Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
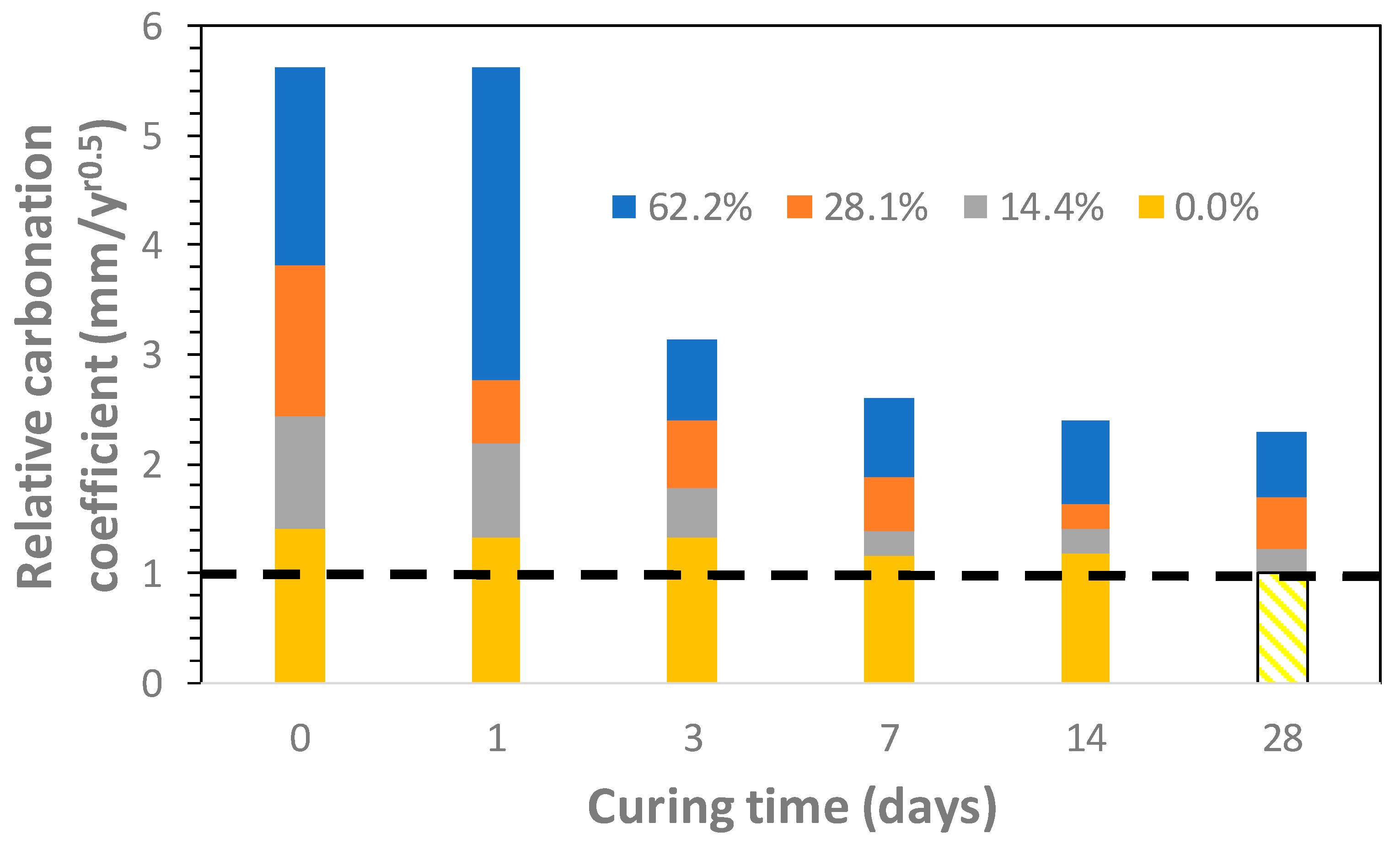
3.1. CO2 Uptake and Carbonation Coefficient
3.2. Compressive Strength
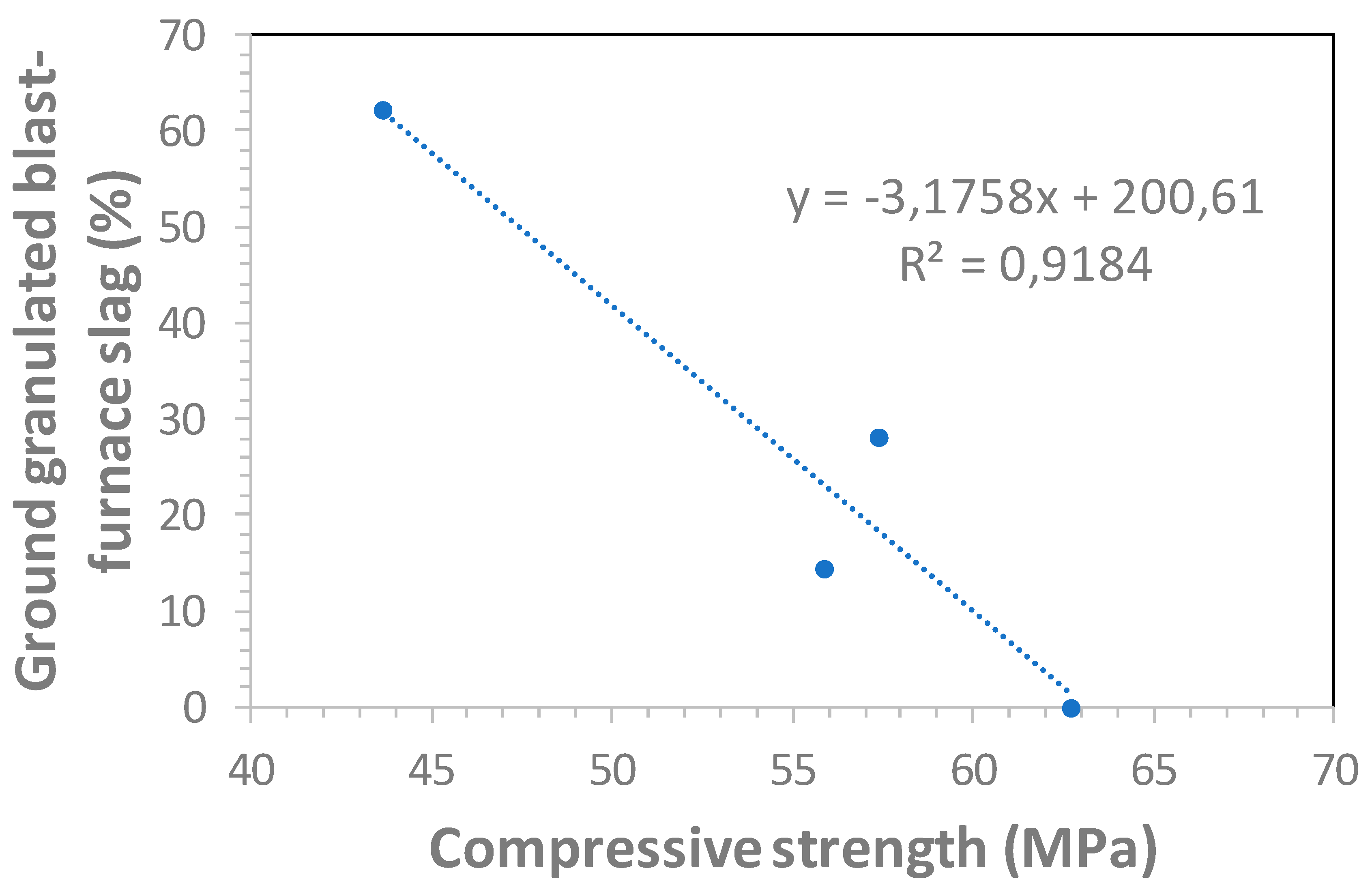
3.3. Carbonation Versus 28-Day Compressive Strength Relationship
4. Conclusions
- Ground granulated blast-furnace slag (GGBFS) mortars cured under controlled conditions, i.e., under poor curing conditions, could help minimize carbon footprints.
- Correlations between the carbonation coefficient and GGBFS content in mortars are heavily reliant on the curing time. Two groups of linear equations were found. The first one corresponds to the lower curing times (0 and 1 days of curing underwater) and the second one corresponds to the longer curing periods (3, 7, 14, and 28 days of curing underwater).
- Carbonation coefficient is indeed increased when GGBFS amount rises. Then, carbonation process in any cement-based material is strongly reliant upon the type of cement, i.e., the kind of additions used in its production.
- The mathematical relationships given in this paper can be used to estimate the carbonation coefficient in function of the GGBFS content and curing.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.-J.; Lee, H.-K. Mineral Sequestration of Carbon Dioxide in Circulating Fluidized Bed Combustion Boiler Bottom Ash. Minerals 2017, 7, 237. [Google Scholar] [CrossRef]
- Klumpen, C.; Radakovitsch, F.; Jess, A.; Senker, J. BILP-19—An Ultramicroporous Organic Network with Exceptional Carbon Dioxide Uptake. Molecules 2017, 22, 1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Wang, X.-Y. Evaluation of the Carbon Dioxide Uptake of Slag-Blended Concrete Structures, Considering the Effect of Carbonation. Sustainability 2016, 8, 312. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IPCC, The Intergovernmental Panel on Climate Change, 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; Institute for Global Environmental Strategies (IGES) on behalf of the IPCC (IGES): Hayama, Japan, 2006; ISBN 4-88788-032-4. [Google Scholar]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Saetta, A.V.; Schrefler, B.A.; Vitaliani, R.V. The carbonation of concrete and the mechanism of moisture, heat and carbon-dioxide flow-through porous materials. Cem. Concr. Res. 1993, 23, 761–772. [Google Scholar] [CrossRef]
- Andrade, C.; Sanjuán, M.A. Updating Carbon Storage Capacity of Spanish Cements. Sustainability 2018, 10, 4806. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef] [Green Version]
- Galán, I.; Andrade, C.; Mora, P.; Sanjuán, M.A. Sequestration of CO2 by Concrete Carbonation. Environ. Sci. Technol. 2010, 44, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Goñi, S.; Gaztañaga, M.; Guerrero, A. Role of cement type on carbonation attack. J. Mater. Res. 2002, 17, 1834–1842. [Google Scholar] [CrossRef]
- Gruyaert, E.; Van den Heede, P.; De Belie, N. Carbonation of slag concrete: Effect of the cement replacement level and curing on the carbonation coefficient– effect of carbonation on the pore structure. Cem. Concr. Compos. 2013, 35, 39–48. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Piñeiro, A.; Rodríguez, O. Ground granulated blast furnace slag efficiency coefficient (k value) in concrete. Applications and limits. Mater. Constr. 2011, 61, 303–313. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Estévez, E.; Argiz, C.; del Barrio, D. Effect of curing time on granulated blast-furnace slag cement mortars carbonation. Cem. Concr. Compos. 2018, 90, 257–265. [Google Scholar] [CrossRef]
- De Belie, N.; Kratky, J.; Van Vlierberghe, S. Influence of pozzolans and slag on the microstructure of partially carbonated cement paste by means of water vapour and nitrogen sorption experiments and BET calculations. Cem. Concr. Res. 2010, 40, 1723–1733. [Google Scholar] [CrossRef]
- Borges, P.H.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Sisomphon, K.; Franke, L. Carbonation rates of concretes containing high volume of pozzolanic materials. Cem. Concr. Res. 2007, 37, 1647–1653. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Effects of Environmental Factors on Concrete Carbonation Depth and Compressive Strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2017, 50, 30–43. [Google Scholar] [CrossRef]
- EN 16757. Sustainability of construction works—Environmental product declarations—Product Category Rules for concrete and concrete elements. Annex BB (informative). In CO2 Uptake by Carbonation—Guidance on Calculation; CEN: Brussels, Belgium, 2017. [Google Scholar]
- Sanjuán, M.A.; Argiz, C. The new European standard on common cements specifications EN 197-1:2011. Mater. Constr. 2012, 62, 425–430. [Google Scholar] [CrossRef]
- EN 197-1. Cement—Part 1: Composition; Specifications and Conformity Criteria for Common Cement; CEN: Brussels, Belgium, 2011. [Google Scholar]
- EN 196-2. Method of Testing Cement—Part 2: Chemical Analysis of Cement; CEN: Brussels, Belgium, 2014. [Google Scholar]
- EN 196-1. Method of Testing Cement—Part 1: Determination of Strength; CEN: Brussels, Belgium, 2016. [Google Scholar]
- CEN/TS 12390-10. Testing Hardened Concrete. Part 10: Determination of the Relative Carbonation Resistance of Concrete; CEN: Brussels, Belgium, 2007. [Google Scholar]
- Konist, A.; Maaten, B.; Loo, L.; Neshumayev, D.; Pihu, T. Mineral sequestration of CO2 by carbonation of Ca-rich oil shale ash in natural conditions. Oil Shale 2016, 33, 248–259. [Google Scholar] [CrossRef]
- Sanjuán, M.A.; Andrade, C.; Cheyrezy, M. Concrete carbonation tests in natural and accelerated conditions. Adv. Cem. Res. 2003, 15, 171–180. [Google Scholar] [CrossRef]



| Cement | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | LOI | IR 1 | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEM I | 21.7 | 3.7 | 4.3 | 66.1 | 1.3 | 3.0 | 0.5 | 0.6 | 1.1 | 0.2 | 0.01 |
| CEM II/A-S | 23.2 | 5.7 | 2.5 | 61.8 | 2.3 | 2.8 | 0.5 | 0.6 | - | - | 0.05 |
| CEM III/A | 24.6 | 6.4 | 2.1 | 57.1 | 3.0 | 2.8 | 0.4 | 0.5 | 0.9 | 0.2 | 0.05 |
| CEM III/B | 29.7 | 8.1 | 2.2 | 51.1 | 4.8 | 2.5 | 0.3 | 0.4 | 0.8 | 0.4 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjuán, M.Á.; Estévez, E.; Argiz, C. Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity. Energies 2019, 12, 2346. https://doi.org/10.3390/en12122346
Sanjuán MÁ, Estévez E, Argiz C. Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity. Energies. 2019; 12(12):2346. https://doi.org/10.3390/en12122346
Chicago/Turabian StyleSanjuán, Miguel Ángel, Esteban Estévez, and Cristina Argiz. 2019. "Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity" Energies 12, no. 12: 2346. https://doi.org/10.3390/en12122346





