solid oxide fuel cell stacks. J. Power Sources 2006, 157, 775–781. [Google Scholar] [CrossRef]
- Baschuk, J.J.; Li, X. Modelling of polymer electrolyte membrane fuel cell stacks based on a hydraulic network approach. Int. J. Energy Res. 2004, 28, 697–724. [Google Scholar] [CrossRef]
- Jiang, R.; Chu, D. Stack design and performance of polymer electrolyte membrane fuel cells. J. Power Sources 2001, 93, 25–31. [Google Scholar] [CrossRef]
- Scholta, J.; Rohland, B.; Trapp, V.; Focken, U. Investigations on novel low−cost graphite composite bipolar plates. J. Power Sources 1999, 84, 231–234. [Google Scholar] [CrossRef]
- Kim, H.; Jung, H.Y.; Jung, H.G.; Kim, J.; Lee, J.H.; Lee, H.W.; Song, H. Fabrication and performance evaluation of multi−cell arrayed planar SOFC stack. ECS Trans. 2007, 7, 311–316. [Google Scholar]
- Ferrari, M.L. Solid oxide fuel cell hybrid system: Control strategy for stand−alone configurations. J. Power Sources 2011, 196, 2682–2690. [Google Scholar] [CrossRef]
- Zaccaria, V.; Tucker, D.; Traverso, A. Operating strategies to minimize degradation in fuel cell gas turbine hybrids. Appl. Energy 2017, 192, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Sharifzadeh, M.; Meghdari, M.; Rashtchian, D. Multi−objective design and operation of Solid Oxide Fuel Cell (SOFC) triple−combined−cycle power generation systems: Integrating energy efficiency and operational safety. Appl. Energy 2017, 185, 345–361. [Google Scholar] [CrossRef]
- Vakouftsi, E.; Marnellos, G.E.; Athanasiou, C.; Coutelieris, F. CFD modeling of a biogas fuelled SOFC. Solid State Ion. 2011, 192, 458–463. [Google Scholar] [CrossRef]
- Park, J.; Li, P.; Bae, J. Analysis of chemical, electrochemical reactions and thermo−fluid flow in methane−feed internal reforming SOFCs: Part II−temperature effect. Int. J. Hydrogen Energy 2012, 37, 8532–8555. [Google Scholar] [CrossRef]
- Djamel, H.; Hafsia, A.; Bariza, Z.; Hocine, B.M.; Kafia, O. Thermal field in SOFC fed by hydrogen: Inlet gases temperature effect. Int. J. Hydrogen Energy 2013, 38, 8575–8583. [Google Scholar] [CrossRef]
- Khani, L.; Mehr, A.S.; Yari, M.; Mahmoudi, S.M.S. Multi−objective optimization of an indirectly integrated solid oxide fuel cell−gas turbine cogeneration system. Int. J. Hydrogen Energy 2016, 41, 21470–21488. [Google Scholar] [CrossRef]
- Eveloy, V.; Karunkeyoon, W.; Rodgers, P.; Al Alili, A. Energy, exergy and economic analysis of an integrated solid oxide fuel cell gas turbineeorganic Rankine power generation system. Int. J. Hydrogen Energy 2016, 41, 13843–13858. [Google Scholar] [CrossRef]
- Azizi, M.A.; Brouwer, J. Progress in solid oxide fuel cell−gas turbine hybrid power systems: System design and analysis, transient operation, controls and optimization. Appl. Energy 2018, 215, 237–289. [Google Scholar] [CrossRef]
- Buonomano, A.; Calise, F.; d’Accadia, M.D.; Palombo, A.; Vicidomini, M. Hybrid solid oxide fuel cells–gas turbine systems for combined heat and power: A review. Appl. Energy 2015, 156, 32–85. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Nguyen, X.V.; Chang, C.T.; Jung, G.B.; Chan, S.H.; Huang, W.C.W.; Hsiao, K.J.; Lee, W.T.; Chang, S.W.; Kao, I.C. Effect of sintering temperature and applied load on anode-supported electrodes for SOFC application. Energies 2016, 9, 701. [Google Scholar] [CrossRef]





© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.-V. Fabrication and Performance Evaluation of Six-Cell Two-Dimensional Configuration Solid Oxide Fuel Cell Stack Based on Planar 6 × 6 cm Anode-Supported Cells. Energies 2019, 12, 3541. https://doi.org/10.3390/en12183541
Nguyen X-V. Fabrication and Performance Evaluation of Six-Cell Two-Dimensional Configuration Solid Oxide Fuel Cell Stack Based on Planar 6 × 6 cm Anode-Supported Cells. Energies. 2019; 12(18):3541. https://doi.org/10.3390/en12183541
Chicago/Turabian StyleNguyen, Xuan-Vien. 2019. "Fabrication and Performance Evaluation of Six-Cell Two-Dimensional Configuration Solid Oxide Fuel Cell Stack Based on Planar 6 × 6 cm Anode-Supported Cells" Energies 12, no. 18: 3541. https://doi.org/10.3390/en12183541




