Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Device
2.2. Procedures
2.2.1. Preparation of the Inoculum and Conditioning of the Anode
2.2.2. Biodegradation of Wastewater
- (a)
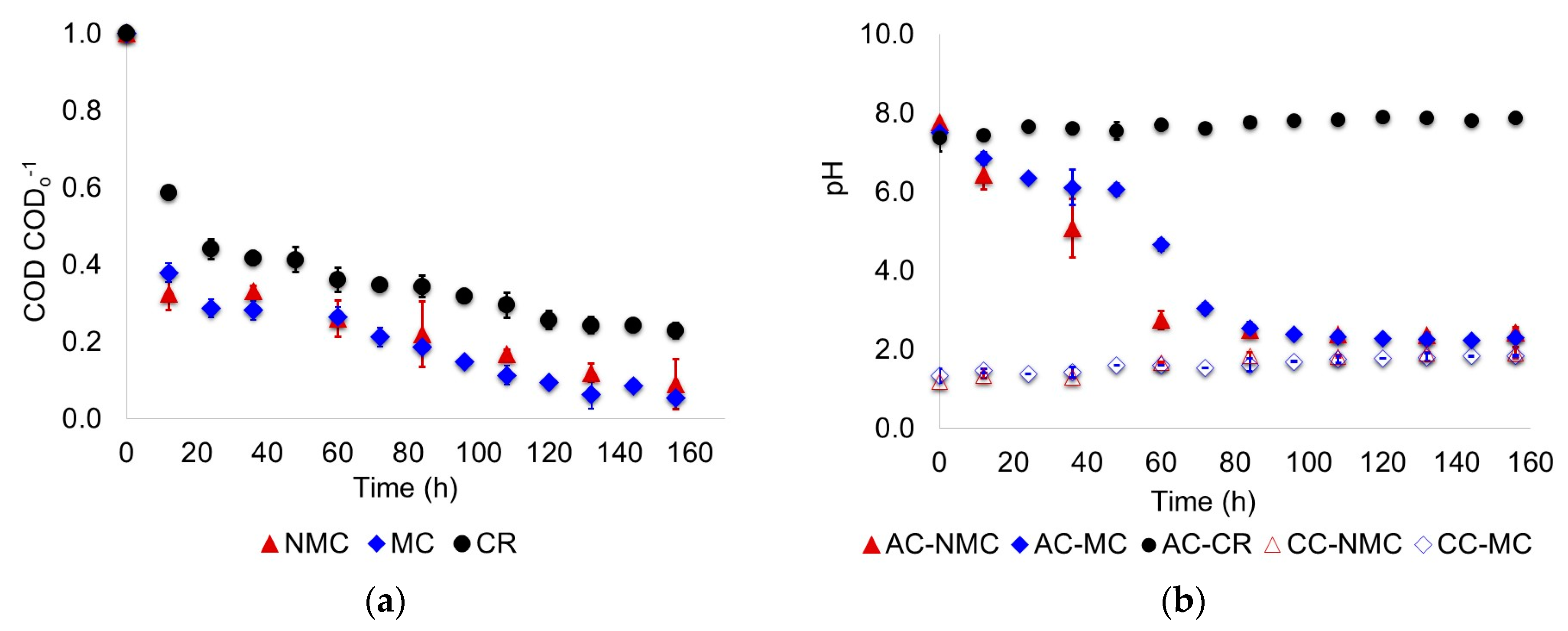
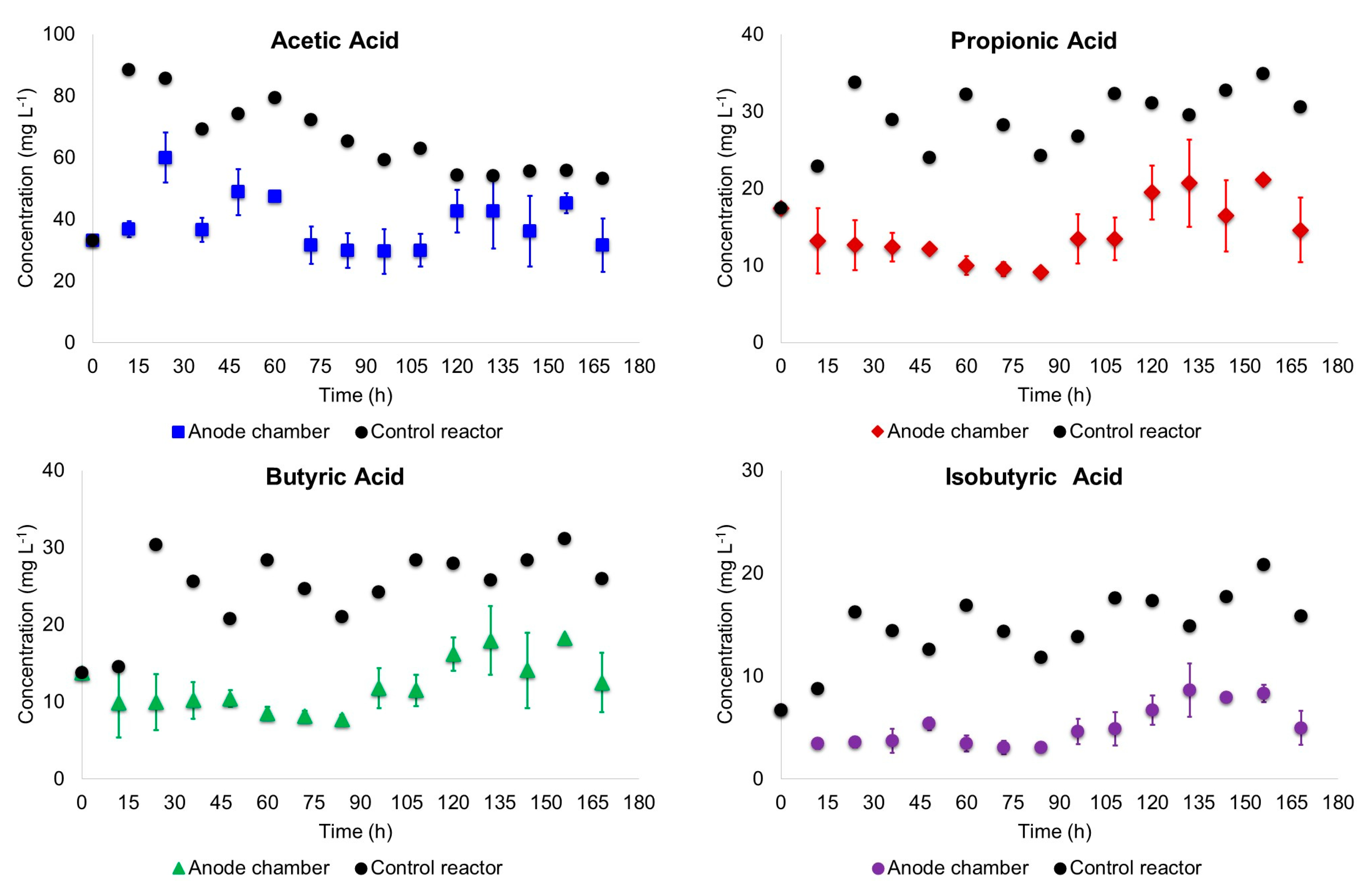
- Supernatant samples of the anode chamber liquor were taken every 12 h. For the scenario MC, mixing in the anode chamber was paused and settling of the suspended biomass was permitted per 1 h; then 5 mL supernatant was taken using plastic syringes and needles (BD Plastik). COD, pH and volatile fatty acids (VFA) of the samples were measured. COD and pH were determined based on the procedures described in the Standard Methods for the Examination of Water and Wastewater [14]. Volatile fatty acids were determined by GC-FID with a HP-FFAP (25 m × 0.32 mm × 0.50 µm) column. The standards (organic acids kit) used to prepare the calibration curves were obtained from Sigma-Aldrich (Toluca, Mexico).
- (b)
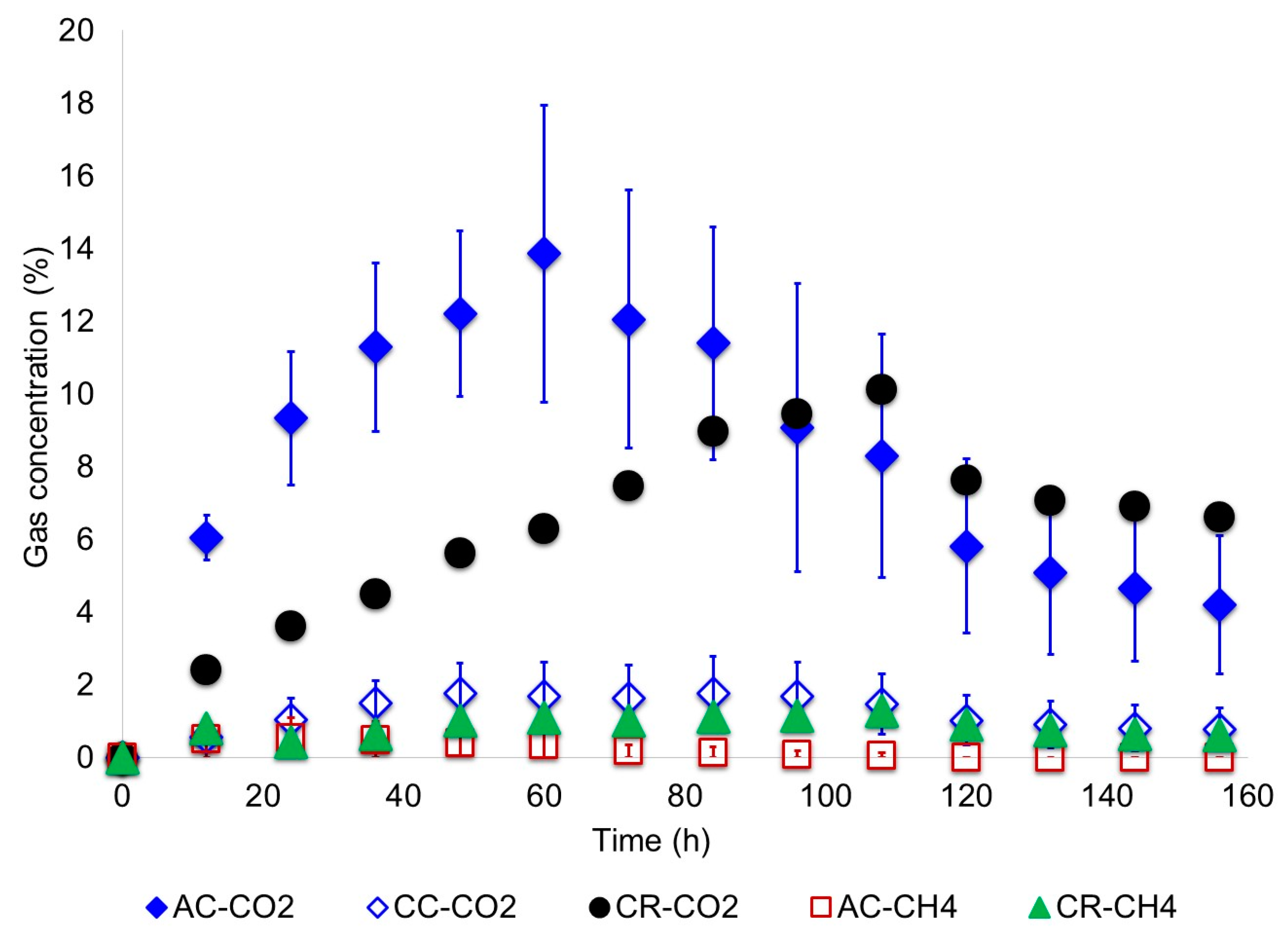
- Gases generated in the anode chamber such as H2, CO2 and CH4 were measured every 12 h. To do that, a 300 μL gas sample was taken with a micro gas-tight syringe provided with a push-pull valve. Then, the sample was analyzed by GC-TCD with a HP-MOLESIEVE (30 m × 0.53 mm × 25 µm) column. Calibration of the chromatographer was conducted with a standard gases mixture of hydrogen (5%), methane (5%), carbon dioxide (5%) and helium (85%) obtained from INFRA Air Products (Mexico).
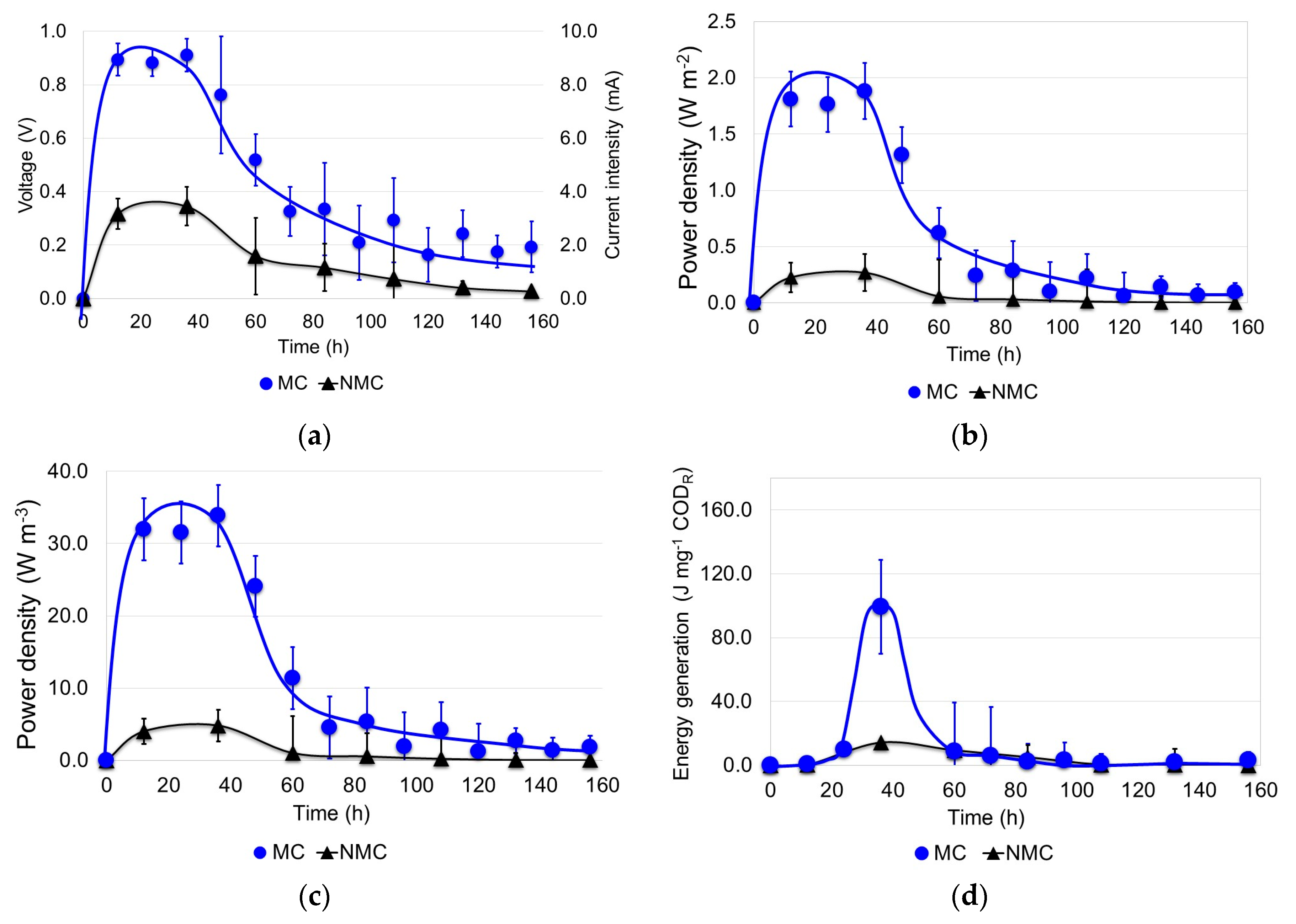
2.2.3. Generation of Electricity
3. Results and Discussion
3.1. Biodegradation of Wastewater
3.2. Generation of Electricity in the MFCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, M.; Yang, J.; Wang, H.; Jin, T.; Hassett, D.J.; Gu, T. Bioelectrochemistry of microbial fuel cells and their potential applications in bioenergy. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdan, The Netherlands, 2014; Volume 9, pp. 131–152. [Google Scholar]
- Virdis, B.; Freguia, S.; Rozendal, R.A.; Rabaey, K.; Yuan, Z.; Keller, J. Microbial fuel cells. In Treatese on Water Science, Water Quality Engineering; Wilderer, P., Hanaki, K., Vereijken, T., Eds.; Elsevier: Amsterdan, The Netherlands, 2011; Volume 4, pp. 641–665. [Google Scholar]
- Logan, B. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Logan, B.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Surajbhan, S.S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of perchlorate and nitrate contaminated water: A review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar]
- Hernández-Fernández, F.J.; De Los Ríos, A.P.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Lozano-Blanco, L.J.; Godínez, C.; Tomás-Alonso, F.; Quesada-Medina, J. Recent progress and perspectives in microbial fuel cells for bioenergy generation and wastewater treatment. Fuel Process Technol. 2015, 138, 284–297. [Google Scholar] [CrossRef]
- Wilkinson, S. “Gastrobots”—Benefits and challenges of microbial fuel cells in food powered robot applications. Auton. Robots 2000, 9, 99–111. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Melhuish, C.; Greenman, J. Artificial metabolism: Towards true energetic autonomy in artificial life. Adv. Artif. Life Proc. 2003, 2801, 792–799. [Google Scholar]
- Shantaram, A.; Beyenal, H.; Raajan, R.; Veluchamy, A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 39, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Tender, L.M.; Gray, S.A.; Groveman, E.; Lowy, D.A.; Kauffman, P.; Melhado, J.; Tyce, R.C.; Flynn, D.; Petrecca, R.; Dobarro, J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar] [CrossRef]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulus, I.A. PEE POWER® urinal II–Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Gajda, I.; Greenman, J.; Santoro, C.; Serov, A.; Melhuish, C.; Atanassov, P.; Ieropoulos, I.A. Improved power and long term performance of microbial fuel cell with Fe-N-C catalyst in air-breathing cathode. Energy 2018, 144, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Solati-Hashjin, M.; Moaddel, H. Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell. J. Membr. Sci. 2010, 365, 286–293. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Huang, L.; Logan, B. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biot. 2008, 80, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Logan, B. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Ostrem, K. Greening Waste: Anaerobic Digestion for Treating the Organic Fraction of Municipal Solid Wastes; Department of Earth and Environmental Engineering, Columbia University: New York, NY, USA, 2004. [Google Scholar]
- Grady, C.P.L.; Daigger, G.Y.; Lim, H.C. Biological Wastewater Treatment, 2nd ed.; Marcel-Dekker: New York, NY, USA, 1999. [Google Scholar]
- Chou, T.Y.; Whiteley, C.G.; Lee, D.J. Anodic potential on dual-chambered microbial fuel cell with sulfate reducing bacteria biofilm. Int. J. Hydrogen Energy 2014, 39, 19225–19231. [Google Scholar] [CrossRef]
- Zhou, M.; Gu, T. The next breakthrough in microbial fuel cells and microbial electrolysis cells for bioenergy and bioproducts. J. Microb. Biochem. Technol. S 2013, 12, 2. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Karra, U.; Troop, E.; Curtis, M.; Scheible, K.; Tenaglier, C.; Patel, N.; Li, B. Performance of plug flow microbial fuel cell (PF-MFC) and complete mixing microbial fuel cell (CM-MFC) for wastewater treatment and power generation. Int. J. Hydrogen Energy 2013, 38, 5383–5388. [Google Scholar] [CrossRef]
- Vizhemehr, A.; Kariminia, H.; Yaghmaei, S. Prediction of electricity generation in a duel chamber microbial fuel cell. Iran. J. Chem. Eng. 2012, 9, 3–11. [Google Scholar]
- Nien, P.C.; Lee, C.Y.; Ho, K.C.; Adav, S.; Liu, L.; Wang, A.; Lee, D.J. Power overshoot in two-chambered microbial fuel cell (MFC). Bioresour. Technol. 2011, 102, 4742–4746. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, B.; Zhou, S.; Zhong, S.; Zhuang, L. Electrocatalytic Activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, P.; Guo, Y.; Zhang, K. Electricity generation during wastewater treatment: An approach using an AFB-MFC for alcohol distillery wastewater. Desalination 2011, 276, 373–378. [Google Scholar] [CrossRef]
- Fornero, J.; Rosenbaum, M.; Angenent, L. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanalysis 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Sharma, V.N. Bioelectricity production from various wastewaters through microbial fuel cell technology. J. Biochem. Tech. 2009, 2, 133–137. [Google Scholar]
- Rozendal, R.; Hamelers, H.; Rabaey, K.; Keller, J.; Buisman, C. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.; Cañizares, P.; Lobato, J.; Paz, R.; Sáez, C.; Linares, J. Production of electricity from the treatment of urban wastewater using a microbial fuel cell. J. Power Sources 2007, 169, 198–204. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006, 70, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.; Hamelers, H.; Buisman, C. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Pham, T.; Chang, I.; Kang, K.; Moon, H.; Cho, K.; Kim, B. Construction and operation of a novel mediator and membrane less microbial fuel cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Rabaey, K.; Lissens, G.; Siciliano, S.; Verstraete, W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechno. Lett. 2003, 25, 1531–1535. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dharmalingam, S. A study of influence on nanocomposite membrane of sulfonated TiO2 and sulfonated polystyrene-ethylene-butylene-polystyrene for microbial fuel cell application. Energy 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Pant, D.; Van, B.G.; Alvarez, G.Y.; Diels, L.; Vanbroekhoven, K. Evaluation of bioelectrogenetic potential of four industrial effluents as substrate for low cost microbial fuel cells operation. Environ. Eng. Manag. J. 2016, 15, 1897–1904. [Google Scholar] [CrossRef]
- Fatemi, S.; Ghoreyshi, A.A.; Rahimnejad, M.; Darzi, G.N.; Pant, D. Sulfide as an alternative electron donor to glucose for power generation in mediator-less microbial fuel cell. J. Environ. Sci. Health. A 2017, 52, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Mashkour, M.; Rahimnejad, M.; Pourali, S.M.; Ezoji, H.; ElMekawy, A.; Pant, D. Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Pro. Nat. Sci. Mater. 2017, 27, 647–651. [Google Scholar] [CrossRef]
- Choudhury, P.; Prasad Uday, U.S.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannelli, N.; Nastro, R.A.; Cigolotti, V.; Minutillo, M.; Falcucci, G. Low pH, high salinity: Too much for Microbial Fuel Cells? Appl. Energy 2017, 192, 543–550. [Google Scholar] [CrossRef]



| Constituent | Unit | NMC | MC | CR | |
|---|---|---|---|---|---|
| Inoculum (MLVSS) | Vol. | mL | 33.0 | 33.0–37.0 | 33.0–37.0 |
| Mass | mg | 112.4 | 112.4–113.4 | 112.4–113.4 | |
| Wastewater | Vol. | mL | 250.0 | 250.0 | 250.0 |
| Total COD | mg·L−1 | 906.7 | 906.7–1253.3 | 906.7–1253.3 | |
| Electrolyte (0.1 M HCl) | Vol. | mL | 283.0 | 283.0–287.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Zavala, M.Á.; Torres Delenne, P.R.; González Peña, O.I. Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses. Energies 2018, 11, 2309. https://doi.org/10.3390/en11092309
López Zavala MÁ, Torres Delenne PR, González Peña OI. Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses. Energies. 2018; 11(9):2309. https://doi.org/10.3390/en11092309
Chicago/Turabian StyleLópez Zavala, Miguel Ángel, Pamela Renée Torres Delenne, and Omar Israel González Peña. 2018. "Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses" Energies 11, no. 9: 2309. https://doi.org/10.3390/en11092309
APA StyleLópez Zavala, M. Á., Torres Delenne, P. R., & González Peña, O. I. (2018). Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses. Energies, 11(9), 2309. https://doi.org/10.3390/en11092309






