Experimental Investigation on Performance Comparison of Solar Water Heating-Phase Change Material System and Solar Water Heating System
Abstract
:1. Introduction
2. Experiments
2.1. Materials
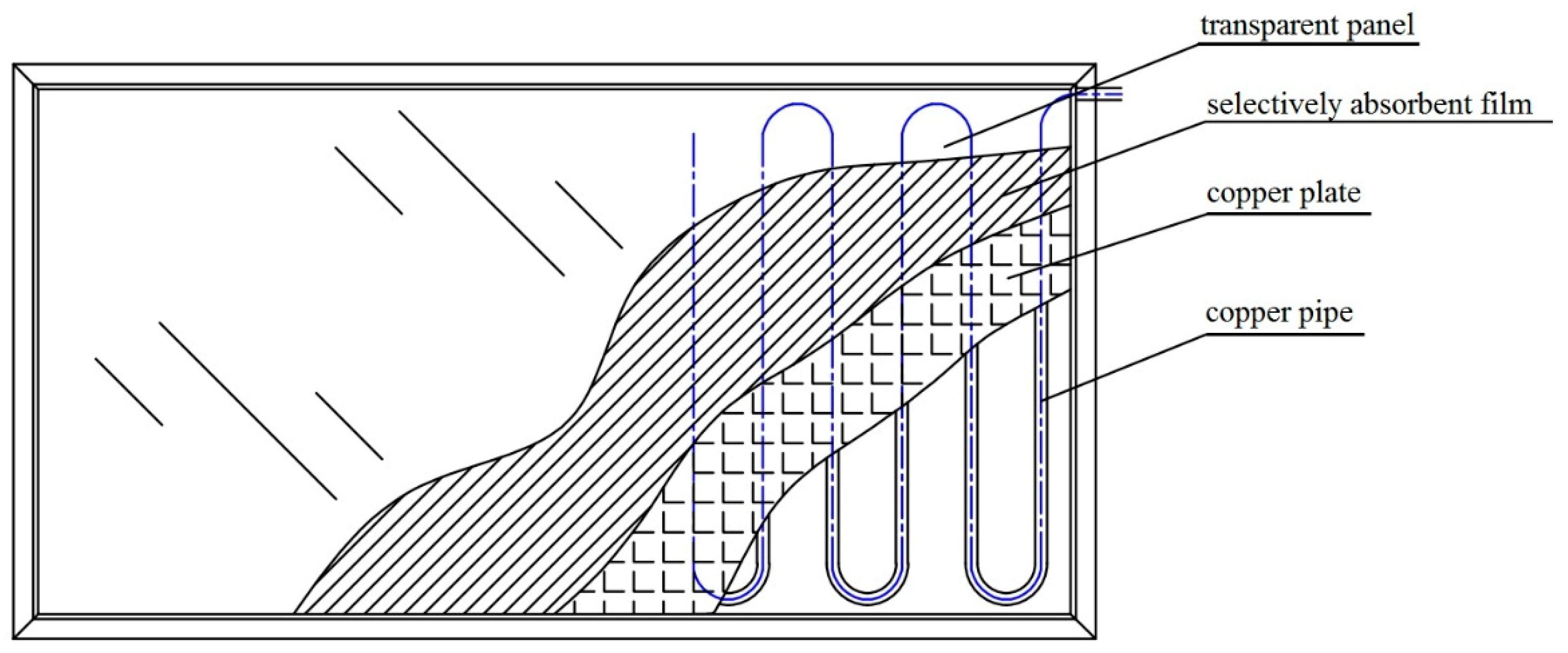
2.2. Experimental Rig
2.3. Experimental Conditions
3. Calculation of Heat Storage and Thermal Efficiency
3.1. Heat Storage
3.2. Thermal Efficiency
4. Results and Discussion
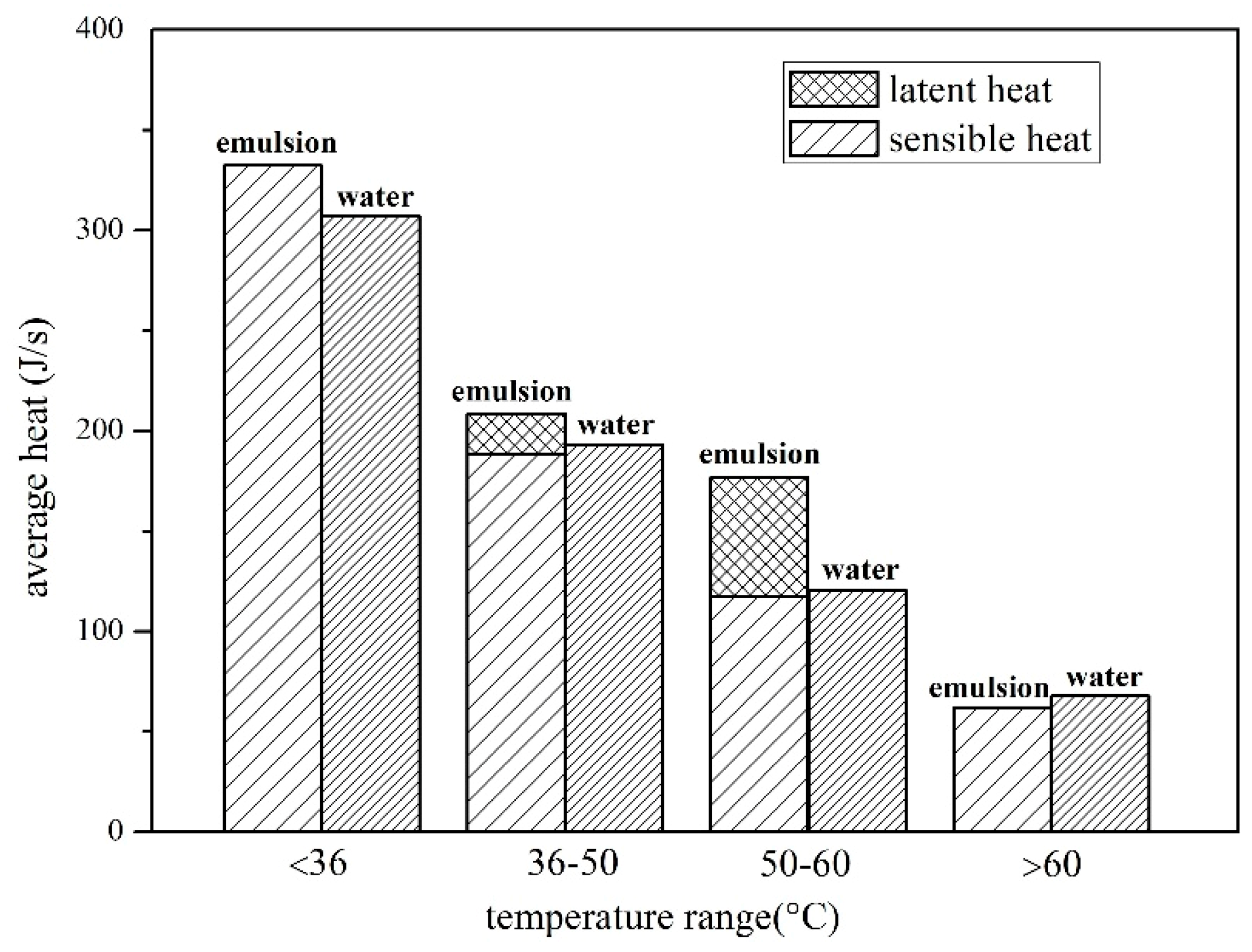
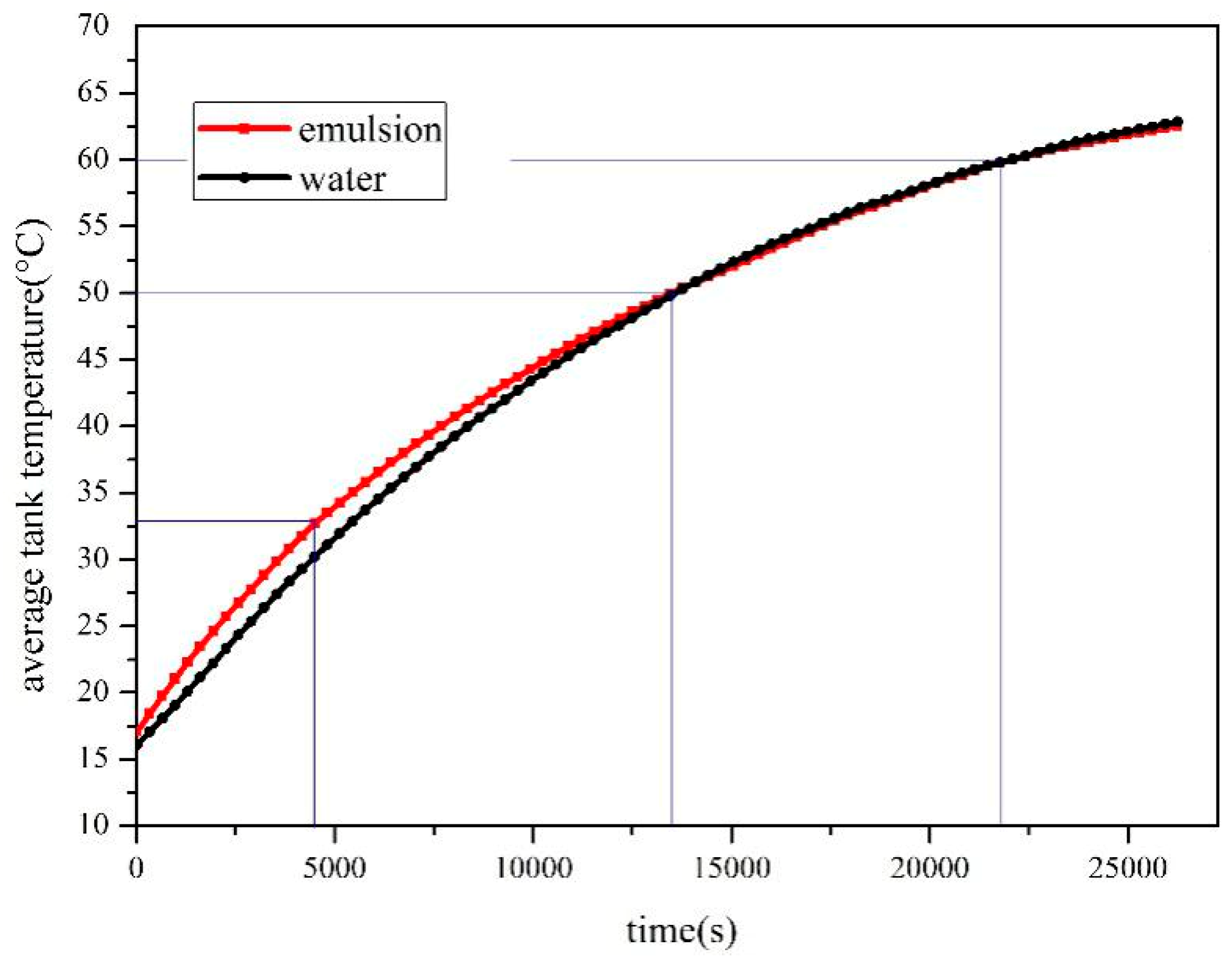
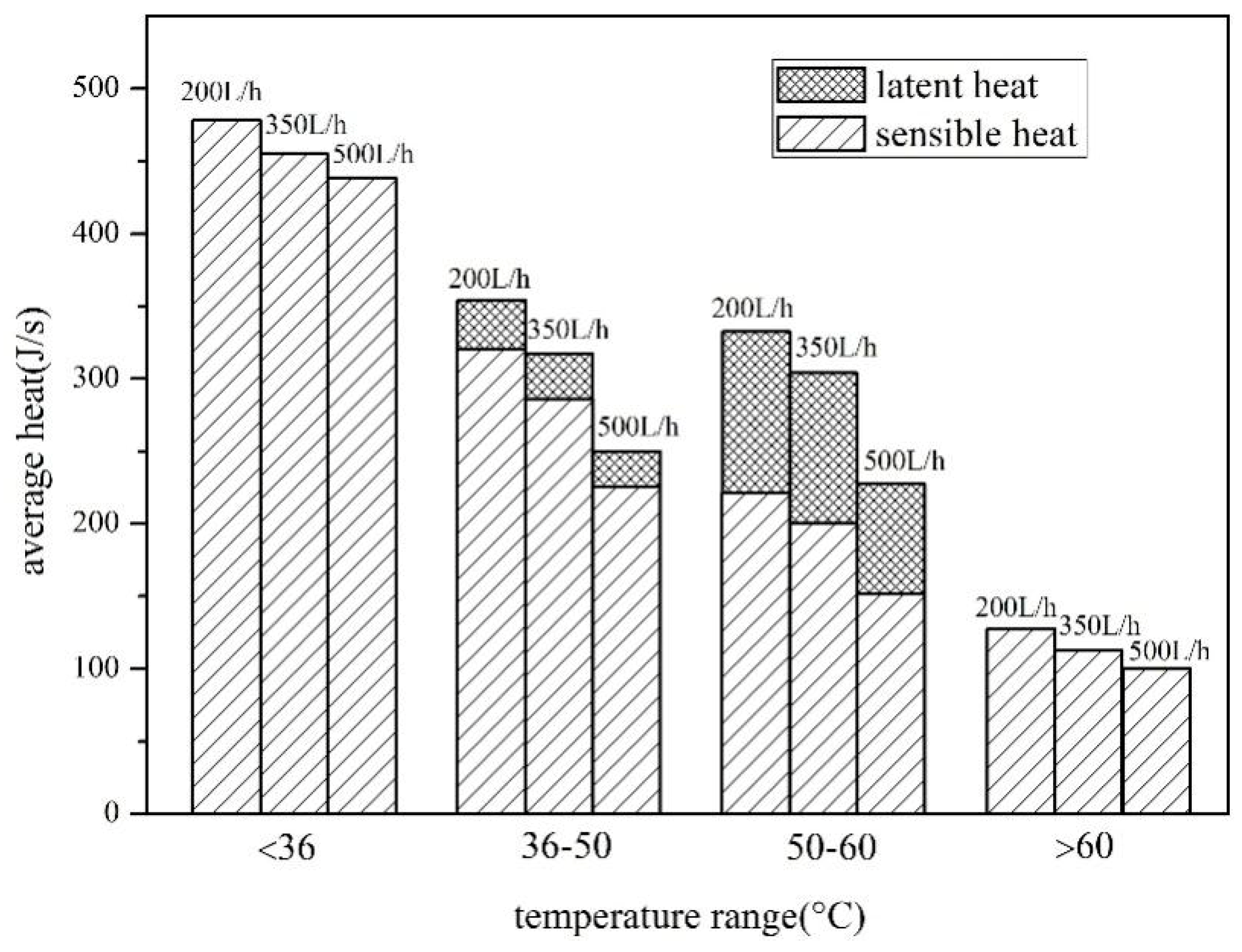
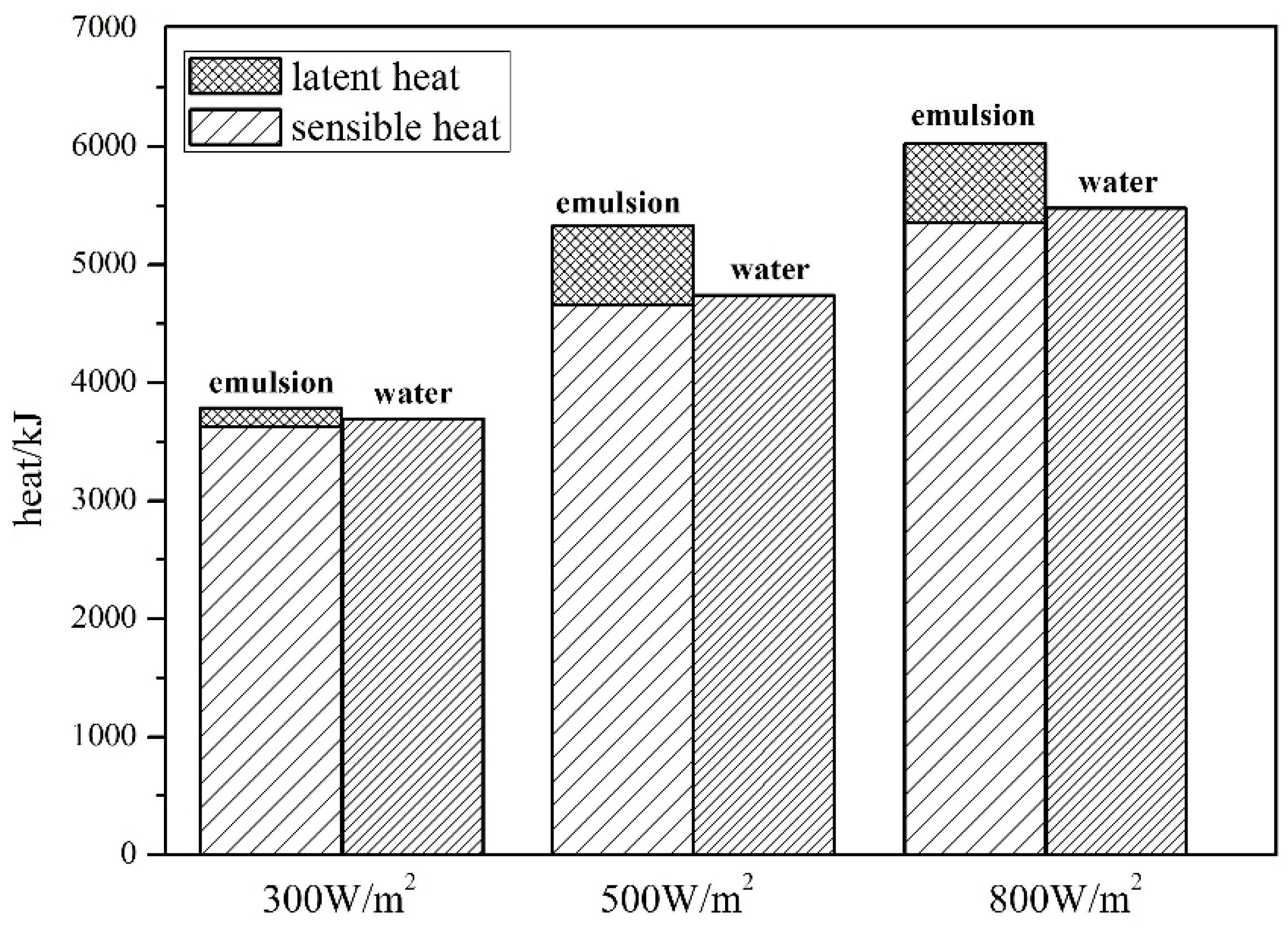
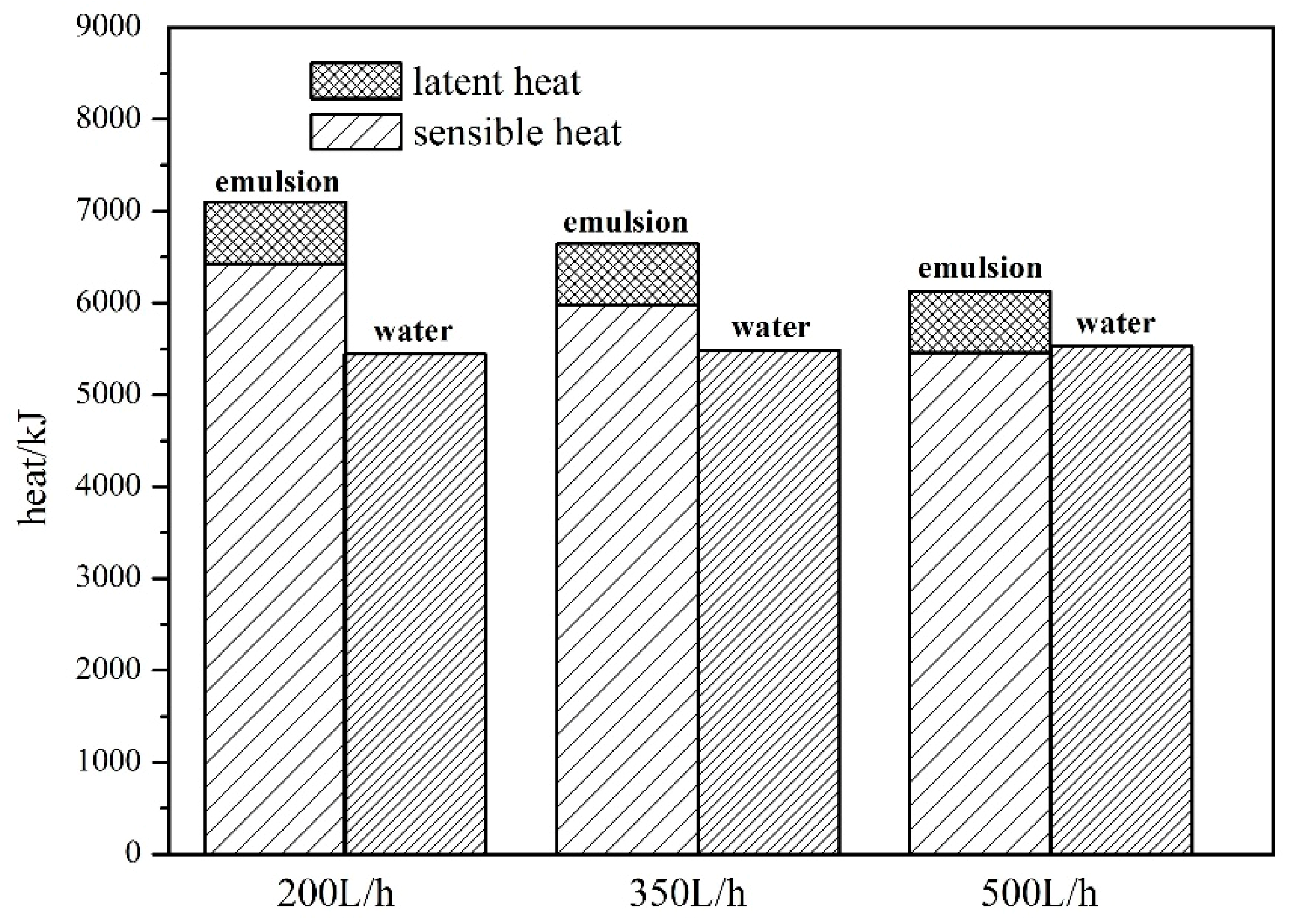
4.1. Comparison of Heat Storage between Water and Composite Paraffin Emulsion
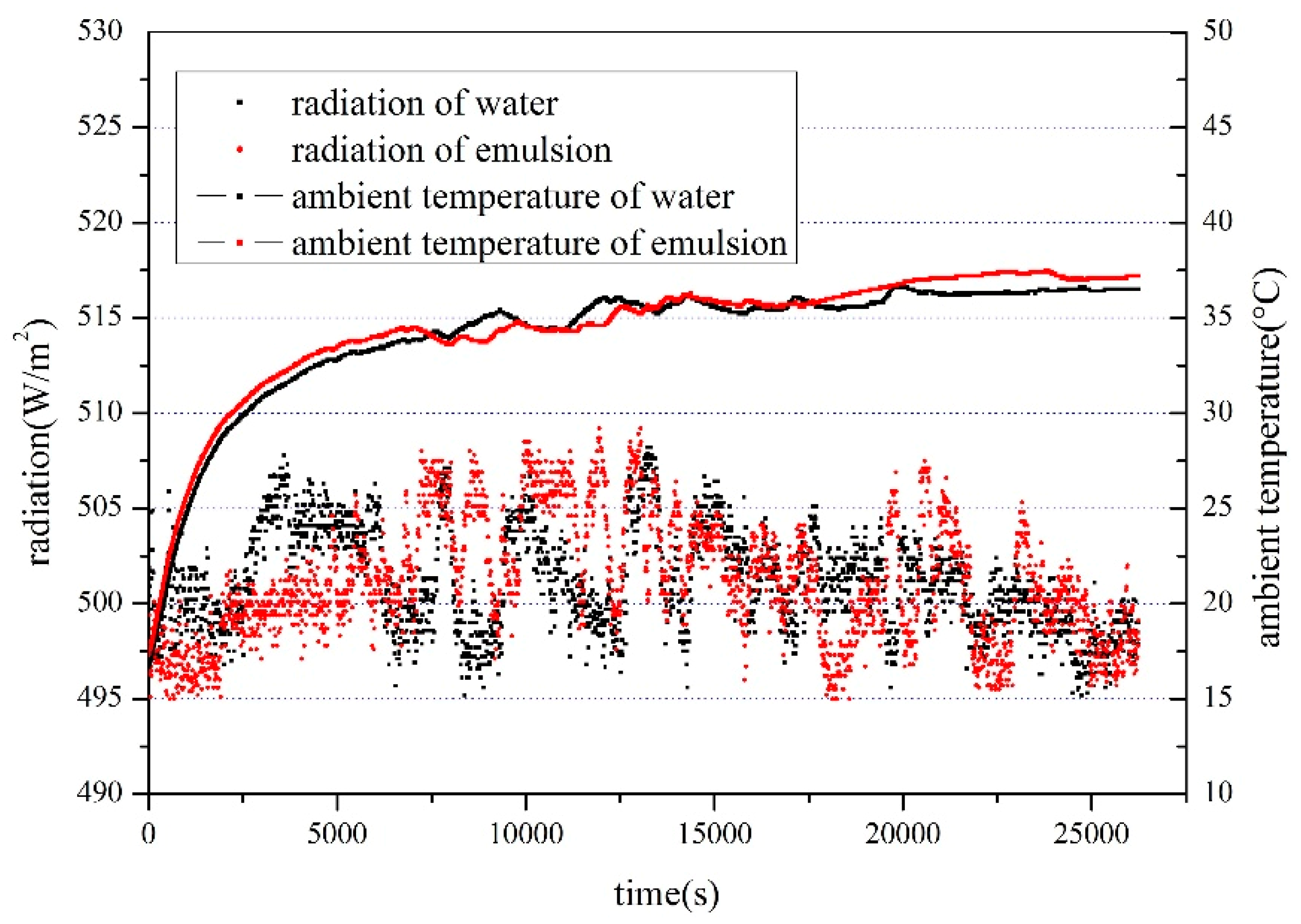
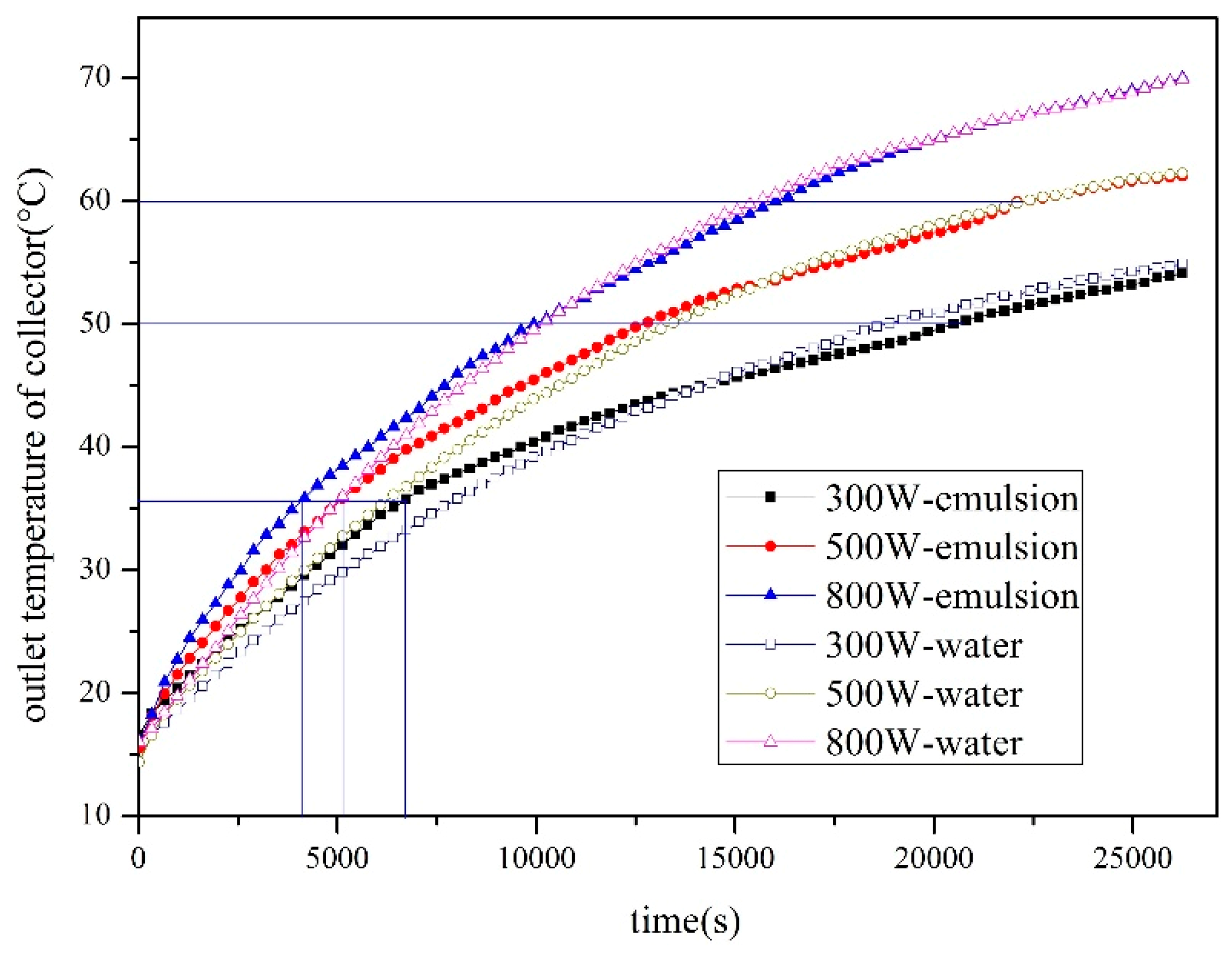
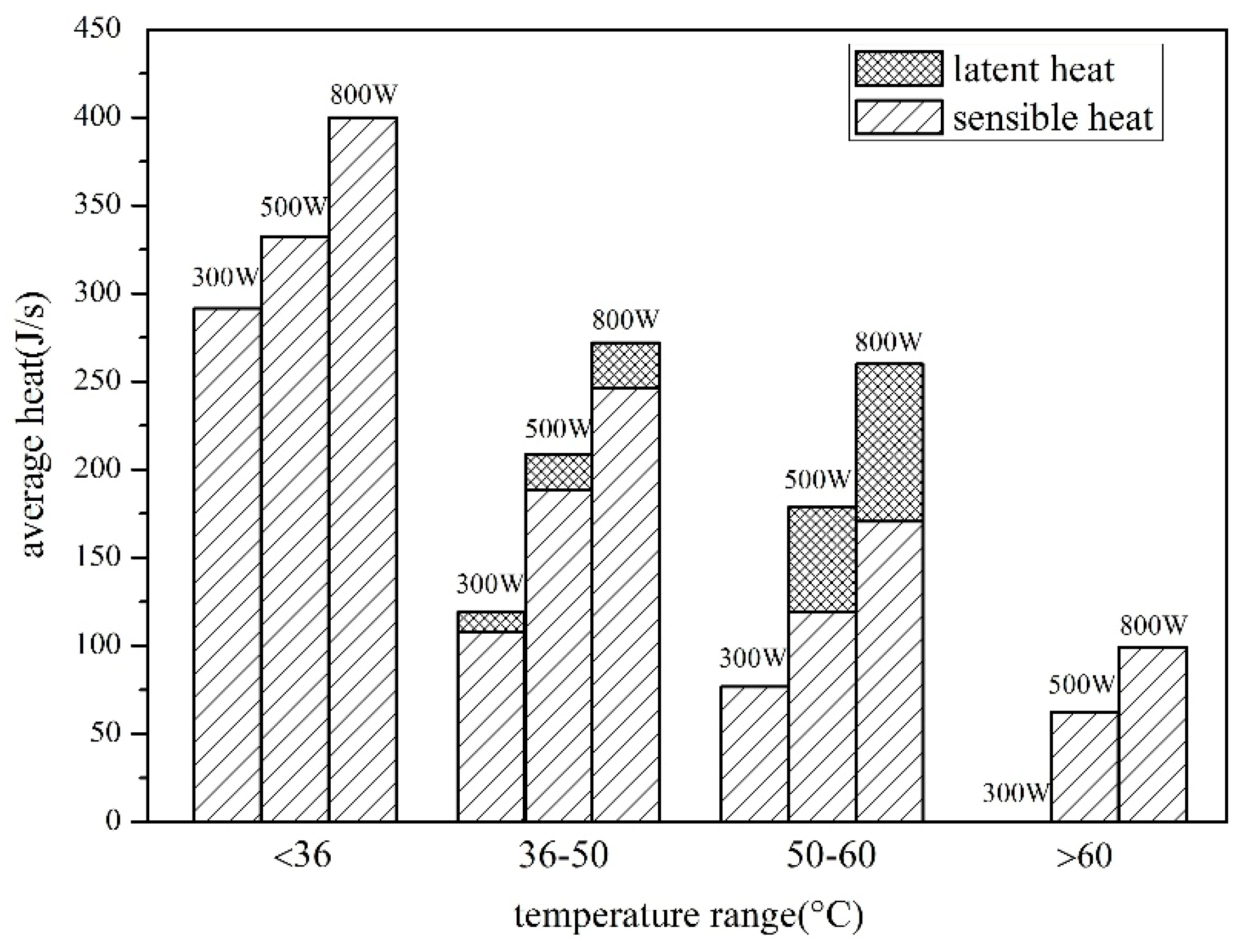
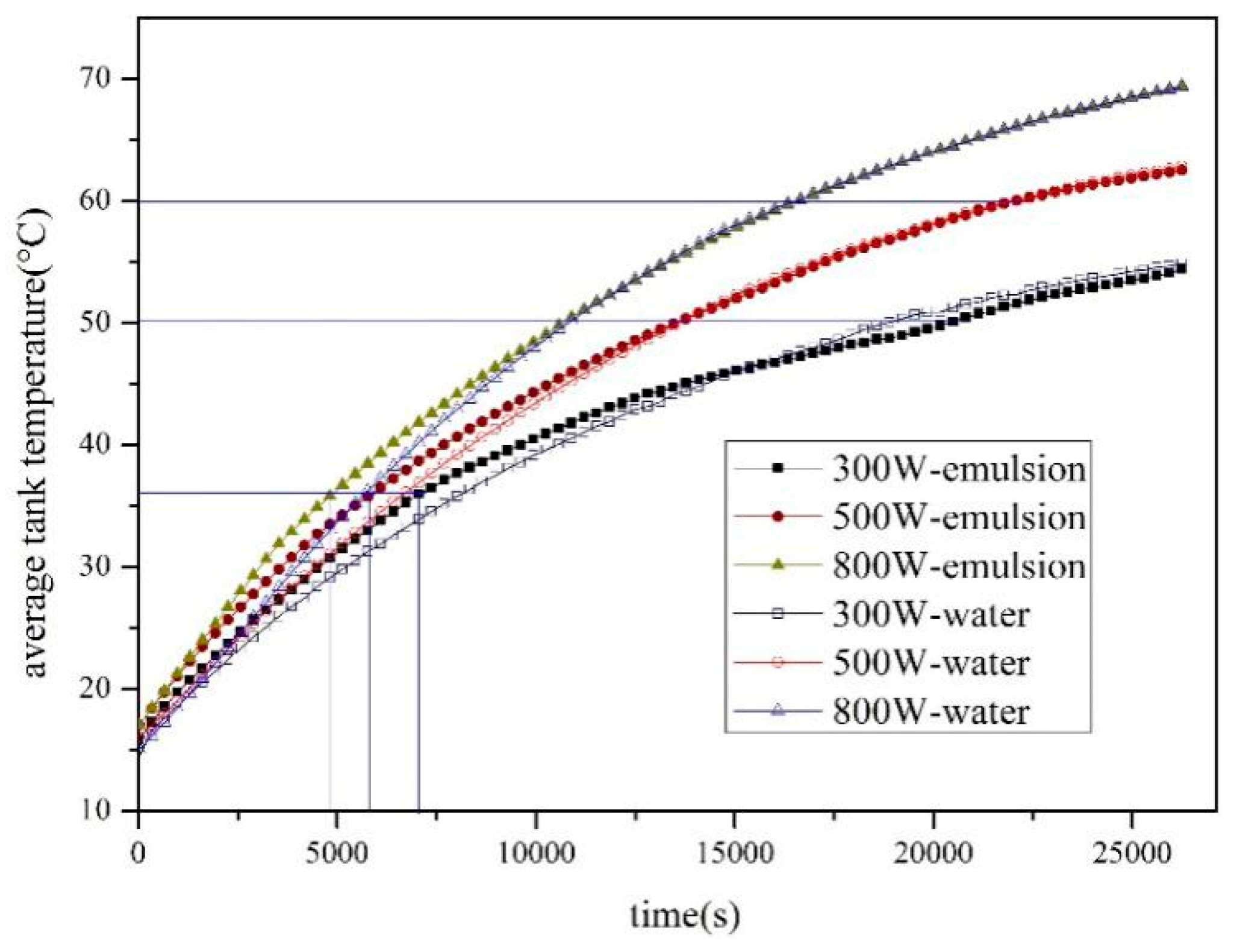
4.1.1. Effect of Solar Radiation on Heat Storage
4.1.2. Effect of Solar Radiation on Heat Storage
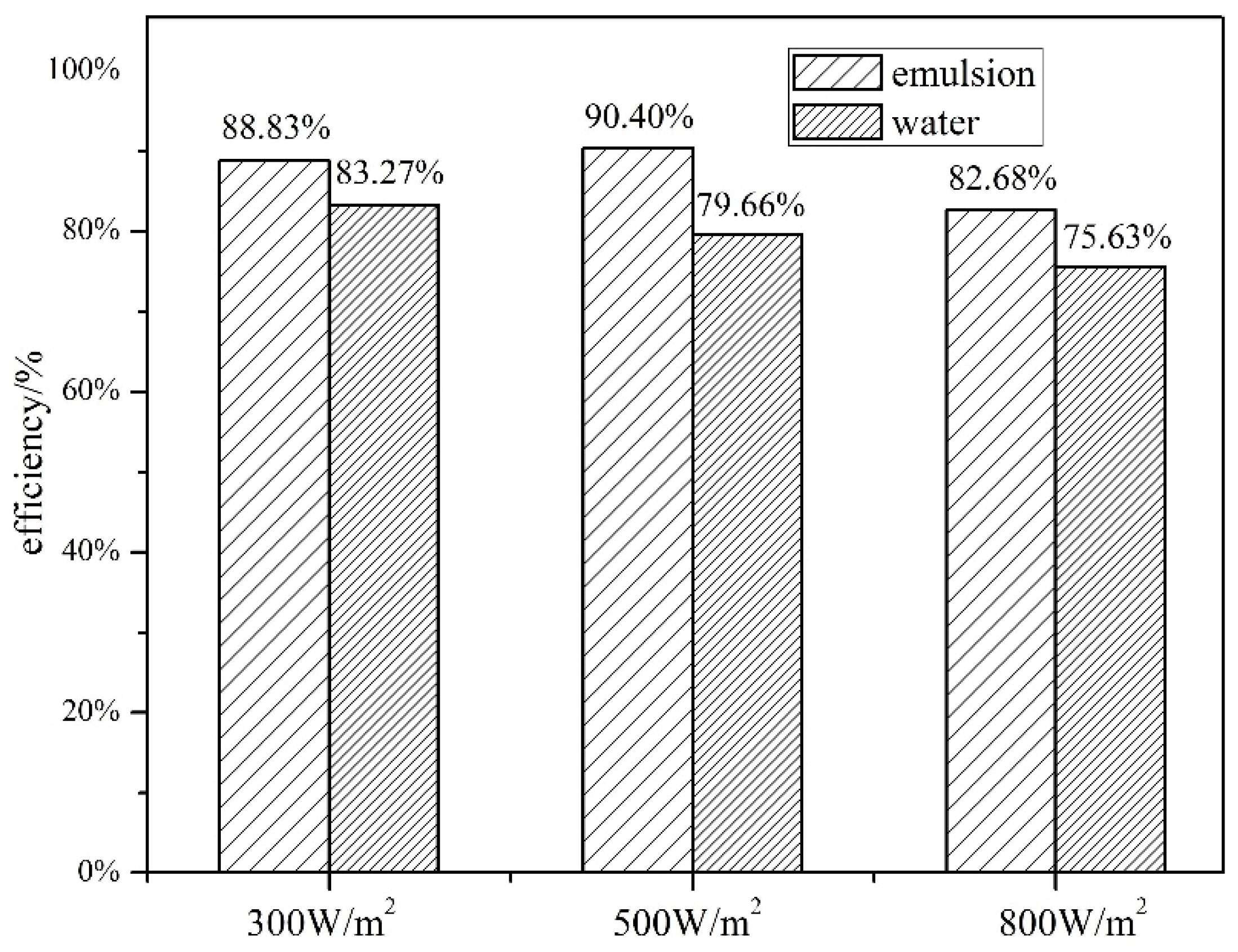
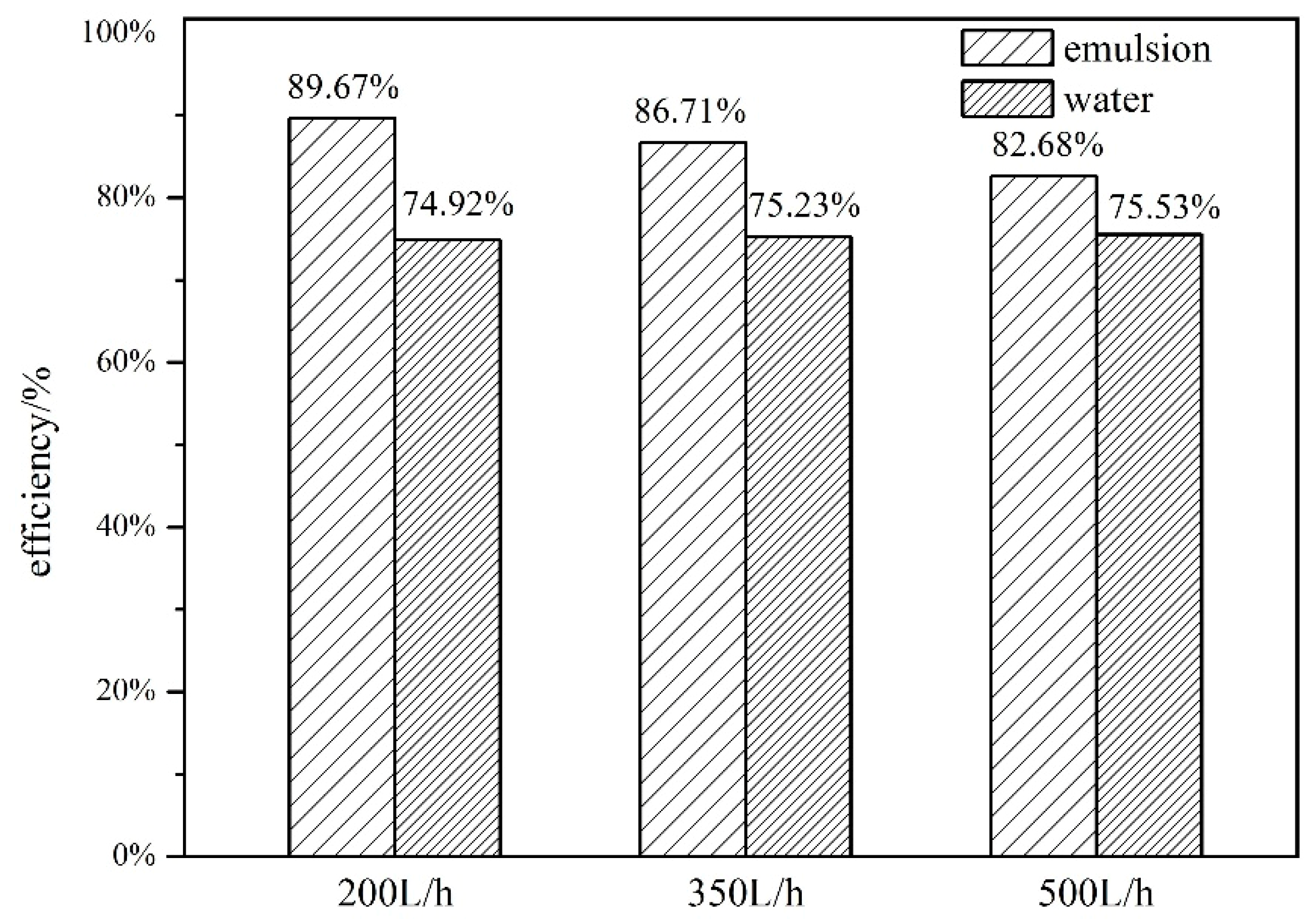
4.2. Comparison of Thermal Efficiency between Water and Composite Paraffin Emulsion
4.2.1. Effect of Solar Radiation on Thermal Efficiency
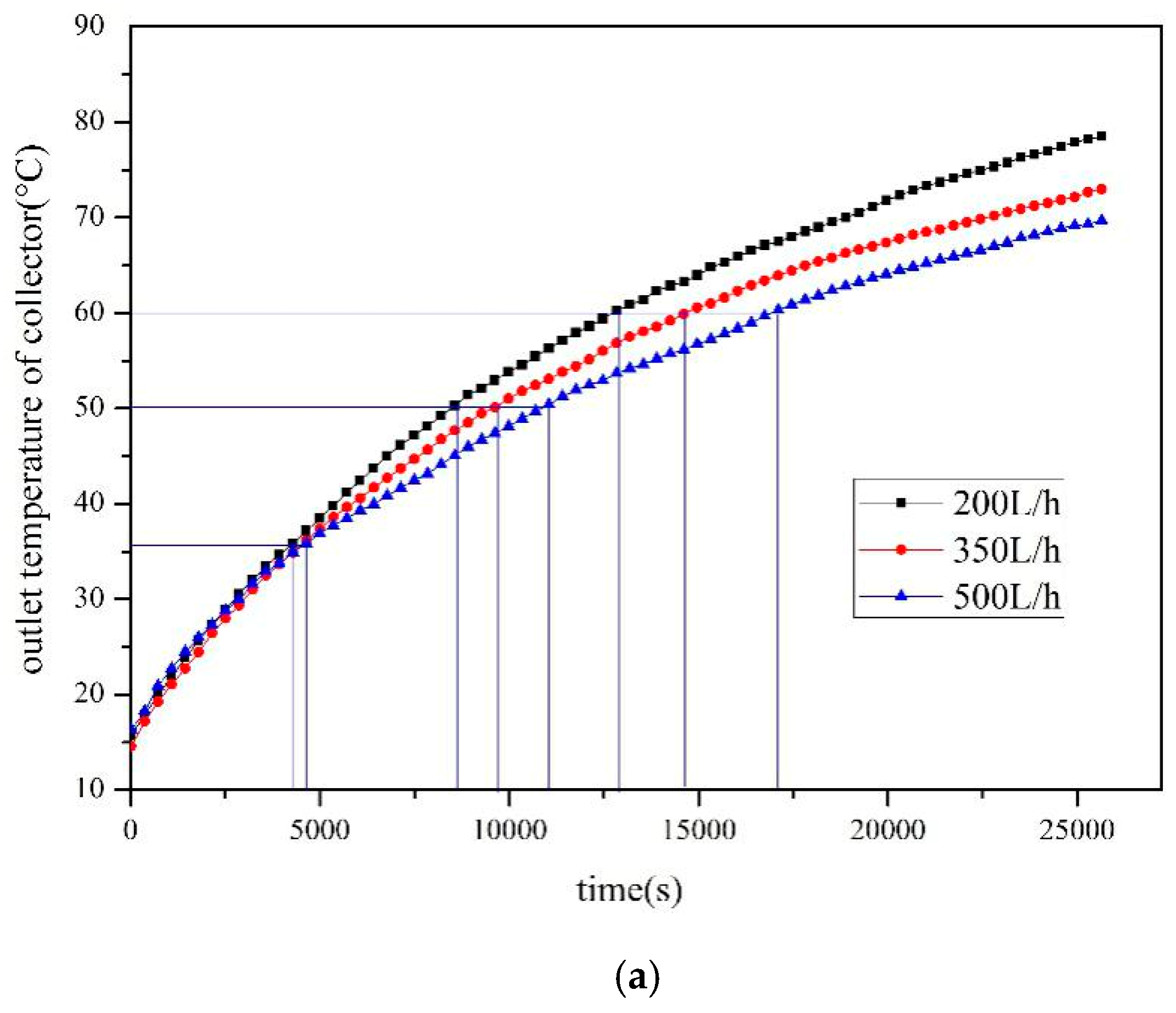
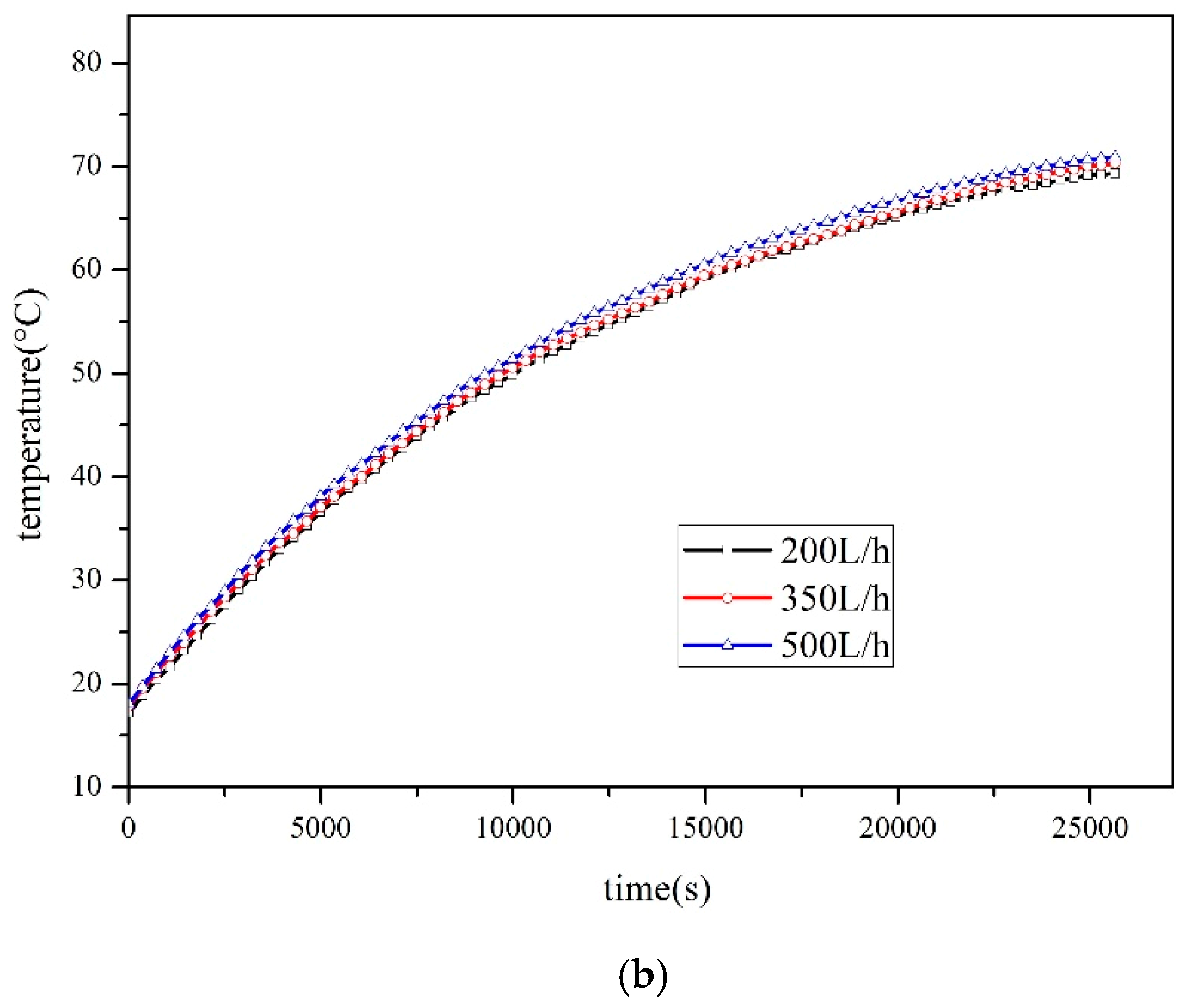
4.2.2. Effect of Flow Rate on Thermal Efficiency
5. Conclusions
- (1)
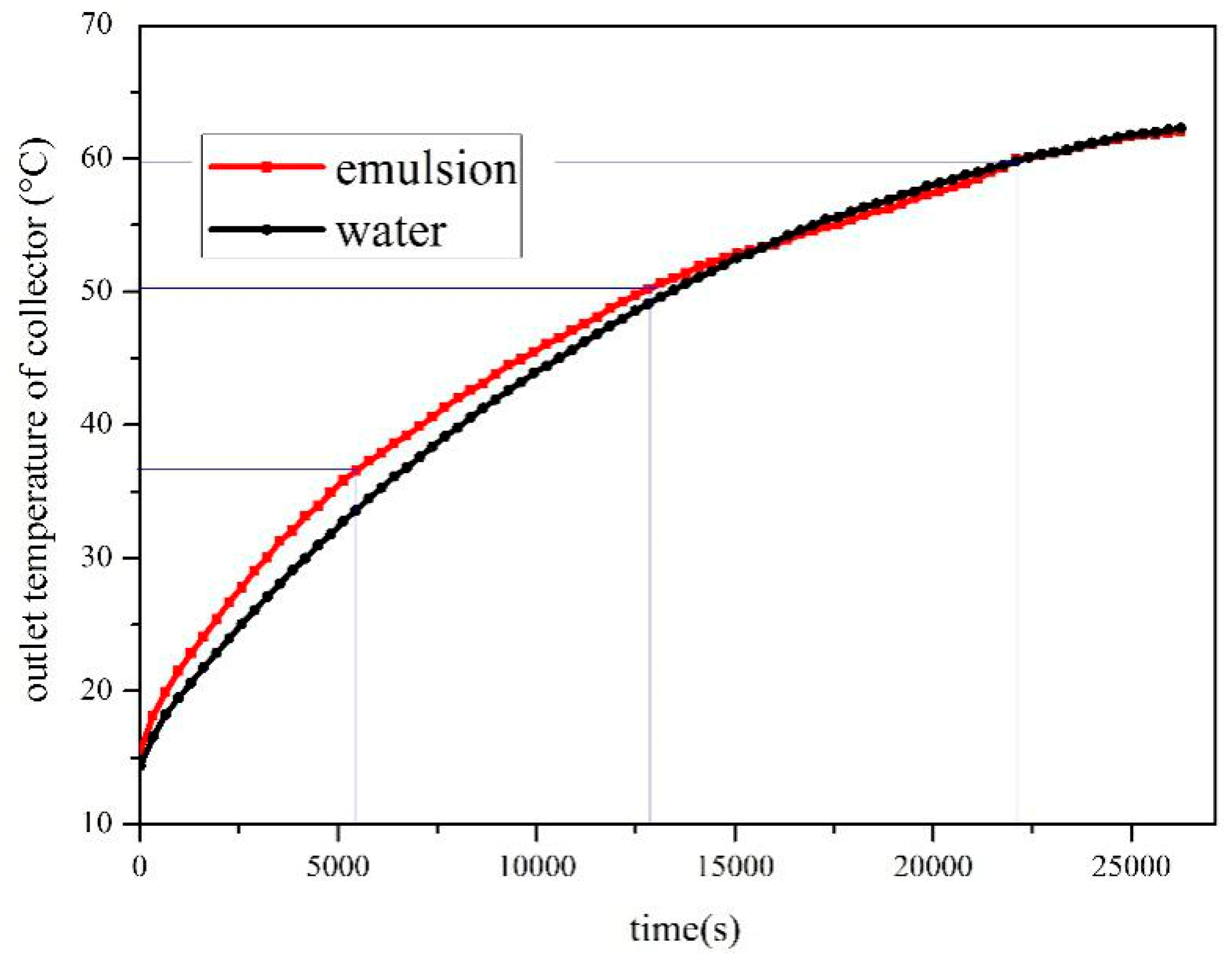
- The SWH-PCM system has a higher temperature rising rate than that of the SWH before all paraffin particles melt into liquid. When the melting process has finished, the SWH-PCM system shows a similar temperature change trend as the SWH system. The SWH-PCM system obtains more heat storage than the SWH system
- (2)
- The heat storage of SWH-PCM system and SWH system all increase with the increase of solar irradiance, while the thermal efficiency has the opposite trend. The thermal efficiency of SWH-PCM system can achieve the maximum value of 90.40% as the solar irradiance of 500 W/m2.
- (3)
- As flow rates increase, the thermal efficiency of the SWH-PCM system decreases slightly, while the thermal efficiency of the SWH system basically is unchanged. The thermal efficiency of the SWH-PCM system can reach up to 89.67% with the flow rate of 200L/h, which is 14.76% higher than that of the SWH system.
Author Contributions
Funding
Conflicts of Interest
References
- Philibert, C. Barriers to Technology Diffusion: The Case of Solar Thermal Technologies; International Energy Agency: Paris, France, 2006. [Google Scholar]
- Boyle, G. Renewable Energy: Power for a Sustainable Future, 3rd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Abhat, A. Low temperature latent thermal energy storage system: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yuan, Y.P.; Zhang, N.; Sun, Q.R.; Cao, X.L.; Sun, L.L. Thermal properties enforcement of carbonate ternary via lithium fluoride: A heat transfer fluid for concentrating solar power systems. Renew. Energy 2017, 111, 523–531. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; Ma, X.L.; Li, P.; Zhao, X.D.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and building applications. Renew. Sustain. Energy Rev. 2017, 77, 246–262. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Qiu, F.; Yang, W.S.; Zhao, X.D. Applications of solar water heating system with phase change material. Renew. Sustain. Energy Rev. 2015, 52, 645–652. [Google Scholar] [CrossRef]
- Ma, F.; Chen, J.; Zhang, P. Experimental study of the hydraulic and thermal performances of nano-sized phase change emulsion in horizontal mini-tubes. Energy 2018, 149, 944–953. [Google Scholar] [CrossRef]
- Zou, D.Q.; Feng, Z.P.; Xiao, R.; Qin, K.; Zhang, J.J.; Song, W.J.; Tu, Q. Preparation and flow characteristic of a novel phase change fluid for latent heat transfer. Sol. Energy Mater. Sol. Cells 2010, 94, 2292–2297. [Google Scholar] [CrossRef]
- Ni, L.; Qv, D.H.; Wang, J.J.; Shang, R.X.; Yao, Y. Forced convective heat transfer for laminar flow of paraffin: A numerical and experimental study. Appl. Therm. Eng. 2017, 113, 1152–1163. [Google Scholar] [CrossRef]
- Saarinen, S.; Puupponen, S.; Meriläinen, A.; Joneidi, A.; Seppälä, A.; Saari, K.; Nissila, T.A. Turbulent heat transfer characteristics in a circular tube and thermal properties of n-decane-in-water nanoemulsion fluids and micelles-in-water fluids. Int. J. Heat Mass Transf. 2015, 81, 246–251. [Google Scholar] [CrossRef]
- Meriläinen, A.; Seppälä, A.; Saari, K.; Seitsonen, J.; Ruokolainen, J.; Puisto, S.; Rostedt, N.; Nissila, T.A. Influence of particle size and shape on turbulent heat transfer characteristics and pressure losses in water-based nanofluids. Int. J. Heat Mass Transf. 2013, 61, 439–448. [Google Scholar] [CrossRef]
- Xie, H.Q.; Li, Y.; Yu, W. Intriguingly high convective heat transfer enhancement of nanofluid coolants in laminar flows. Phys. Lett. A 2010, 374, 2566–2568. [Google Scholar] [CrossRef]
- Morimoto, T.; Kumano, H. Flow and heat transfer characteristics of phase change emulsions in a circular tube: Part 2. Turbulent flow. Int. J. Heat. Mass Transf. 2018, 117, 903–911. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, J.; Wang, Z.X.; Yang, W.S.; Zhao, X.D. Experimental investigation of the performance of the novel HP-BIPV/T system for use in residential buildings. Energy Build. 2016, 130, 295–308. [Google Scholar] [CrossRef]
- Serale, G.; Baronetto, S.; Goia, F.; Perino, M. Characterization and energy performance of a slurry PCM-based solar thermal collector: A numerical analysis. Energy Procedia 2014, 48, 223–232. [Google Scholar] [CrossRef]
- Qiu, Z.Z.; Ma, X.L.; Zhao, X.D.; Li, P.; Samira, A. Experimental investigation of the energy performance of a novel micro-encapsulated phase change material (MPCM) slurry based PV/T system. Appl. Energy 2016, 165, 260–271. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, T.; Shi, Y.; Li, L. An investigation on rheology and heat transfer characteristics for a phase change emulsion. J. Eng. Thermophys. 2001, 22, 589–592. [Google Scholar]
- Mikkola, V.; Puupponen, S.; Saari, K.; Ala-Nissila, T.; Seppälä, A. Thermal properties and convective heat transfer of phase changing paraffin nanofluids. Int. J. Therm. Sci. 2017, 117, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, J.; Fang, X.; Zhang, Z. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2016, 147, 101–107. [Google Scholar] [CrossRef]
- Xiang, N.; Yuan, Y.P.; Sun, L.L.; Cao, X.L.; Zhao, J. Simultaneous decrease in supercooling and enhancement of thermal conductivity of paraffin emulsion in medium temperature range with graphene as additive. Thermochim. Acta 2018, 664, 16–25. [Google Scholar] [CrossRef]
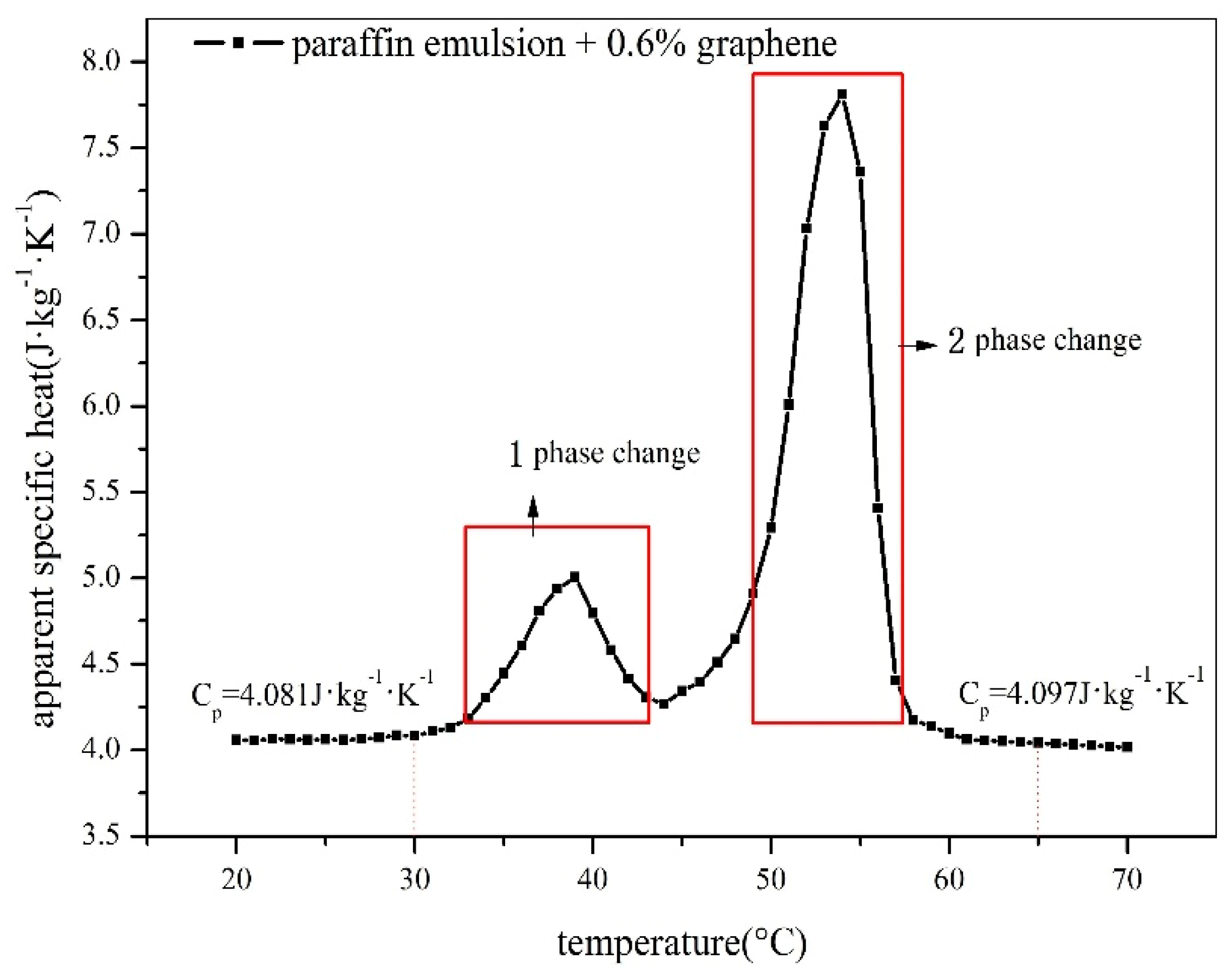



| Performance Parameters | Process | Phase Change Temperature/°C | Phase Change Enthalpy/kJ·kg−1 | Thermal Conductivity/W·m−1·K−1 | Super Cooling/°C | Viscosity/Pa·s | |
|---|---|---|---|---|---|---|---|
| Graphene composite paraffin emulsion (0.6%wt graphene, 20%wt paraffin emulsion) | Melting | 1 | 36.0 | 6.23 | 0.472(30 °C) | 2.13 | 0.438– 0.00615 (30 °C–60 °C) |
| 2 | 50.4 | 20.58 | |||||
| Solidification | 56.48 | 25.51 | |||||
| Parameter | Measurement Equipment |
|---|---|
| Temperature | K-type thermocouples range: −200–1300 °C, accuracy: ±0.2 °C |
| Flow rate | LYDF-DN10 electromagnetic flowmeter range: 30–600 L/h, accuracy: 0.5% |
| Solar radiation (Ambient temperature) | Delta-T SPN1 pyranometer range: 0–2000 W/m2, accuracy: ±5 W/m2 (range: −40–80 °C, accuracy: ±0.4 °C) |
| Layer | Parameter | Value |
|---|---|---|
| Covering glass | Thickness | 5 mm |
| Material | Tempered glass | |
| Air layer | Thickness | 20 mm |
| Vacuum coating | length × width × thickness | 1050 × 514 × 2 mm |
| Thermal conductive silica gel | Thickness | 2 mm |
| Thermal conductivity coefficient | 1.2 W/(m·K) | |
| Absorber plate | Material | Copper |
| Thickness | 2 mm | |
| Absorbent film | material | Blue titanium |
| thickness | 0.2 mm | |
| absorptance | 95% | |
| Tube | Material Diameter | Copper 12 mm |
| Distance of adjacent tubes | 70 mm | |
| Insulation material | Material | Rock wool |
| Thickness | 40 mm | |
| Thermal conductivity coefficient | 0.039 W/(m·K) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Xiang, N.; Yuan, Y.; Cao, X. Experimental Investigation on Performance Comparison of Solar Water Heating-Phase Change Material System and Solar Water Heating System. Energies 2019, 12, 2347. https://doi.org/10.3390/en12122347
Sun L, Xiang N, Yuan Y, Cao X. Experimental Investigation on Performance Comparison of Solar Water Heating-Phase Change Material System and Solar Water Heating System. Energies. 2019; 12(12):2347. https://doi.org/10.3390/en12122347
Chicago/Turabian StyleSun, Liangliang, Nan Xiang, Yanping Yuan, and Xiaoling Cao. 2019. "Experimental Investigation on Performance Comparison of Solar Water Heating-Phase Change Material System and Solar Water Heating System" Energies 12, no. 12: 2347. https://doi.org/10.3390/en12122347





