Desert Palm Date Seeds as a Biodiesel Feedstock: Extraction, Characterization, and Engine Testing
Abstract
:1. Introduction
2. Materials and Methods
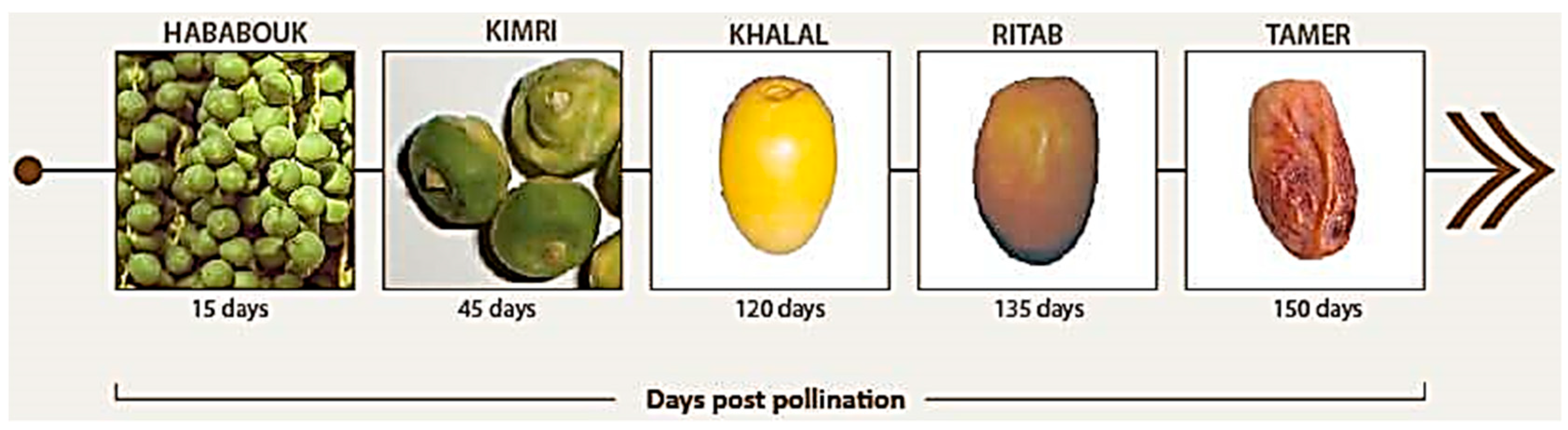
2.1. Date Seed Collection and Characterization
2.2. DSO Extraction
2.3. DSO Characterization and Evaluation
2.4. DSO Transesterification
2.5. Engine Test Rig
3. Results and Discussion
3.1. DSO Biodiesel Characterization and Evaluation
3.1.1. Chemical Element Content
3.1.2. Cetane Number
3.1.3. Cloud Point
3.1.4. Flash Point
3.1.5. Water and Sediment
3.1.6. Viscosity
3.1.7. Sulfated Ash
3.1.8. Carbon Residue
3.1.9. Distillation
3.1.10. Acidity
3.1.11. Stability
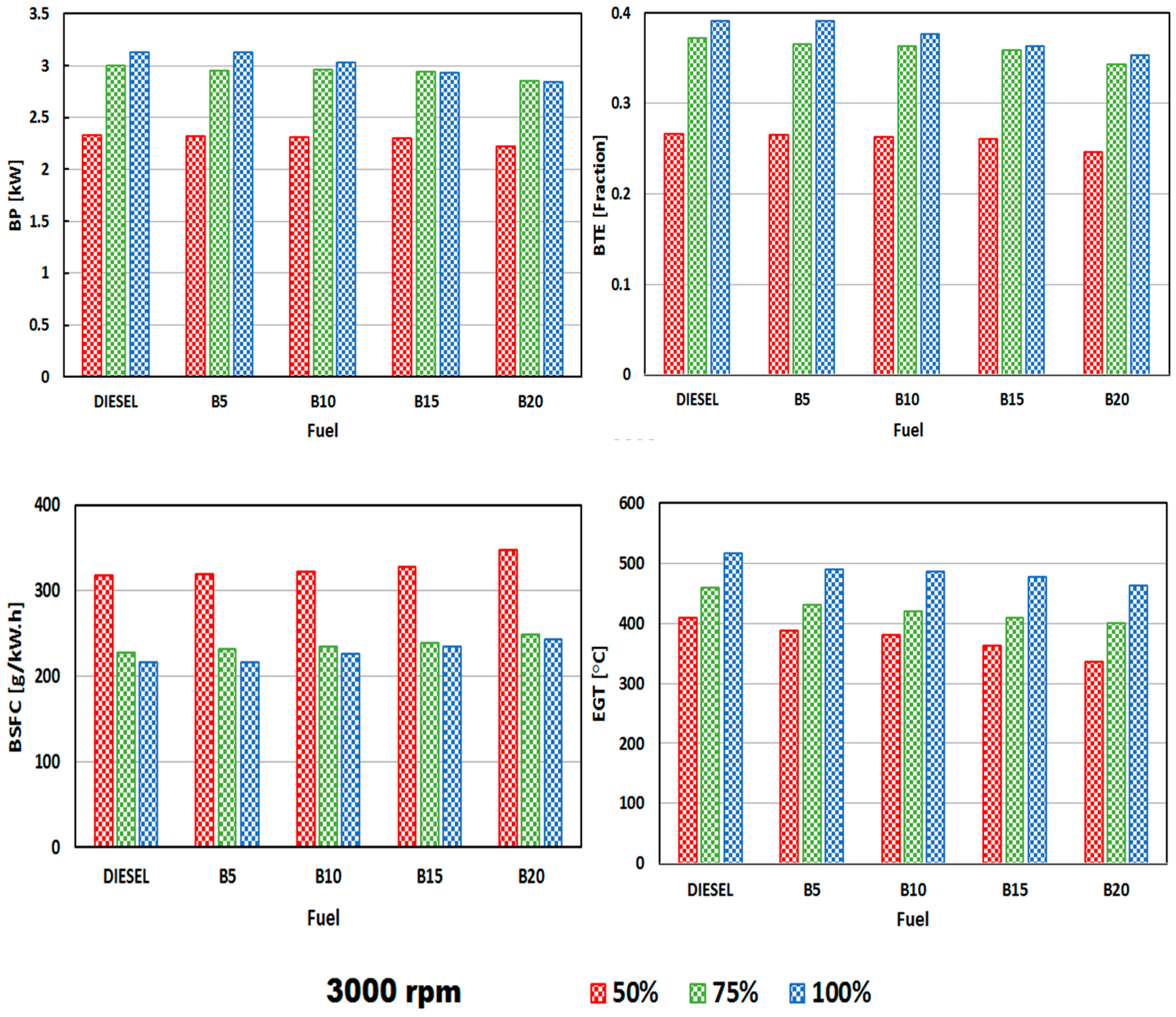
3.2. Engine Performance
3.3. Engine Tailpipe Emissions
4. Conclusions
- (1)
- At the same temperature, time, and solvent to seed ratios, hexane consistently outperformed petroleum ether in oil yields. The highest oil extraction (10.74 wt.%) was observed with hexane at 60 °C, 4 h extraction time, and a solvent:seed ratio of 6.
- (2)
- A DSO biodiesel yield of 92% was recorded at the following transesterification conditions: 55 °C, 9:1 AOMR, 1 wt.% CMf, and 90 min.
- (3)
- Phosphoric acid treatment of the DSO reduced levels of Mg, Ca, and P in the biodiesel to the ASTM D6751 allowable level.
- (4)
- The cloud point of the DSO was relatively high (9.4 °C), so the use of cold flow improvers would be necessary when this fuel is used in cold climates.
- (5)
- The BP, BTE, and BSFC values of the DSO biodiesel blends were comparable with the baseline diesel, though the latter was superior.
- (6)
- All biodiesel blends produced lower levels of CO2, CO, and HC emissions.
- (7)
- All biodiesel blends produced higher levels of NOx emissions.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kontorovich, A.E. Estimate of Global Oil Resource and the Forecast for Global Oil Production in the 21st Century. Russ. Geol. Geophys. 2009, 50, 237–242. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S.; Kanapkienė, I.; Labeckas, G.; Slavinskas, S.; Kanapkienė, I. Study of the effects of Biofuel-Oxygen of various origins on a CRDI diesel engine combustion and emissions. Energies 2019, 12, 1241. [Google Scholar] [CrossRef]
- Arunkumar, M.; Kannan, M.; Murali, G. Experimental Studies on Engine Performance and Emission Characteristics Using Castor Biodiesel as Fuel in CI Engine. Renew. Energy 2019, 131, 737–744. [Google Scholar] [CrossRef]
- Hirner, F.S.; Hwang, J.; Bae, C.; Patel, C.; Gupta, T.; Agarwal, A.K. Performance and emission evaluation of a small-bore biodiesel compression-ignition engine. Energy 2019, 183, 971–982. [Google Scholar] [CrossRef]
- Scheffran, J. The Global Demand for Biofuels: Technologies, Markets and Policies. In Biomass to Biofuels: Strategies for Global Industries; Vertes, A.A., Qureshi, N., Yukawa, H., Blaschek, H.P., Eds.; Wiley: West Sussex, UK, 2010; pp. 141–165. [Google Scholar]
- Luque, R.; Lin, C.S.K.; Wilson, K.; Clark, J.H. Handbook of Biofuels Production: Processes and Technologies, 2nd ed.; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental Impacts of Biodiesel Production from Waste Spent Coffee Grounds and Its Implementation in a Compression Ignition Engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M.; Choi, S.; Shin, J.; Park, C.; Cho, S.-K.; Kim, Y.M.; Lee, M.; Yang, M.; Choi, S.; et al. Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process. Energies 2019, 12, 2360. [Google Scholar] [CrossRef]
- Azeem, M.W.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of Biodiesel from Low Priced, Renewable and Abundant Date Seed Oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]
- Akasha, I.A.M. Extraction and Characterisation of Protein Fraction from Date Palm (Phoenix dactylifera L.) Seeds. Ph.D. Thesis, Heriot-Watt University, Edinburgh, Scotland, 2004. [Google Scholar]
- Johnson, D.V.; Al-Khayri, J.M.; Jain, S.M. Introduction: Date Production Status and Prospects in Africa and the Americas. In Date Palm Genetic Resources and Utilization; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 3–18. [Google Scholar]
- Al-Yahyai, R.; Manickavasagan, A. An Overview of Date Palm Production. In Dates Production, Processing, Food, and Medicinal Values; Manickavasagan, A., Mohamed Essa, M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 3–12. [Google Scholar]
- Mrabet, A.; Ferchichi, A.; Chaira, N.; Mohamed, B.S.; Baaziz, Z.; Penny, T.M. Physico-Chemical Characteristics and Total Quality of Date Palm Varieties Grown in the Southern of Tunisia. Pak. J. Biol. Sci. 2008, 11, 1003–1008. [Google Scholar] [CrossRef]
- Al-shahib, W.; Marshall, R.J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations Organization, FAO. FAO Statistical Year Book, World Food and Agriculture. Available online: http://www.fao:faostat/en/#data/QC (accessed on 22 August 2018).
- Atia, A.; Abdelkarim, D.; Younis, M.; Alhamdan, A. Effects of Gibberellic Acid (GA3) and Salicylic Acid (SA) Postharvest Treatments on the Quality of Fresh Barhi Dates at Different Ripening Levels in the Khalal Maturity Stage during Controlled Atmosphere Storage. Int. J. Agric. Biol. Eng. 2018, 11, 211–219. [Google Scholar] [CrossRef]
- Afiq, A.; Rahman, A.; Man, C. Date Seed and Date Seed Oil. Int. Food Res. J. 2013, 20, 2035–2043. [Google Scholar]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and Functional Characteristics of Dates, Syrups, and Their by-Products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Besbes, S.; Drira, L.; Blecker, C.; Deroanne, C.; Attia, H. Adding value to hard date (Phoenix dactylifera L.): Compositional, functional and sensory characteristics of date jam. Food Chem. 2009, 112, 406–411. [Google Scholar] [CrossRef]
- The British Petroleum Company, BP. BP Statistical Review of World Energy, June 2017. Available online: https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review-2017/bp-statistical-review-of-world-energy-2017-full-report.pdf (accessed on 23 August 2018).
- Besbes, S.; Blecker, C.; Deroanne, C.; Drira, N.-E.; Attia, H. Date Seeds: Chemical Composition and Characteristic Profiles of the Lipid Fraction. Food Chem. 2004, 84, 577–584. [Google Scholar] [CrossRef]
- Vandepopuliere, J.M.; Al-Yousef, Y.; Lyons, J.J. Dates and date pits as ingredients in broiler starting and Coturnix quail breeder diets. Poult. Sci. 1995, 74, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.A.; Lee, C.Y. Usage of Date (Phoenix dactylifera L.) Seeds in Human Health and Animal Feed. Nuts Seeds Health Dis. Prev. 2011. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kasapis, S.; Al-Kharusi, N.S.Z.; Al-Marhubi, I.M.; Khan, A.J. composition characterisation and thermal transition of date pits powders. J. Food Eng. 2007, 80, 1–10. [Google Scholar] [CrossRef]
- Ali-Mohamed, A.Y.; Khamis, A.S.H. Mineral Ion Content of the Seeds of Six Cultivars of Bahraini Date Palm (Phoenix dactylifera). J. Agric. Food Chem. 2004, 52, 6522–6525. [Google Scholar] [CrossRef]
- Devshony, S.; Eteshola, E.; Shani, A. Characteristics and Some Potential App.lications of Date Palm (Phoenix dactylifera L.) Seeds and Seed Oil. J. Am. Oil Chem. Soc. 1992, 69, 595–597. [Google Scholar] [CrossRef]
- Jamil, F.; Al-Muhtaseb, A.H.; Al-Haj, L.; Al-Hinai, M.A.; Hellier, P.; Rashid, U. Optimization of oil extraction from waste “Date Pits” for biodiesel production. Energy Convers. Manag. 2016, 117, 264–272. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Taher, H.; Al Dhaheri, S.; Wajeeh, S.; Nour, M.; El-Najjar, E. Biodiesel production from oils extracted from date pits. Green Sustain. Chem. 2017, 7, 48. [Google Scholar] [CrossRef]
- Nehdi, I.; Omri, S.; Khalil, M.I.; Al-Resayes, S.I. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crops Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Hattab, T.A.; Al-hydary, I.A.D. Extraction of date palm seed oil (Phoenix dactylifera) by soxhlet app.aratus. Int. J. Adv. Eng. Technol. 2015, 8, 261. [Google Scholar]
- Yousuf, R.G.; Winterburn, J.B. Waste date seed oil extract as an alternative feedstock for poly (3–Hydroxybutyrate) Synthesis. Biochem. Eng. J. 2017, 127, 68–76. [Google Scholar] [CrossRef]
- Akaagerger, S.M.; Giwa, S.O.; Ibrahim, M.; GIWA, A. Production of biodiesel from desert date seed oil. Int. J. ChemTech Res. 2016, 9, 453–463. [Google Scholar]
- Farooq, M.; Ramli, A.; Naeem, A.; Mahmood, T.; Ahmad, S.; Humayun, M.; Islam, M.G.U. Biodiesel production from date seed oil (Phoenix dactylifera L.) via egg shell derived heterogeneous catalyst. Chem. Eng. Res. Des. 2018, 132, 644–651. [Google Scholar] [CrossRef]
- Knothe, G. The Biodiesel Handbook, 1st ed.; Knothe, G., Krahl, J., Gerpen, J.V., Eds.; AOCS Press: Champaign, IL, USA, 2005. [Google Scholar]
- Canakci, M.; Van Gerpen, J. Biodiesel Production from Oils and Fats with High Free Fatty Acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel Processing and Production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Nitayaphat, W.; Jintakosol, T.; Engkaseth, K. Removal of methylene blue from aqueous solution by coffee residues. Chiang Mai J. Sci. 2015, 42, 407–416. [Google Scholar]
- American Society for Testing and Materials, ASTM. ASTM Standard D6751, Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Hansen, A.C.; Kyritsis, D.C.; Lee, C.f.F. Characteristics of Biofuels and Renewable Fuel Standards. In Biomass to Biofuels: Strategies for Global Industries; Vertes, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; Wiley: West Sussex, UK, 2010; pp. 1–26. [Google Scholar]
- Van Gerpen, J. Biodiesel from Vegetable Oils. In Biomass to Biofuels: Strategies for Global Industries; Vertes, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; Wiley: West Sussex, UK, 2010; pp. 141–163. [Google Scholar]
- Lomonaco, D.; Maia, F.J.N.; Clemente, C.S.; Mota, J.P.F.; Costa, A.E.; Mazzetto, S.E. Thermal Studies of New Biodiesel Antioxidants Synthesized from a Natural Occurring Phenolic Lipid. Fuel 2012, 97, 552–559. [Google Scholar] [CrossRef]
- Suda, K.J. Vegetable Oil or Diesel Fuel-A Flexible Option; SAE Paper 840004; SAE International: Warrendale, PA, USA, 1984. [Google Scholar]
- Barsic, N.J.; Humke, A.L. Performance and Emissions Characteristics of a Naturally Aspirated Diesel Engine with Vegetable Oil Fuels; SAE Paper 810262; SAE International: Warrendale, PA, USA, 1981. [Google Scholar]
- Espinosa, E.A.M.; Rodríguez, R.P.; Sierens, R.; Verhelst, S. Emulsification of Waste Cooking Oils and Fatty Acid Distillates as Diesel Engine Fuels: An Attractive Alternative. Int. J. Sustain. Energy Plan. Manag. 2016, 9, 3–16. [Google Scholar] [CrossRef]
- Hawkins, C.S.; Fuls, J. Comparative Combustion Studies on Various Plants Oil Esters and the Long Term Effects of an Ethyl Ester on a Compression Ignition Engine. In Proceedings of the International Conference on Plant and Vegetable Oils as Fuels, Fargo, ND, USA, 23–26 September 1982; pp. 312–328. [Google Scholar]
- Zhou, W.; Konar, S.K.; Boocock, D.G.B. Ethyl Esters from the Single-Phase Base-Catalyzed Ethanolysis of Vegetable Oils. J. Am. Oil Chem. Soc. 2003, 80, 367–371. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S.; Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Lee, J.-S.; Saka, S. Biodiesel Production by Heterogeneous Catalysts and Supercritical Technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H. A Critical Review of Bio-Diesel as a Vehicular Fuel. Energy Convers. Manag. 2008, 49, 2727–2741. [Google Scholar] [CrossRef]
- Musa, I.A. The Effects of Alcohol to Oil Molar Ratios and the Type of Alcohol on Biodiesel Production Using Transesterification Process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel Fuel Production by Transesterification of Oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel Fuel from Rapeseed Oil as Prepared in Supercritical Methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification Kinetics of Soybean Oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Sanford, S.; White, J.; Shah, P. Feedstock and Biodiesel Characteristics Report; Renewable Energy Group: Ames, IA, USA, 2009. [Google Scholar]
- Lee, I.; Johnson, L.A.; Hammond, E.G. Use of Branched-Chain Esters to Reduce the Crystallization Temperature of Biodiesel. J. Am. Oil Chem. Soc. 1995, 72, 1155–1160. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of Blending Canola, Palm, Soybean, and Sunflower Oil Methyl Esters on Fuel Properties of Biodiesel. Energy Fuels 2008, 22, 4301–4306. [Google Scholar] [CrossRef]
- Bhale, P.V.; Deshpande, N.V.; Thombre, S.B. Improving the Low Temperature Properties of Biodiesel Fuel. Renew. Energy 2009, 34, 794–800. [Google Scholar] [CrossRef]
- Fu, J. Flash Points Measurements and Prediction of Biofuels and Biofuel Blends with Aromatic Fluids. Fuel 2019, 241, 892–900. [Google Scholar] [CrossRef]
- Bazooyar, B.; Ghorbani, A.; Shariati, A. Physical Properties of Methyl Esters Made from Alkali-Based Transesterification and Conventional Diesel Fuel. Energy Sources Part A Recover. Util. Environ. Eff. 2015, 37, 468–476. [Google Scholar] [CrossRef]
- Ayetor, G.K.; Sunnu, A.; Parbey, J. Effect of Biodiesel Production Parameters on Viscosity and Yield of Methyl Esters: Jatropha Curcas, Elaeis Guineensis and Cocos Nucifera. Alex. Eng. J. 2015, 54, 1285–1290. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel Properties and Emission Characteristics of Biodiesel Produced from Unused Algae Grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Al-Otoom, A.; Allawzi, M.; Ajlouni, A.; Abu-Alrub, F.; Kandah, M. The Use of Oil Shale Ash in the Production of Biodiesel from Waste Vegetable Oil. J. Renew. Sustain. Energy 2012, 4, 063123. [Google Scholar] [CrossRef]
- Barbieri Gonzaga, F.; Pereira Sobral, S. A New Method for Determining the Acid Number of Biodiesel Based on Coulometric Titration. Talanta 2012, 97, 199–203. [Google Scholar] [CrossRef]
- Aydin, H.; Bayindir, H. Performance and Emission Analysis of Cottonseed Oil Methyl Ester in a Diesel Engine. Renew. Energy 2010, 35, 588–592. [Google Scholar] [CrossRef]
- Luján, J.M.; Bermúdez, V.; Tormos, B.; Pla, B. Comparative analysis of a di diesel engine fuelled with biodiesel blends during the European MVEG-A Cycle: Performance and Emissions (II). Biomass Bioenergy 2009, 33, 948–956. [Google Scholar] [CrossRef]
- Özener, O.; Yüksek, L.; Ergenç, A.T.; Özkan, M. Effects of soybean biodiesel on a di diesel engine performance, emission and combustion characteristics. Fuel 2014, 115, 875–883. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Li, R.-J. Engine performance and emission characteristics of marine fish-oil biodiesel produced from the discarded parts of marine fish. Fuel Process. Technol. 2009, 90, 883–888. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M.; Lim, H.C. A study on the performance and emission of a diesel engine fueled with karanja biodiesel and its blends. Energy 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]







| Combination Identifier | Solvent | Solvent: Ground Seed Ratio [wt/wt] | Temperature [°C] | Time [h] | Ground Particle Size [mm] | Highest Yield [wt.%] | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Hexane | 16.37 | 80 | 12 | 2.36 | 11.7 | [28] |
| 2 | Hexane | 16.37 | 80 | 12 | 0.5 | ||
| 3 * | Hexane | 16.37 | 80 | 12 | 0.35 | ||
| 1 | Hexane | 2 | 50 | 5 | 0.25–0.3 | 16.5 | [27] |
| 2 | Hexane | 3 | 60 | 6 | 0.25–0.3 | ||
| 3 * | Hexane | 4 | 70 | 7 | 0.25–0.3 | ||
| 1 * | Petroleum ether | 6.53 | 40–60 | 1.5 | 1–2 | 12.67 | [21] |
| 1 * | Hexane | 5.24 | 40–50 | 8 | 1 | 10.36 | [29] |
| 1 | Methanol | 3.94 | 50 | 1 | 0.45 | 8.5 | [30] |
| 2 | 2-opropanol | 3.925 | 50 | 2 | 1 | ||
| 3 | Chloroform | 7.5 | 50 | 4 | 1.2 | ||
| 4 * | Hexane | 3.27 | 50 | 6 | 0.45 | ||
| 5 | Toluene | 4.33 | 50 | 4 | 0.45 | ||
| 1 * | Chloroform/methanol | 11.43 | 160 | 0.5–4 | 4 | 9.3 | [31] |
| 2 | Hexane | 6.55 | 120 | 0.5–4 | 4 | ||
| 3 | Petroleum ether | 6.53 | 100 | 0.5–4 | 4 | ||
| 1 * | Hexane | 16.37 | 55 | 4 | 0.6 | 4.5 | [32] |
| 2 | Hexane | 16.37 | 55 | 7 | 1.8 |
| Alcohol | Catalyst | Alcohol: Oil Molar Ratio | CMF % [wt./wt.] | Time [h] | Temp. [C] | Highest Yield [wt.%] | Ref. |
|---|---|---|---|---|---|---|---|
| Methanol | HCL | 6 | 25 a, 50 a, 100 a,* | 1.5 | 60 | 61 | [9] |
| KOH | 3 | 0.125, 0.25 *, 0.5 | 1.5 | 60 | 80.5 | ||
| Immobilized enzyme | 5 | 3, 4 *, 5 | 10 | 40 | 80 | ||
| Methanol | NaOH | 3, 4 *, 5 | 0.3 *, 0.5 | 0.5, 0.75, 1, 1.25 * | 45, 55, 65, 75 * | 91 | [32] |
| Methanol | NaOH | 5 | 10 | 6 | 40 | 30 | [28] |
| Methanol | KOH | 6 | 1 | 1 | 65 | [27] | |
| Methanol | Synthesized catalysts | 12 | 5 | 1.5 | 93.5 | [33] |
| Component | Amount |
|---|---|
| Dry matter (dm), [%] | |
| Oil in dm, [%] | |
| Ash in dm, [%] | |
| Potassium, [mg/100 g dm] | |
| Phosphorus, [mg/100 g dm] | |
| Magnesium, [mg/100 g dm] | |
| Calcium, [mg/100 g dm] | |
| Sodium, [mg/100 g dm] | |
| Iron, [mg/100 g dm] |
| Property | Unit | Value |
|---|---|---|
| Density @ 40 | 897.4 | |
| Viscosity @ 40 | 33.14 | |
| Higher heating value (HHV) | 32.63 | |
| Moisture | wt.% | 0.022 |
| Magnesium | ppm | 2.82 |
| Calcium | ppm | 6.26 |
| Sulphur | ppm | 2.14 |
| Phosphorous | ppm | 14.16 |
| Free fatty acids | % | 0.42 |
| Acid numbers (AcNo) | mgKOH/g | 0.29 |
| Saponification number (SaNo) | mgKOH/g | 194.56 |
| Unsaponifiable fraction | % | 0.25 |
| FA | Structure | Content (%) | Molecular Formula | Molar Mass (g/mole) |
|---|---|---|---|---|
| Linoleic acid | C18:2 | 9.95 | C18H32O2 | 280.45 |
| Palmitic acid | C16:0 | 12.01 | C16H32O2 | 256.43 |
| Oleic acid | C18:1 | 44.73 | C18H34O2 | 282.47 |
| Arachidic acid | C20:0 | 0.21 | C20H40O2 | 312.54 |
| linolenic acid | C18:3 | 0.11 | C18H30O2 | 278.44 |
| Behenic acid | C22:0 | 0.46 | C22H44O2 | 340.59 |
| Lignoceric acid | C24:0 | 0.24 | C24H48O2 | 368.65 |
| Stearic acid | C18:0 | 4.54 | C18H36O2 | 284.48 |
| Erucic acid | C22:1 | 0.38 | C22H42O2 | 338.58 |
| Myristic acid | C14:0 | 10.14 | C14H28O2 | 228.38 |
| Palmitoleic acid | C16:1 | 0.09 | C16H30O2 | 254.41 |
| Lauric acid | C12:0 | 16.14 | C12H24O2 | 200.32 |
| Caprylic acid | C8:0 | 0.34 | C8H16O2 | 144.21 |
| Capric acid | C10:0 | 0.47 | C10H20O2 | 172.27 |
| Specifications | Unit | Description/Value |
|---|---|---|
| Engine model | Lombardini 15-LD-225 | |
| Bore | mm | 69 |
| Stroke | mm | 60 |
| Displacement volume | L | 0.224 |
| Compression ratio | 21:1 | |
| Rated power @ 3600 rpm | kW | 3.5 |
| Rated torque @ 2400 rpm | N·m | 10.4 |
| Intake valve opening | Crank angle BTDC | 6 |
| Intake valve closing | Crank angle ABDC | 22° |
| Exhaust valve opening | Crank angle BBDC | 58° |
| Exhaust valve closing | Crank angle ATDC | 10° |
| Blend | Lower Heating Value [MJ/kg] | Density [kg/m3] | Viscosity [mm2/s] | Cetane Number | C/H Mass Ratio | Oxygen Content [wt.%] |
|---|---|---|---|---|---|---|
| B5 | 43.6 | 839.1 | 2.7 | 51.6 | 6.2 | 0.6 |
| B10 | 43.4 | 842.2 | 2.8 | 52.1 | 6.1 | 1.2 |
| B15 | 43.2 | 845.3 | 2.9 | 52.7 | 6.1 | 1.8 |
| B20 | 43 | 848.4 | 3.0 | 53.2 | 6.0 | 2.5 |
| Exhaust Gas | Accuracy | Display Resolution | Range |
|---|---|---|---|
| CO2 [%] | 0.3 | 0.1 | 0–20 |
| CO [%] | 0.06 | 0.01 | 0–10 |
| NOx [ppm] | 25 | 1 | 0–5000 |
| HC [ppm] | 4 | 1 | 0–2000 |
| Property | Units | Standard | DSO Biodiesel | ASTM D975 Diesel | ASTM D6751 Biodiesel |
|---|---|---|---|---|---|
| Sulfur | ppm ( | ASTM D 5453 | 0.93 | 1D and 2D: S15 15 mg/kg S500 0.05% S5000 0.50% | 15 ppm for S15 grade; 500 ppm for S500 grade |
| Fuel Filter Blocking Potential/Cold Soak Filterability Seconds | s | ASTM D 7501 | - | 200 | |
| Monoglyceride content | % weight | EN 14105 | - | 0.70% max | |
| Calcium & Magnesium combined | mg/kg | EN 14538 | 3.27 | 5.0 | |
| Flash Point | ASTM D 93 | 164 | 1D: 38 2D: 52 | 130 min | |
| Water & sediment | % volume | ASTM D 2709 | 0.019 | 0.05% max | 0.050% max |
| Kinematic viscosity | ASTM D 445 | 4.38 | 1D: 1.3–2.4 2D: 1.9–4.1 | 1.9–6.0 | |
| Sulphated ash | % mass | ASTM D 874 | < 0.02 | 0.020% max | |
| Copper strip corrosion | ASTM D 130 | 1a | No. 3 max | No. 3 max | |
| Cetane number | ASTM D 613 | 62 | 40 min | 47 min | |
| Cloud point | ASTM D 2500 | +9.4 | Report | ||
| Carbon residue | % weight | ASTM D 4530 | 0.023 | 1D: 0.15% max 2D: 0.35% max | 0.050% max |
| Acid number | Mg KOH | ASTM D 664 | 0.29 | 0.5 max | |
| Free glycerine | % weight | EN 14105 | - | 0.02% max | |
| Total glycerine | % weight | EN 14105 | - | 0.25% max | |
| Phosphorous content | % mass | ASTM D 4951 | 0.0002 | 0.001% max | |
| Distillation 90% recovered | ASTM D 1160 | 352.4 | 1D: 288 max 2D 282-338°C | 360 max | |
| 2D: 282–338 | |||||
| Sodium & potassium combined | ppm ( | EN 14538 | 3.2 | 5 max | |
| Oxidation stability | Hr @ 110 | EN 15751 | 7.4 | 6 min |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamil, M.; Ramadan, K.; Olabi, A.G.; Ghenai, C.; Inayat, A.; Rajab, M.H. Desert Palm Date Seeds as a Biodiesel Feedstock: Extraction, Characterization, and Engine Testing. Energies 2019, 12, 3147. https://doi.org/10.3390/en12163147
Kamil M, Ramadan K, Olabi AG, Ghenai C, Inayat A, Rajab MH. Desert Palm Date Seeds as a Biodiesel Feedstock: Extraction, Characterization, and Engine Testing. Energies. 2019; 12(16):3147. https://doi.org/10.3390/en12163147
Chicago/Turabian StyleKamil, Mohammed, Khalid Ramadan, Abdul Ghani Olabi, Chaouki Ghenai, Abrar Inayat, and Mugdad H. Rajab. 2019. "Desert Palm Date Seeds as a Biodiesel Feedstock: Extraction, Characterization, and Engine Testing" Energies 12, no. 16: 3147. https://doi.org/10.3390/en12163147






