The Viscosity and Combustion Characteristics of Single-Droplet Water-Diesel Emulsion
Abstract
:1. Introduction
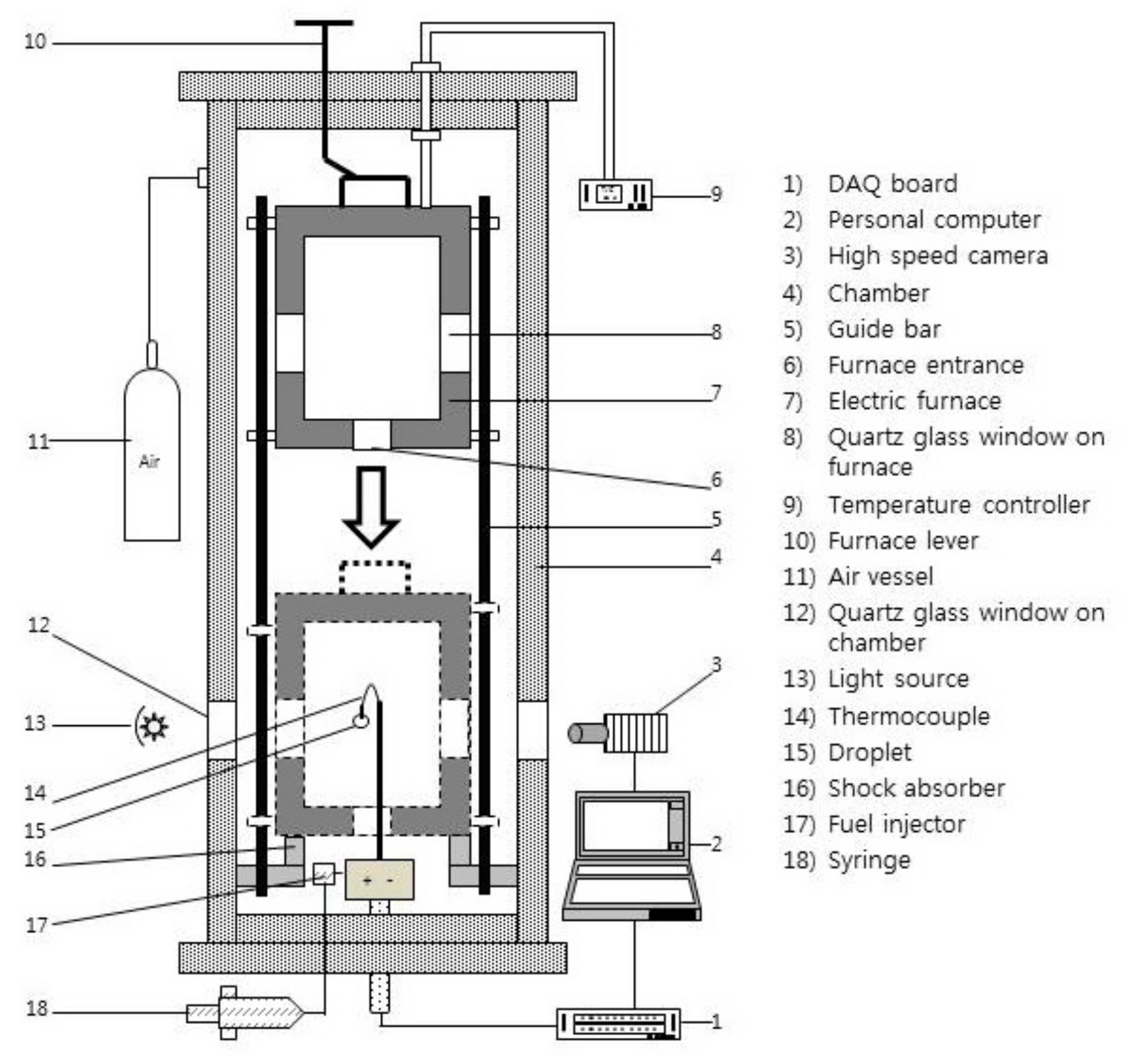
2. Experimental Apparatus
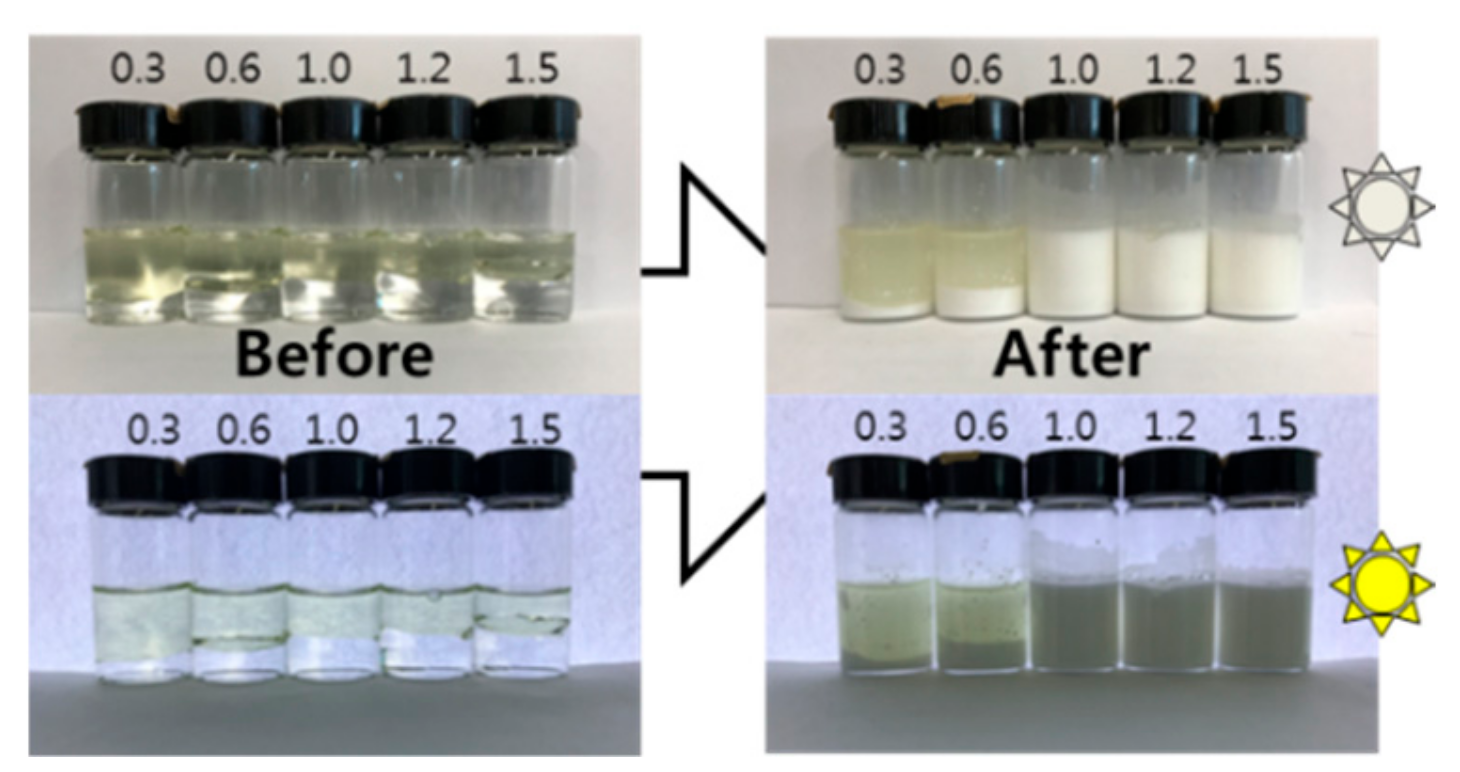
2.1. Preparation of Emulsion Fuel
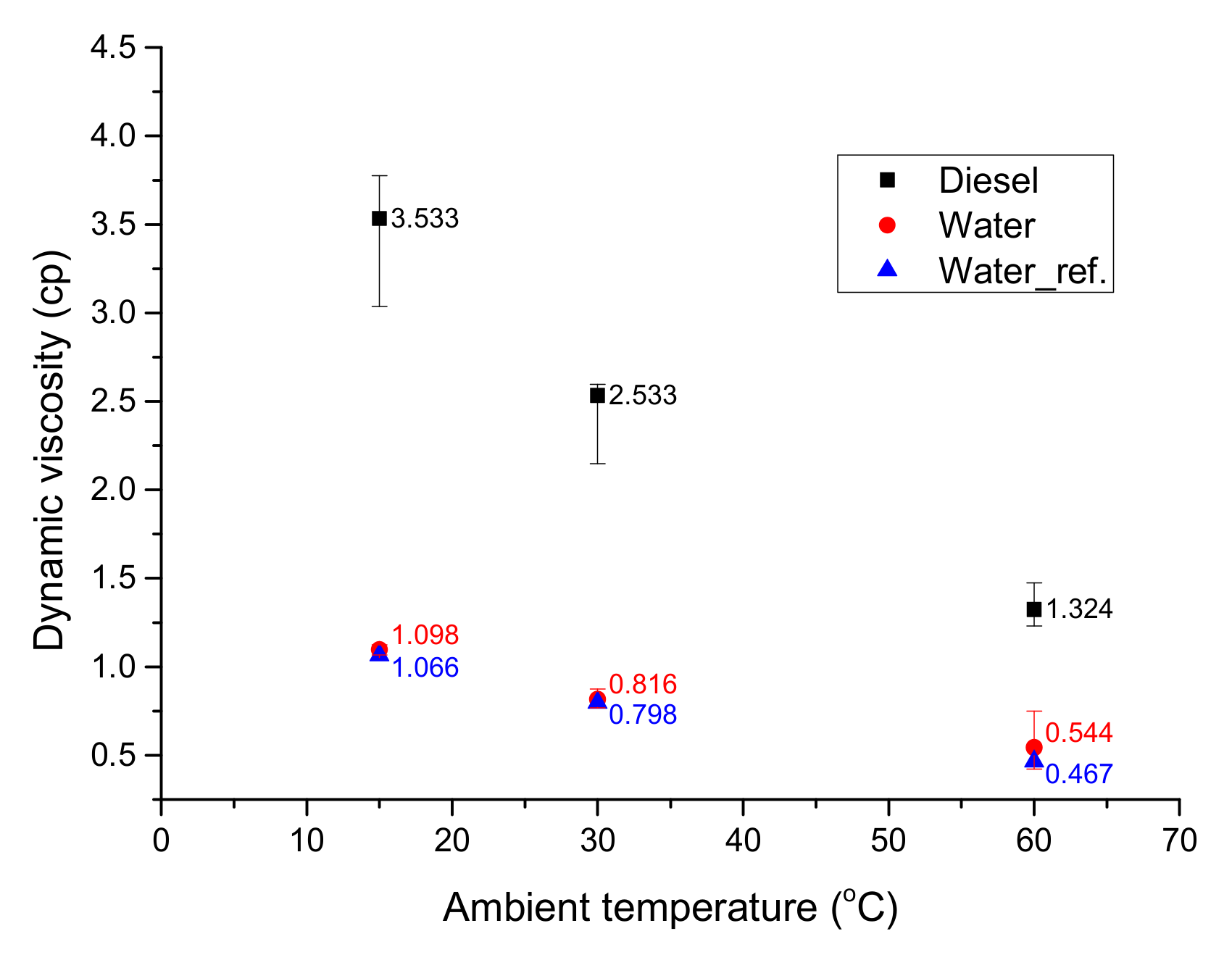
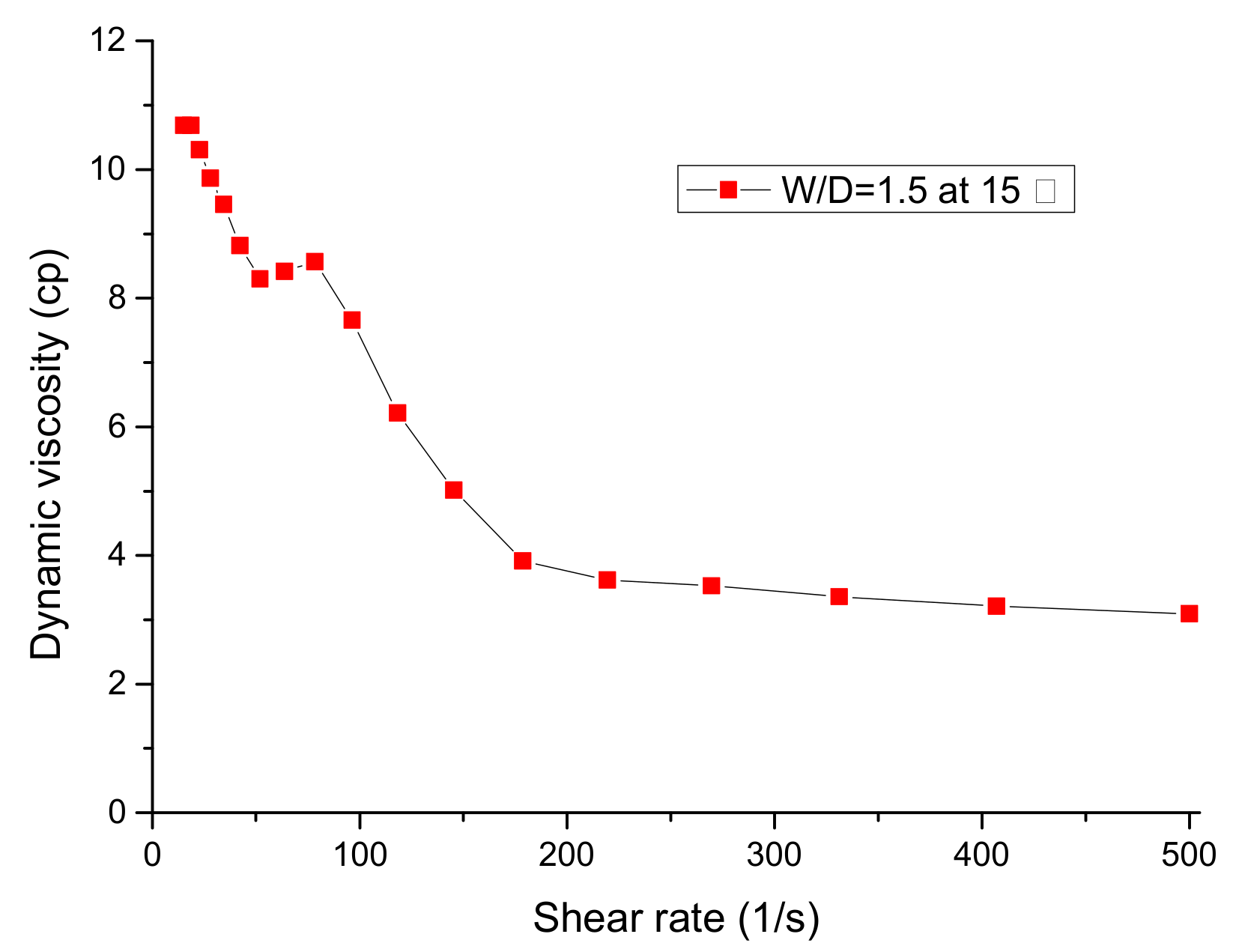
2.2. Viscosity Measurements
2.3. Constant-Volume Combustion Chamber
3. Results
3.1. Viscosity of Emulsion Fuels
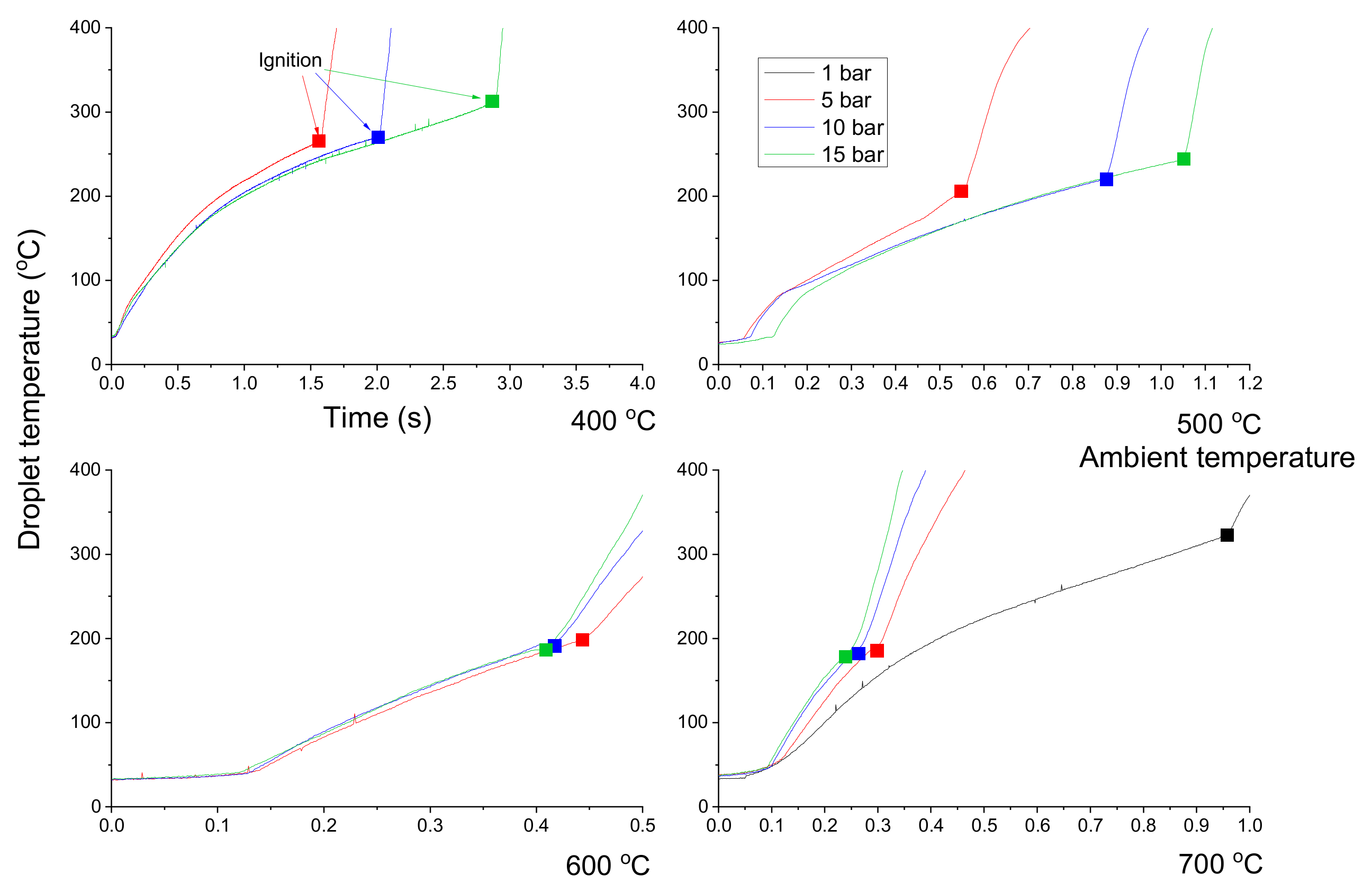
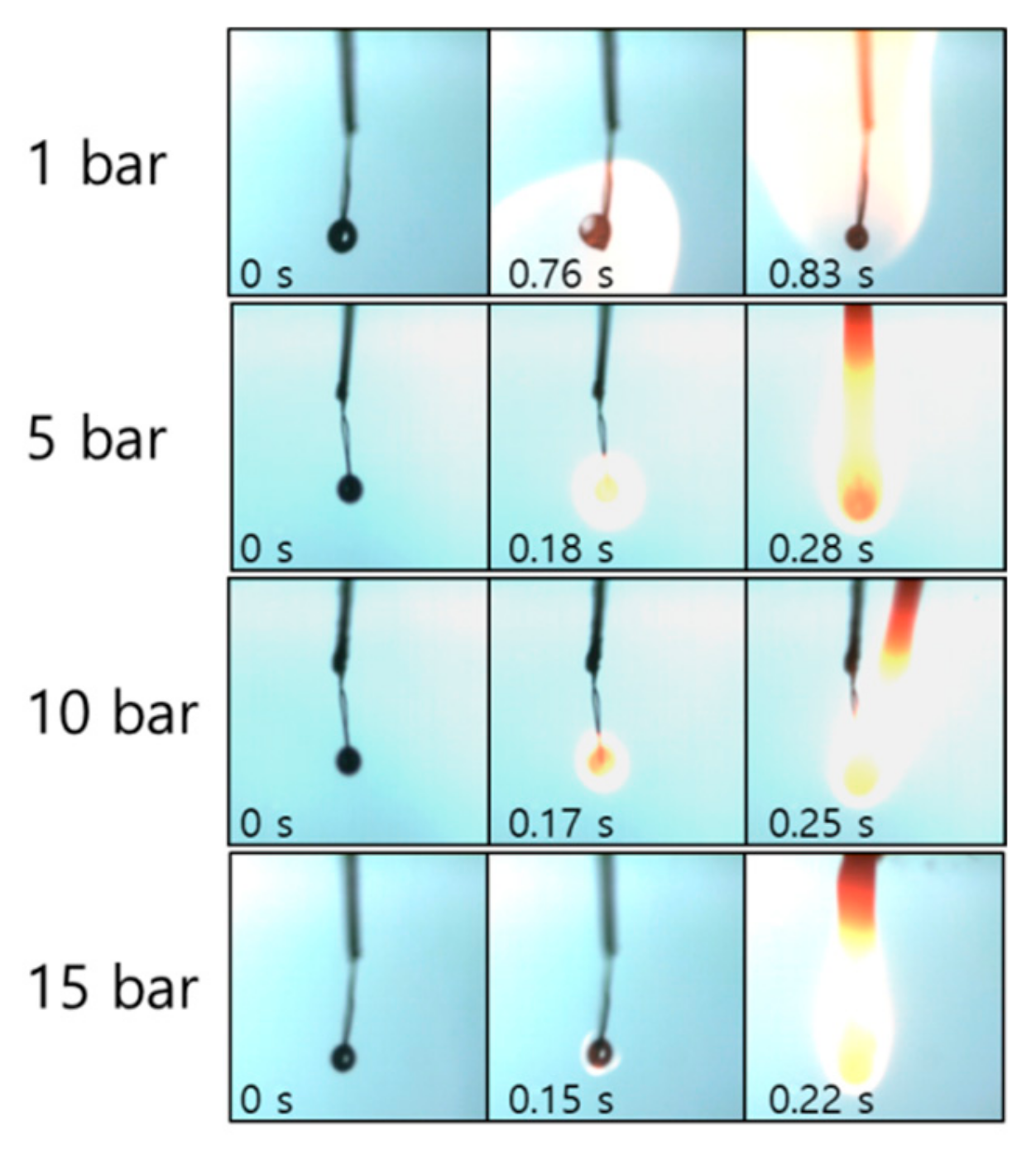
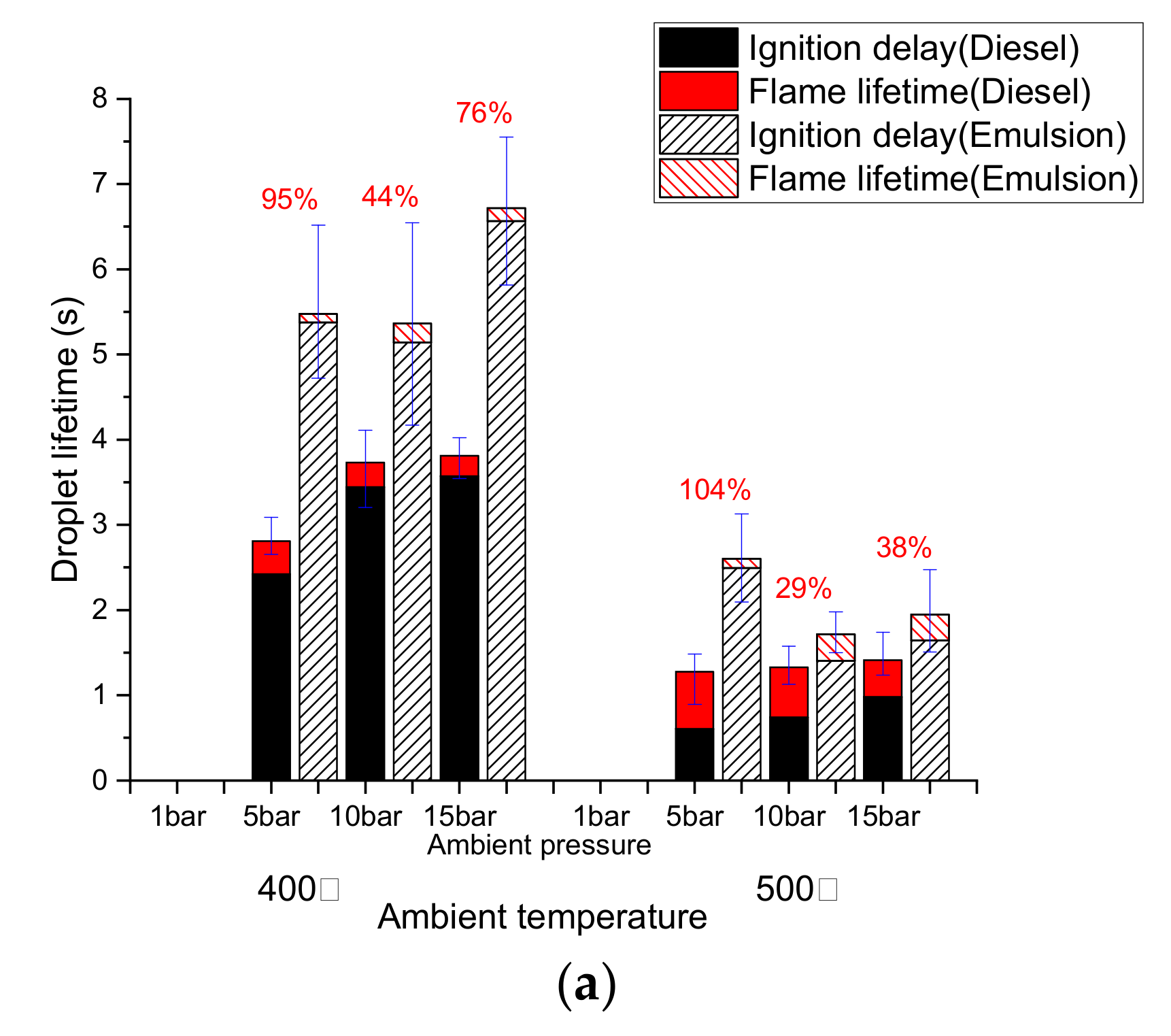
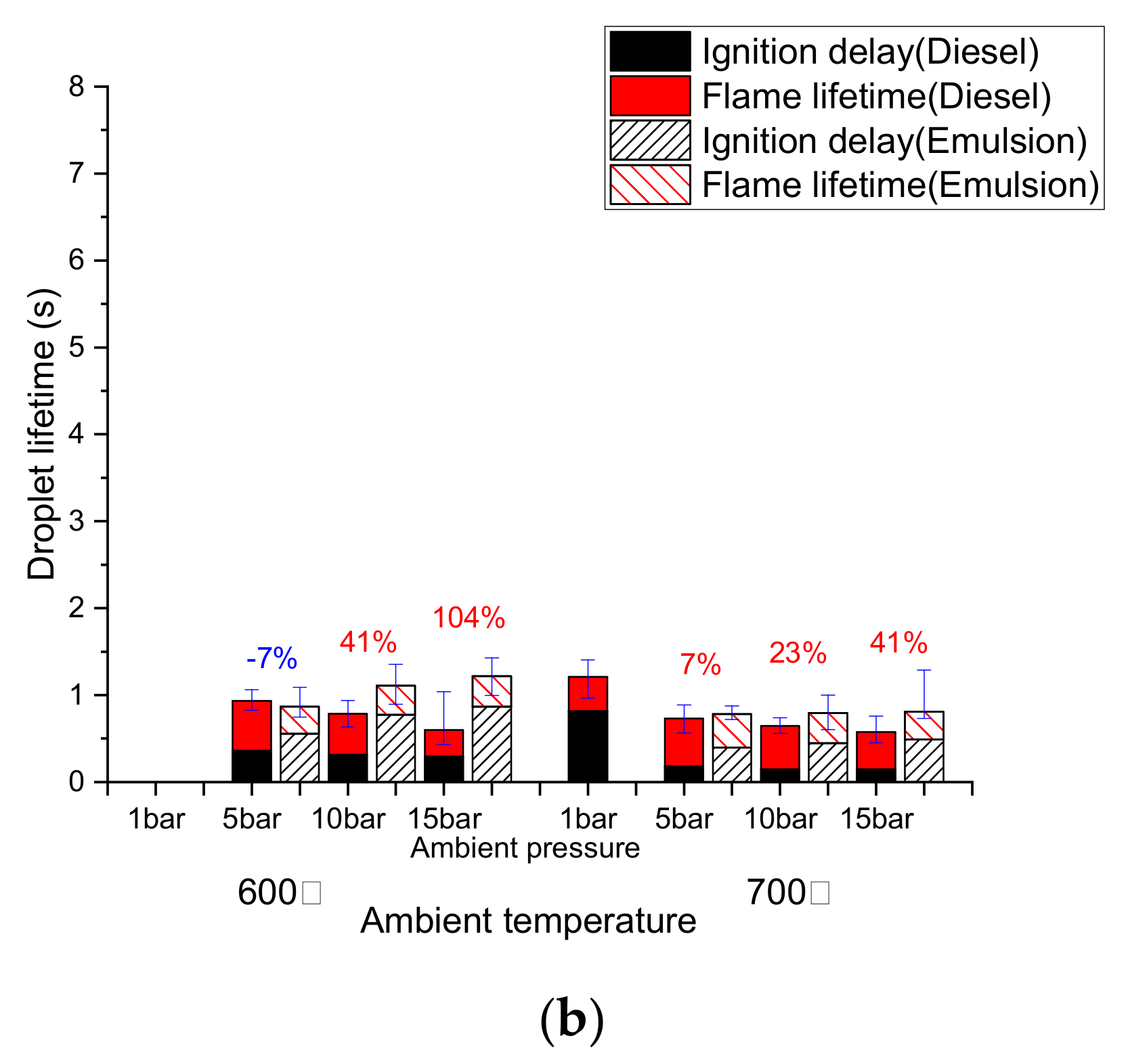
3.2. Diesel Droplet Combustion
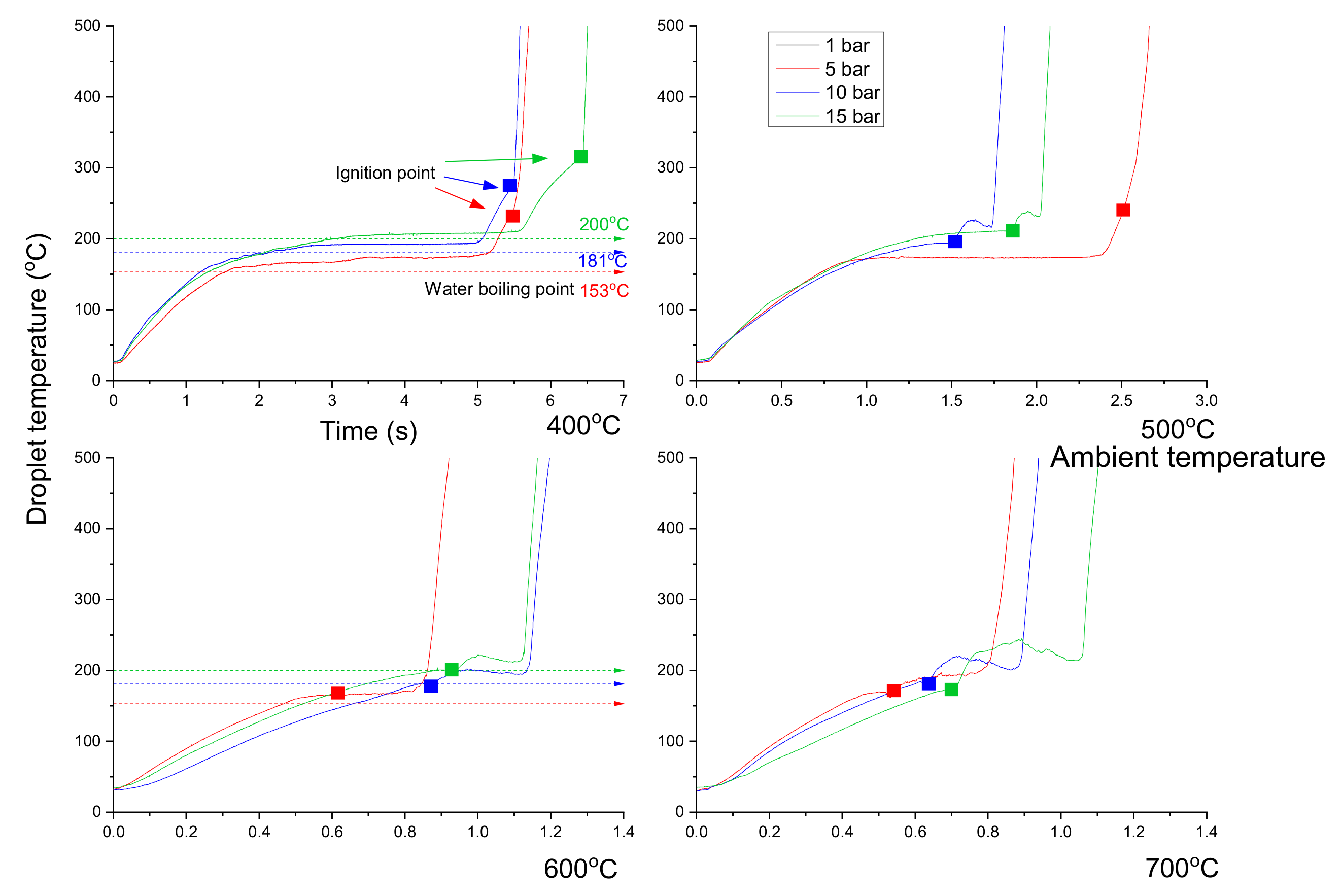
3.3. Emulsion Droplet Combustion
3.4. Comparison of the Combustion Behavior of Diesel and Emulsion Fuels
4. Conclusions
- Emulsions formed readily as the water content rose above a 1:1 water:diesel mass ratio. The optimum emulsion fuel was 1:1 water:diesel mass ratio containing 0.2 wt% mixed surfactant (HLB 14) as prepared via 10 min of sonication.
- The viscosity of the W:D = 1.0 emulsion was the highest because droplet dispersion increased the effective concentration as the shear rate rose. After addition of 0.2 wt% mixed surfactant (HLB 14), the viscosity was independent of mixing time.
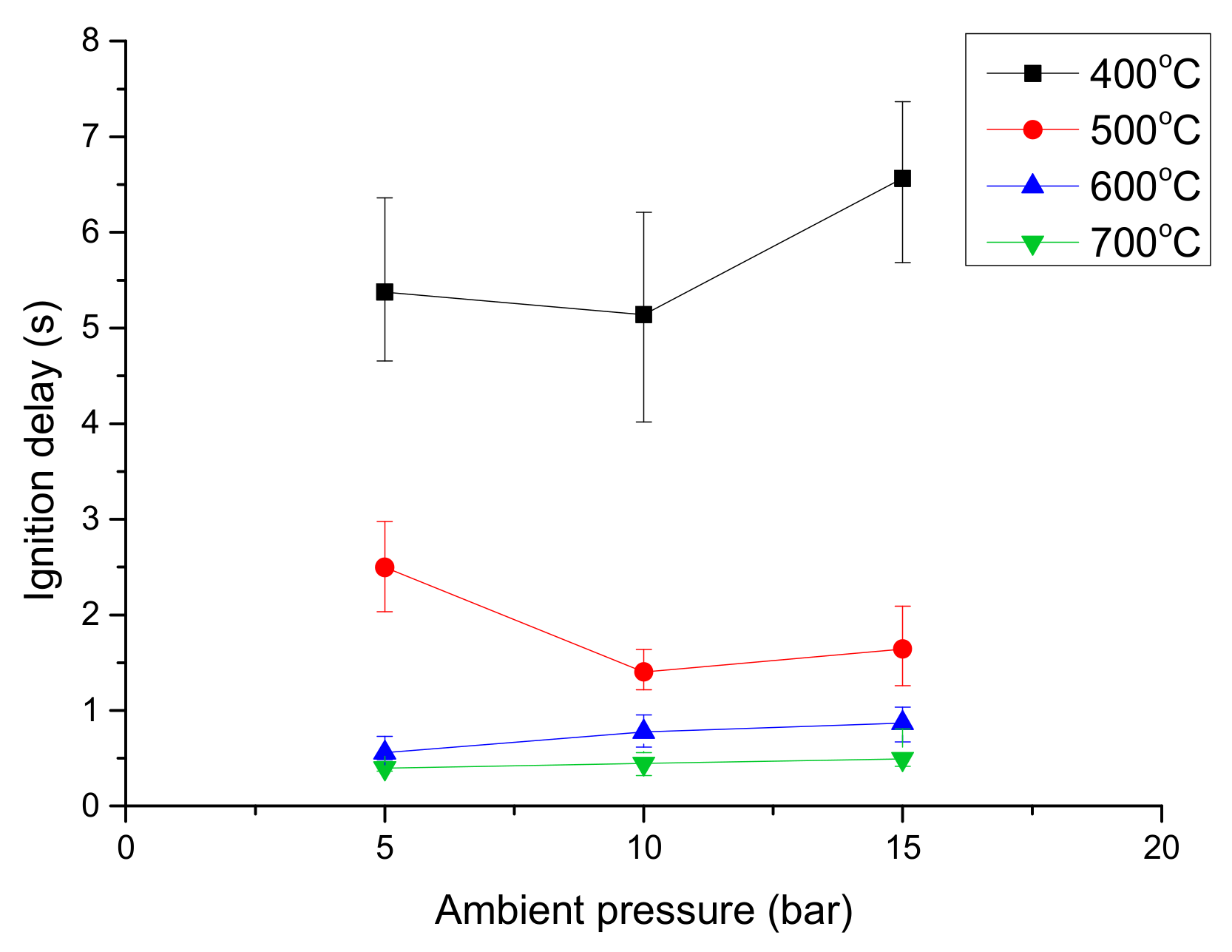
- The ignition delay of a diesel droplet increased below 500 °C as the pressure rose, but decreased at temperatures above 600 °C. However, the ignition delay of an emulsion droplet decreased above 600 °C, as the pressure decreased due to the effect of the micro-explosion.
- The total lifetime of an emulsion droplet was higher than that of a diesel droplet except under 5 bar, 600 °C condition. Under this condition, the fast flame lifetime of the emulsion droplet represented a shorter total droplet lifetime than diesel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selim, M.Y.E.; Ghannam, M.T. Combustion study of stabilized water-in-diesel fuel emulsion. Energy Sources Part A 2009, 32, 256–274. [Google Scholar] [CrossRef]
- Kim, H.M.; Won, J.H.; Baek, S.W. Evaporation of a single emulsion fuel droplet in elevated temperature and pressure conditions. Fuel 2018, 226, 172–180. [Google Scholar] [CrossRef]
- Vellaiyan, S.; Amirthagadeswaran, K.S. Zinc oxide incorporated water-in-diesel emulsion fuel: Formulation, particle size measurement, and emission characteristics assessment. Pet. Sci. Technol. 2016, 34, 114–122. [Google Scholar] [CrossRef]
- Randazzo, M.L.; Sodre, J.R. Exhaust emissions from a diesel powered vehicle fuelled by soybean biodiesel blends (B3–B20) with ethanol as an additive (B20E2–B20E5). Fuel 2011, 90, 90–103. [Google Scholar] [CrossRef]
- Kichatov, B.; Korshunov, A.; Kiverin, A.; Son, E. Experimental study of foamed emulsion combustion: Influence of solid microparticles, glycerol and surfactant. Fuel Process. Technol. 2017, 166, 77–85. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental understanding on the dynamics of micro-explosion and puffing in ternary emulsion droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Ithnin, A.M.; Ahmad, M.A.; Bakar, M.A.A.; Rajoo, S.; Yahya, W.J. Combustion performance and emission analysis of diesel engine fuelled with water-in-diesel emulsion fuel made from low-grade diesel fuel. Energy Convers. Manag. 2015, 90, 375–382. [Google Scholar] [CrossRef]
- Jang, G.M.; Kim, N.I. Relationships between dynamic behavior and properties of a single droplet of water-emulsified n-dodecane. Fuel 2018, 220, 130–139. [Google Scholar] [CrossRef]
- Elsanusi, O.A.; Roy, M.M.; Sidhu, M.S. Experimental investigation on a diesel engine fueled by diesel-biodiesel blends and their emulsions at various engine operating conditions. Appl. Energy 2017, 203, 582–593. [Google Scholar] [CrossRef]
- Reham, S.S.; Masjuki, H.H.; Kalam, M.A.; Shancita, I.; Fattah, I.M.; Ruhul, A.M. Study on stability, fuel properties, engine combustion, performance and emission characteristics of biofuel emulsion. Renew. Sustain. Energy Rev. 2015, 52, 1566–1579. [Google Scholar] [CrossRef]
- Jeong, I.C.; Lee, K.H. Auto-ignition and micro-explosion behavior of droplet arrays of water-in-fuel emulsion. Int. J. Automot. Technol. 2008, 9, 735–740. [Google Scholar] [CrossRef]
- Ghassemi, H.; Baek, S.W.; Khan, Q.S. Experimental study on evaporation of kerosene droplets at elevated pressures and temperatures. Combust. Sci. Technol. 2006, 178, 1669–1684. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Li, Z.; Du, W.; Yin, Z.; Xu, Z. Evaporation and combustion characteristics of hydrocarbon fuel droplet in sub- and super-critical environments. Fuel 2018, 220, 763–768. [Google Scholar] [CrossRef]
- Faik, A.; Zhang, Y. Multicomponent fuel droplet combustion investigation using magnified high speed backlighting and shadowgraph imaging. Fuel 2018, 221, 89–109. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Wang, B.; Wei, Y. Superheat limit and micro-explosion in droplets of hydrous ethanol-diesel emulsions at atmospheric pressure and diesel-like conditions. Energy 2018, 154, 535–543. [Google Scholar] [CrossRef]
- Genot, C.; Kabri, T.H.; Meynier, A. Stabilization of omega-3 oils and enriched foods using emulsifiers. In Food Enrichment with Omega-3 Fatty Acids; Woodhead Publishing: Sawston, Cambridge, UK, 2013; pp. 150–193. [Google Scholar]
- Noor El-Din, M.R.; El-Hamouly, S.H.; Mohamed, H.M.; Mishrif, M.R.; Ragab, A.M. Water-in diesel fuel nanoemulsions: Preparation, stability, and physical properties. Egypt. J. Pet. 2013, 22, 517–530. [Google Scholar] [CrossRef]
- Xie, F.; Brooks, B.W. Phase behaviour of a non-ionic surfactant-polymeric solution-water system during the phase inversion process. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 27–32. [Google Scholar] [CrossRef]
- Liang, X.; Wu, J.; Yang, X.; Tu, Z.; Wang, Y. Investigation of oil-in-water emulsion stability with relevant interfacial characteristics simulated by dissipative particles dynamics. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 107–114. [Google Scholar] [CrossRef]
- Rastogi, P.; Mehta, P.S.; Kaisare, N.S.; Basavaraj, M.G. Kinetic stability of surfactant stabilized water-in-diesel emulsion fuels. Fuel 2019, 236, 1415–1422. [Google Scholar]
- Fan, X.; Hu, W.; Yang, J.; Xu, X.; Gao, J. A New emulsifier behavior of the preparation for micro-emulsified diesel oil. Pet. Sci. Technol. 2008, 26, 2125–2136. [Google Scholar] [CrossRef]
- Won, J.H.; Baek, S.W.; Kim, H.M. Autoignition and combustion behavior of emulsion droplet under elevated temperature and pressure conditions. Energy 2018, 163, 800–810. [Google Scholar] [CrossRef]
- Harada, T.; Watanabe, H.; Suzuki, Y.; Kamata, H.; Matsushita, Y.; Aoki, H.; Miura, T. A numerical investigation of evaporation characteristics of a fuel droplet suspended from a thermocouple. Int. J. Heat Mass Transf. 2011, 54, 649–655. [Google Scholar] [CrossRef]
- Khan, Q.S.; Baek, S.W.; Ghassemi, H. On the autoignition and combustion characteristics of kerosene droplets at elevated pressure and temperature. Combust. Sci. Technol. 2007, 179, 2437–2451. [Google Scholar] [CrossRef]
- Kim, H.M.; Baek, S.W.; Chang, D.J. Auto-ignition characteristics of single n-heptane droplet in a rapid compression machine. Combust. Sci. Technol. 2014, 186, 912–927. [Google Scholar] [CrossRef]
- Joshi, R.M.; Pegg, M.J. Flow properties of biodiesel fuel blends at low temperatures. Fuel 2007, 86, 143–151. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Available online: https://www.engineeringtoolbox.com/water-dynamic-kinematic-viscosity-d_596.html (accessed on 12 February 2019).
- Benayoune, M.; Khezzar, L.; Al-Rumhy, M. Viscosity of water in oil emulsions. Pet. Sci. Technol. 1998, 16, 767–784. [Google Scholar] [CrossRef]
- Singh, B.P.; Pandey, B.P. Ultrasonication for breaking water-in-oil emulsions. Proc. Indian Natl. Sci. Acad. USA 1992, 58, 181–194. [Google Scholar]
- Antonov, V.N. Features of preparation of water-fuel emulsions for diesel engines. Chem. Technol. Fuels Oils 1983, 19, 606–609. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W. Autoignition and combustion characteristics of kerosene droplets with dilute concentrations of aluminum nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 774–787. [Google Scholar] [CrossRef]
- Lee, D.G.; Won, J.H.; Kim, H.M.; Baek, S.W. Autoignition behavior of an ethanol-methylcellulose gel droplet in a hot environment. Energies 2018, 11, 2168. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 2nd ed.; McGraw-Hill: Singapore, 2005; pp. 337–394. [Google Scholar]






| Property | Diesel |
|---|---|
| Boiling point (°C) | 130–360 |
| Flash point (°C) | Higher than 40 |
| Autoignition point (°C) | Higher than 260 |
| Density at 15 °C (kg/m3) | 815–835 |
| Cetane number | Higher than 52 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.; Baek, S.W.; Kim, H.; Lee, H. The Viscosity and Combustion Characteristics of Single-Droplet Water-Diesel Emulsion. Energies 2019, 12, 1963. https://doi.org/10.3390/en12101963
Won J, Baek SW, Kim H, Lee H. The Viscosity and Combustion Characteristics of Single-Droplet Water-Diesel Emulsion. Energies. 2019; 12(10):1963. https://doi.org/10.3390/en12101963
Chicago/Turabian StyleWon, Jonghan, Seung Wook Baek, Hyemin Kim, and Hookyung Lee. 2019. "The Viscosity and Combustion Characteristics of Single-Droplet Water-Diesel Emulsion" Energies 12, no. 10: 1963. https://doi.org/10.3390/en12101963




