Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
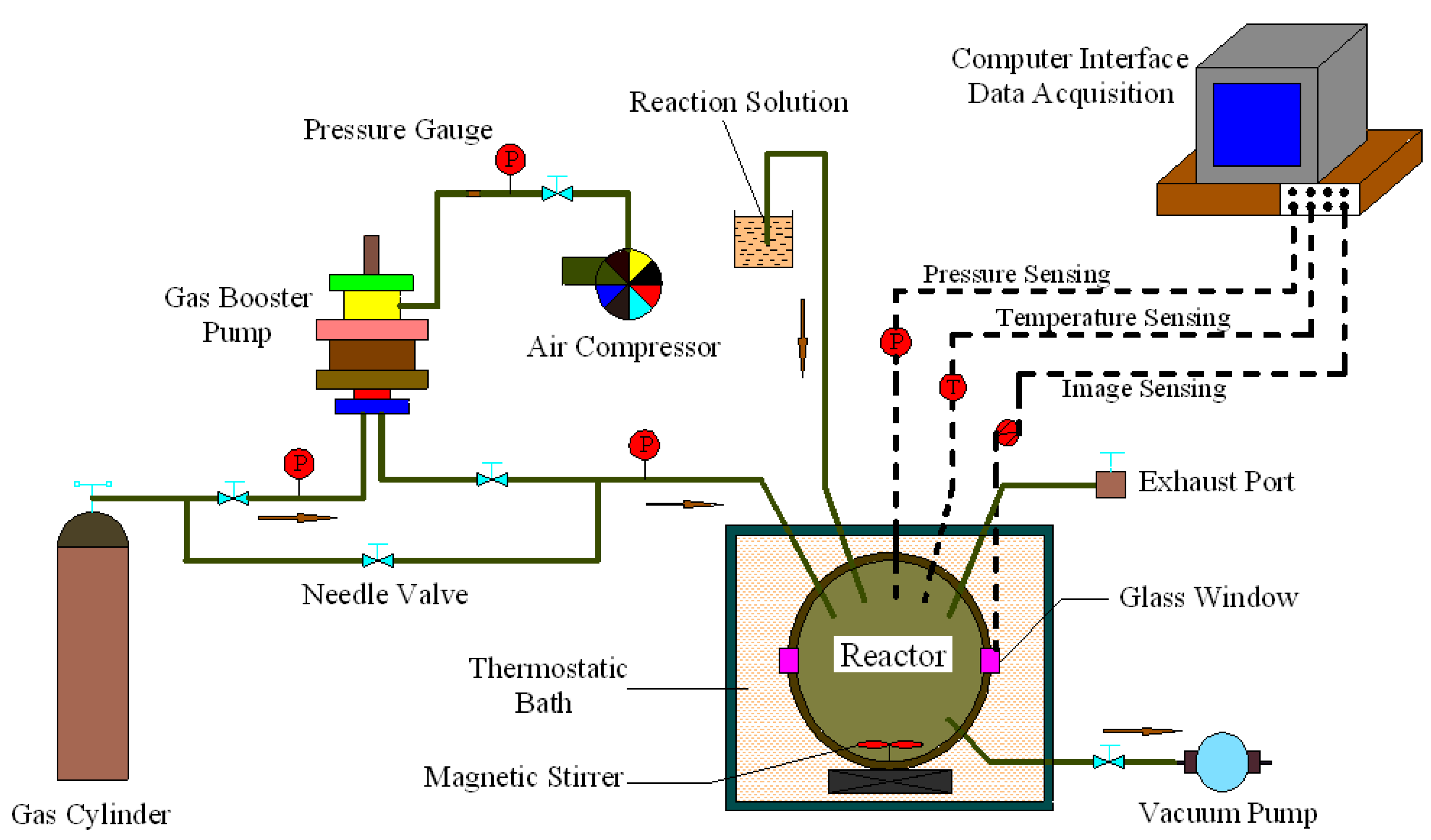
2.2. Apparatus
2.3. Procedure
3. Results and Discussion
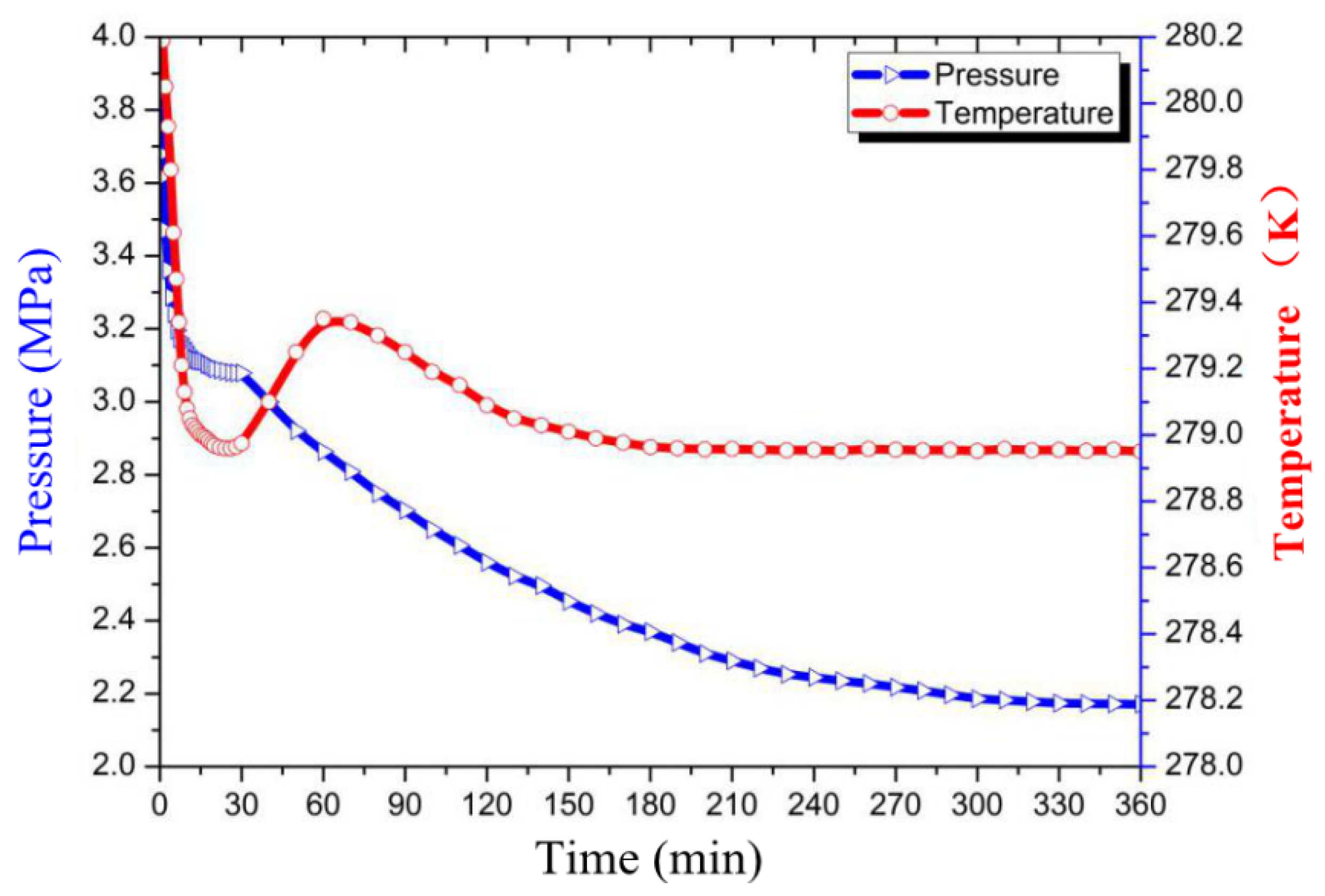
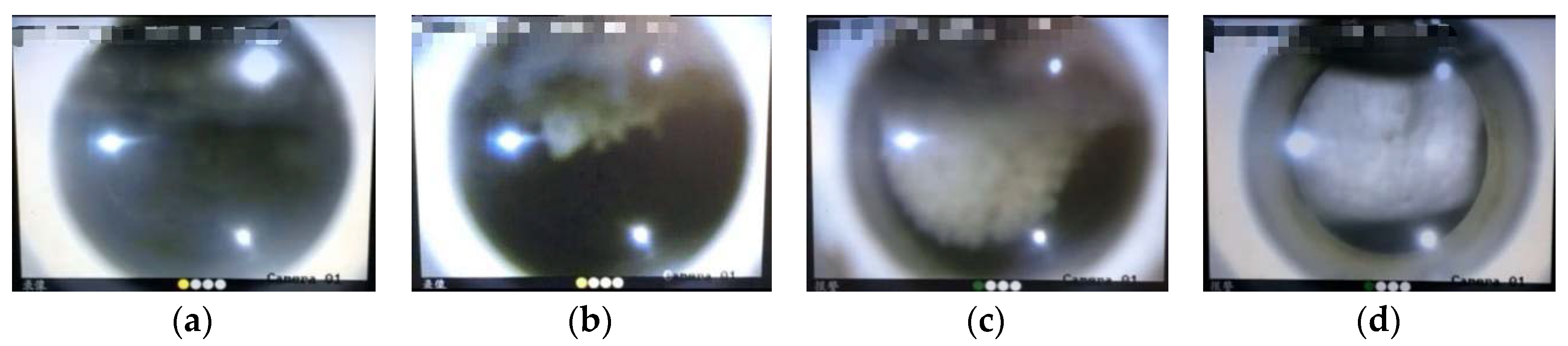
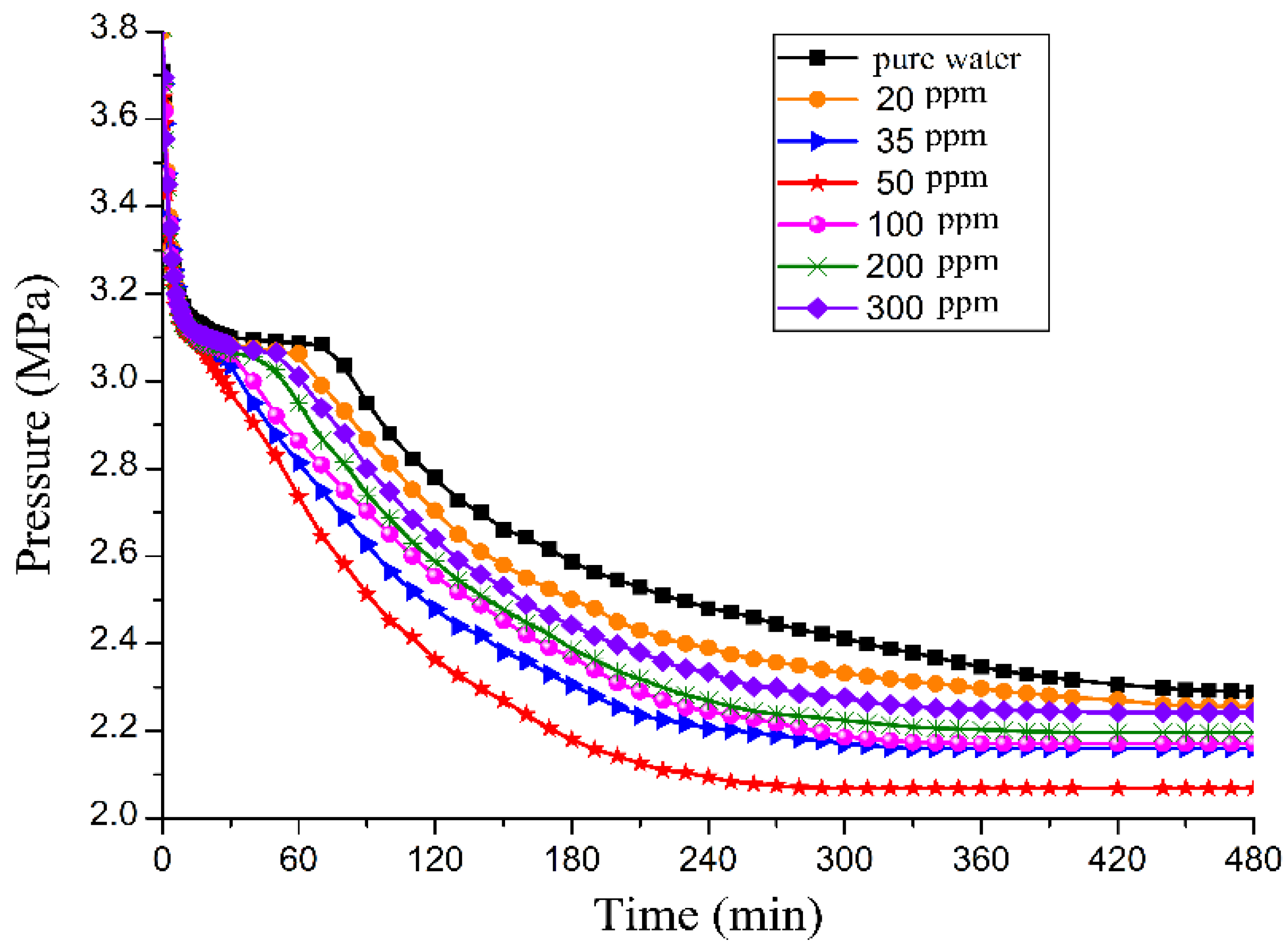
3.1. Effect of GO on CO2 Hydrate Formation Process
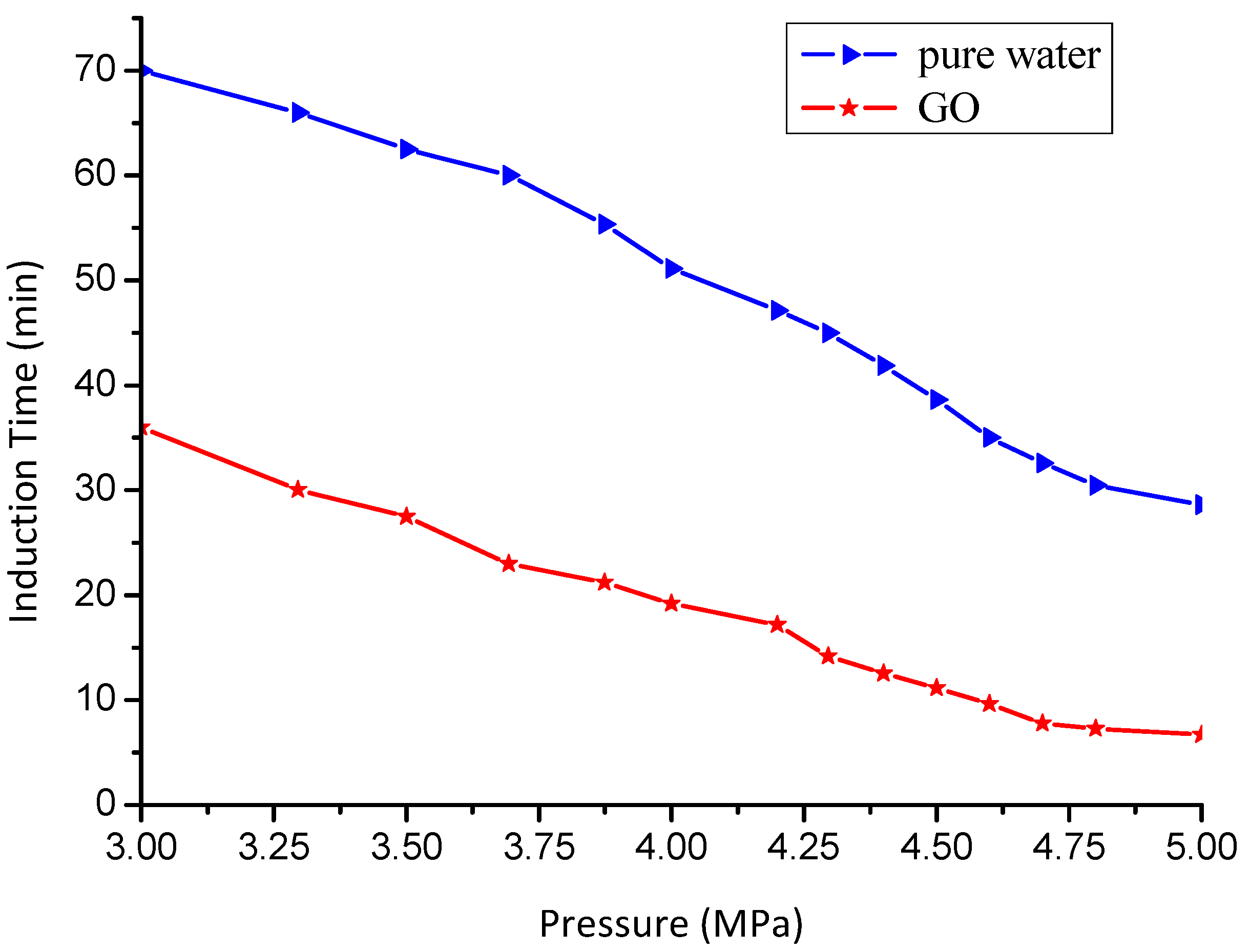
3.2. Effect of GO on the Induction Time
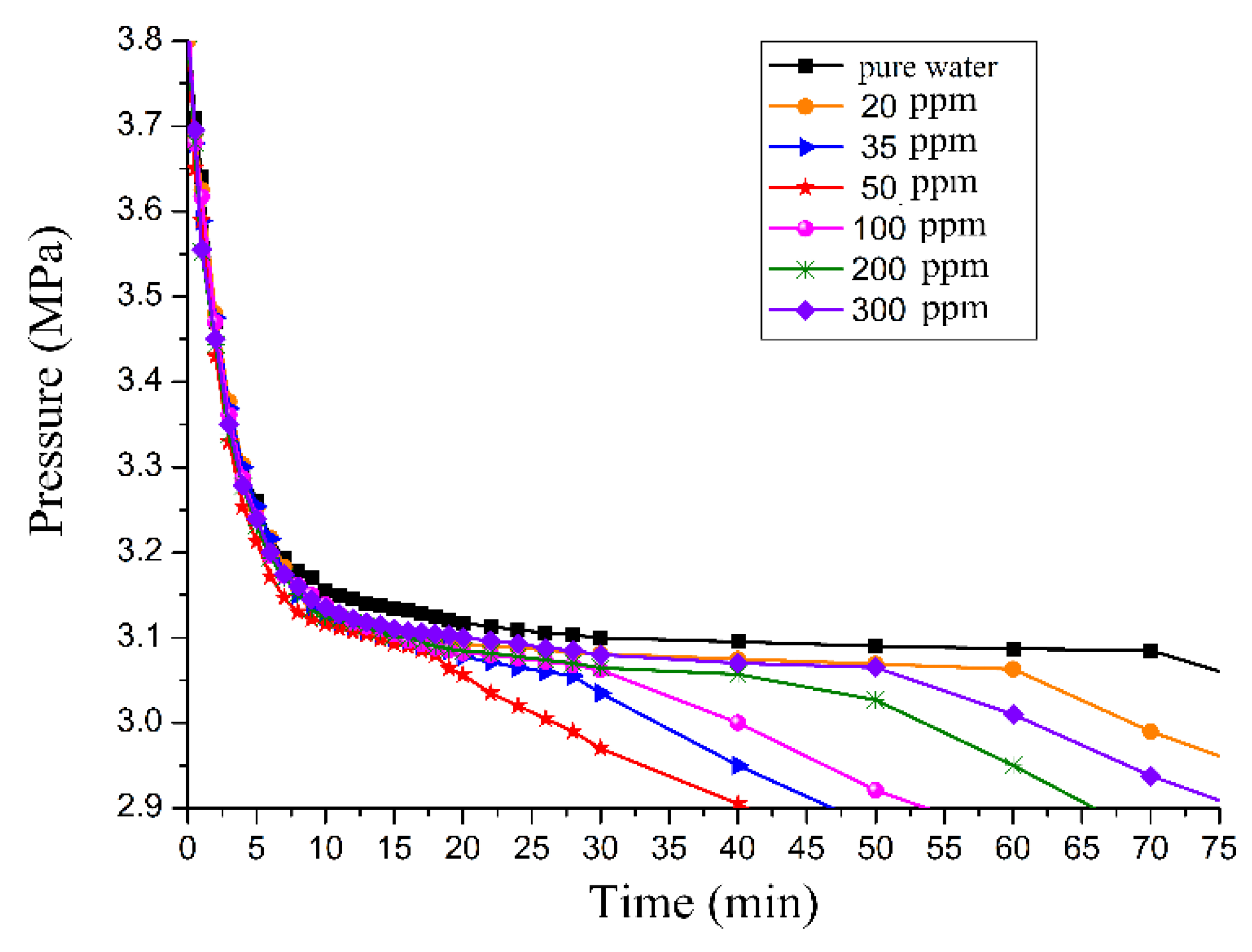
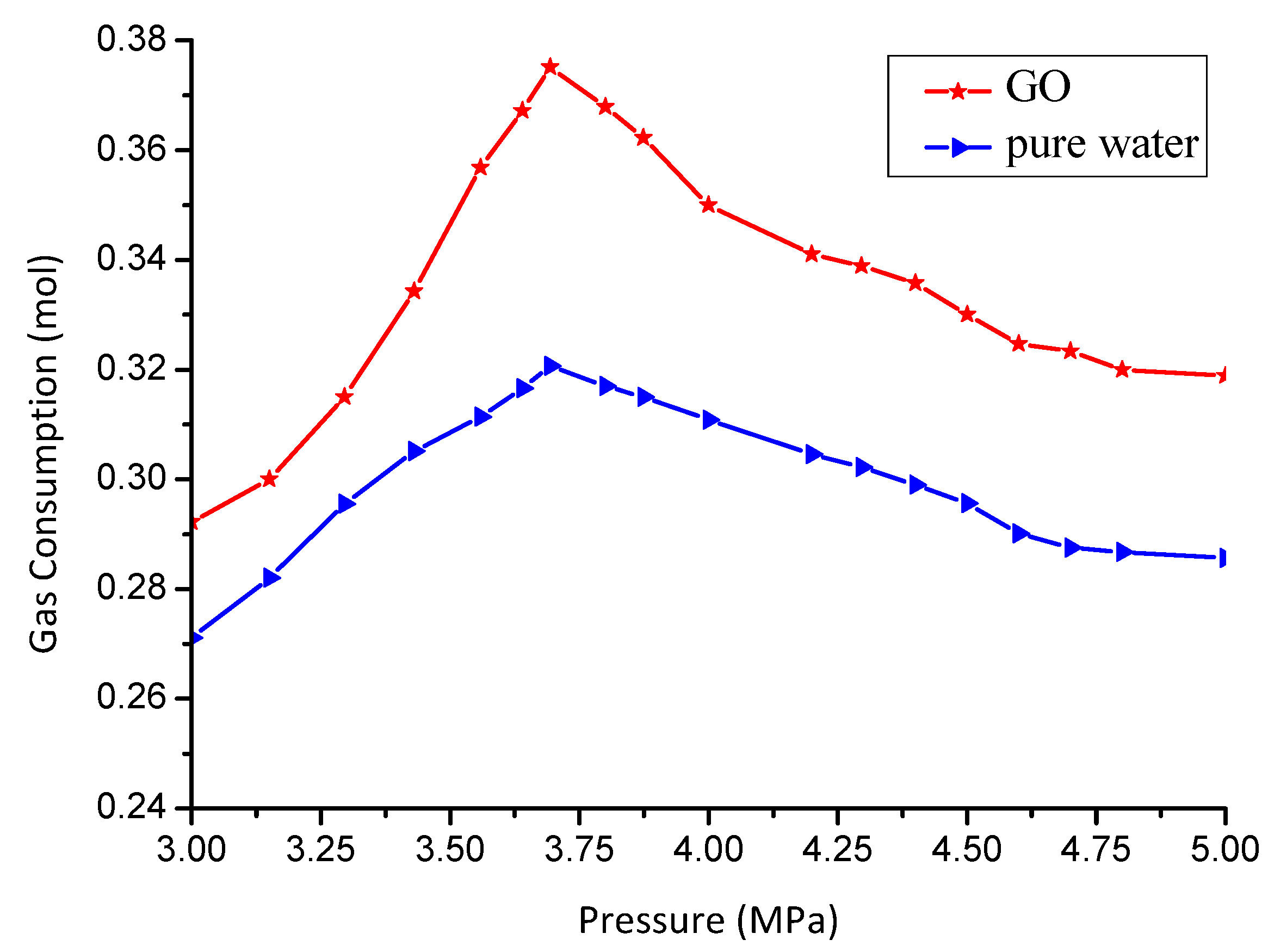
3.3. Effect of GO on Gas Consumption
3.4. Effect of the GO Concentration on Promotion
3.5. Promotion Mechanism
- (1)
- GO can improve the efficiency of gas hydrate nucleation. The molecular structure of GO is characterized as an oxidized graphite monolayer, whose structure generally maintains a planar structure. It is composed of a similar aromatic ring structure formed by carbon-carbon double bonds. There are abundant hydroxyl and epoxy groups on its surface, and substantial carboxyl groups at the edge of the plane structure. These functional groups help form hydrogen bonds with water molecules, so it can promote the hydration of the gas, and can provide a lot of nucleation sites for the gas molecules. The crystal nucleation involves heterogeneous nucleation and homogeneous nucleation. The former forms the hydrate crystal nuclei more easily, because the requirements for the super-saturation and super-cooling are fairly reduced in heterogeneous nucleation system. GO nanoparticles increase the inhomogeneity of the hydrate formation system, thus providing an environment for heterogeneous nucleation, which makes it easier for hydrate nucleation.
- (2)
- GO can effectively enhance the heat transfer. Gas hydrate formation is an exothermic process, thus if reaction heat can be removed in time and effectively then the hydrate formation rate will be accelerated. The thickness of monolayer GO is about 0.7–1.6 nm [34]. Compared with the traditional surfactants and the other nanoparticles, GO has excellent features such as a great specific surface area and thermal conductivity, so the heat-transfer performance increases with the addition of GO nanoparticles. The heat generated during hydrate formation can be removed in a timely way so as to form a more uniform and more suitable temperature field. In this way, GO promotes the growth of hydrate crystal nuclei and shortens the generation period.
- (3)
- GO can enhance the mass transfer dramatically. GO is an amphipathic compound, in other words, it presents a range of hydrophilic to hydrophobic properties from the edge of graphene sheet to the center. Therefore, it can act like a surfactant to reduce the energy between the interfaces, enlarge the contact area between phases, increase the gas solubility in the liquid phase, and accelerate the hydrate growth rate. In addition, due to the large surface area, GO can better carry molecules from the gas phase into the liquid phase so as to form the hydrate crystal nucleus.
- (4)
- The diameter of a GO particle is extremely small. The tinier the particle size is, the greater the micromotion intensity will be, thus, heat and mass transfer efficiency are improved. According to the experimental results, the additive concentration also significantly influences the promoting effect in gas hydrate formation. The promotion effect of a low GO concentration was better than that of a high concentration. This could be interpreted as due to the fact that when the concentration of GO reached a certain degree, the dissolved gas and GO particles easily form micelles which would be absorbed onto the hydrate surface, leading to a reduction of the surface energy, and then it was very difficult for the gas to disperse into the liquid phase [27]. Besides, a high GO suspension concentration means that the number of particles in a unit volume of solution increased. The intermolecular interaction became stronger due to the smaller molecular spacing and the quite lower molecular weight of GO, which leads to an increased concentration and viscosity of the solution, and even contributes to the phenomena of agglomeration and precipitation. In the end, the heat and mass transfer efficiency of the interfaces were severely reduced. However, a high concentration of GO suspension was not conducive to enhanced gas solubility and promotion of crystal nucleus growth, therefore, there exists an optimal GO concentration that best promotes gas hydrate formation.
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.F.A.; Dinis, M.A.P.; de Sousa, M.J.L. Review of European energy policies regarding the recent “carbon capture, utilization and storage” technologies scenario and the role of coal seams. Environ. Earth Sci. 2015, 74, 2553–2561. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Lee, Y.; Lee, Y.; Seo, Y. Hydrate-based pre-combustion capture of carbon dioxide in the presence of a thermodynamic promoter and porous silica gels. Int. J. Greenh. Gas Control 2013, 14, 193–199. [Google Scholar] [CrossRef]
- Ricaurte, M.; Dicharry, C.; Broseta, D.; Renaud, X.; Torré, J.P. CO2 removal from a CO2–CH4 gas mixture by clathrate hydrate formation using THF and SDS as water-soluble hydrate promoters. Ind. Eng. Chem. Res. 2013, 52, 899–910. [Google Scholar] [CrossRef]
- Li, A.; Jiang, L.; Tang, S. An experimental study on carbon dioxide hydrate formation using a gas-inducing agitated reactor. Energy 2017, 134, 629–637. [Google Scholar] [CrossRef]
- Lv, Q.N.; Li, X.S.; Xu, C.G.; Chen, Z.Y. Experimental investigation of the formation of cyclopentane-methane hydrate in a novel and large-size bubble column reactor. Ind. Eng. Chem. Res. 2012, 51, 5967–5975. [Google Scholar] [CrossRef]
- Rossi, F.; Filipponi, M.; Castellani, B. Investigation on a novel reactor for gas hydrate production. Appl. Energy 2012, 99, 167–172. [Google Scholar] [CrossRef]
- Moeini, H.; Bonyadi, M.; Esmaeilzadeh, F.; Rasoolzadeh, A. Experimental study of sodium chloride aqueous solution effect on the kinetic parameters of carbon dioxide hydrate formation in the presence/absence of magnetic field. J. Nat. Gas Sci. Eng. 2018, 150, 231–239. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, N.J. Study on methane hydrate formation using ultrasonic waves. J. Ind. Eng. Chem. 2013, 19, 1668–1672. [Google Scholar] [CrossRef]
- Da Silva Lirio, C.F.; Pessoa, F.L.P.; Uller, A.M.C. Storage capacity of carbon dioxide hydrates in the presence of sodium dodecyl sulfate (SDS) and tetrahydrofuran (THF). Chem. Eng. Sci. 2013, 96, 118–123. [Google Scholar] [CrossRef]
- Li, R.; Li, X.S.; Chen, Z.Y.; Zhang, Y.; Xu, C.G.; Xia, Z.M. Anti-Agglomerator of Tetra-n-Butyl Ammonium Bromide Hydrate and Its Effect on Hydrate-Based CO2 Capture. Energies 2018, 11, 399. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Z.Z.; Luo, S.J.; Fu, S.F.; Wang, L.; Shi, X.S.; Guo, R.B. Effects of different anionic surfactants on methane hydrate formation. Chem. Eng. Sci. 2015, 137, 896–903. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Wang, N.; Wang, X.; Gao, J.; Li, H. Dispersion behavior and thermal conductivity characteristics of Al2O3–H2O nanofluids. Curr. Appl. Phys. 2009, 9, 131–139. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Esfahany, M.N. Experimental study of turbulent convective heat transfer and pressure drop of dilute CuO/water nanofluid inside a circular tube. Int. Commun. Heat Mass 2010, 37, 214–219. [Google Scholar] [CrossRef]
- Pineda, I.T.; Lee, J.W.; Jung, I.; Kang, Y.T. CO2 absorption enhancement by methanol-based Al2O3, and SiO2 nanofluids in a tray column absorber. Int. J. Refri. 2012, 35, 1402–1409. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, J.W.; Kang, Y.T. CO2 absorption characteristics of nanoparticle suspensions in methanol. J. Mech. Sci. Technol. 2012, 26, 2285–2290. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2013, 53, 206–211. [Google Scholar] [CrossRef]
- Haghtalab, A.; Mohammadi, M.; Fakhroueian, Z. Absorption and solubility measurement of CO2 in water-based ZnO and SiO2 nanofluids. Fluid Phase Equilib. 2015, 392, 33–42. [Google Scholar] [CrossRef]
- Prasad, P.S.; Chari, V.D.; Sharma, D.V.; Murthy, S.R. Effect of silica particles on the stability of methane hydrates. Fluid Phase Equilib. 2012, 318, 110–114. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and dissociation of HFC134a gas hydrate in nano-copper suspension. Energy Convers. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Najibi, H.; Shayegan, M.M.; Heidary, H. Experimental investigation of methane hydrate formation in the presence of copper oxide nanoparticles and SDS. J. Nat. Gas Sci. Eng. 2015, 23, 315–323. [Google Scholar] [CrossRef]
- Aliabadi, M.; Rasoolzadeh, A.; Esmaeilzadeh, F.; Alamdari, A. Experimental Study of Using CuO Nanoparticles as a Methane Hydrate Promoter. J. Nat. Gas Sci. Eng. 2015, 27, 1518–1522. [Google Scholar] [CrossRef]
- Arjang, S.; Manteghian, M.; Mohammadi, A. Effect of synthesized silver nanoparticles in promoting methane hydrate formation at 4.7 MPa and 5.7 MPa. Chem. Eng. Res. Des. 2013, 91, 1050–1054. [Google Scholar] [CrossRef]
- Chari, V.D.; Sharma, D.V.; Prasad, P.S.; Murthy, S.R. Methane hydrates formation and dissociation in nano silica suspension. J. Nat. Gas Sci. Eng. 2013, 11, 7–11. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.H.; Rahmati-Abkenar, M. Kinetic study of carbon dioxide hydrate formation in presence of silver nanoparticles and SDS. Chem. Eng. J. 2014, 237, 387–395. [Google Scholar] [CrossRef]
- Park, S.S.; An, E.J.; Lee, S.B.; Chun, W.G.; Kim, N.J. Characteristics of methane hydrate formation in carbon nanofluids. J. Ind. Eng. Chem. 2012, 18, 443–448. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M.; Manakov, A.Y.; Rodionova, T.V.; Paukshtis, E.A.; Asanov, I.P.; Bulavchenko, A.I. Promotion and inhibition of gas hydrate formation by oxide powders. J. Mol. Liq. 2015, 204, 118–125. [Google Scholar] [CrossRef]
- Choi, J.W.; Chung, J.T.; Kang, Y.T. CO2 hydrate formation at atmospheric pressure using high efficiency absorbent and surfactants. Energy 2014, 78, 869–876. [Google Scholar] [CrossRef]
- Mohammadi, M.; Haghtalab, A.; Fakhroueian, Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles. J. Chem. Thermodyn. 2016, 96, 24–33. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Promoting effect of Al2O3/ZnO based nanofluids stabilized by SDS surfactant on CH4+C2H6+C3H8 hydrate formation. J. Ind. Eng. Chem. 2016, 35, 357–368. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Yu, S.; Mao, L.; Wang, G. Preparation and properties of graphene oxide and theirthermal conductivity nanofluids. Func. Mater. 2015, 46, 16138–16141. [Google Scholar] [CrossRef]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 92–94. [Google Scholar] [CrossRef]
- Yaodi, M. Method for determining gas compression factor in engineering design. Chem. Eng. Des. 2006, 16, 17–18. [Google Scholar]
- Ye, P.; Liu, D.P.; Shi, J.J. Study on driving force for carbon dioxide hydrate formation. Nat. Gas. Chem. Ind. 2013, 2, 38–41. [Google Scholar] [CrossRef]
- Lai, Q.; Luo, X. Qualitative and Quantitative Analysis of Graphene Oxides by UV Spectroscopy. Chin. J. Mater. Res. 2015, 2, 155–160. [Google Scholar]
- Li, Y.; Zhu, C.; Wang, W. Promoting Effects of Surfactants on Carbon Dioxide Hydrate Formation and Kinetics. Petrochem. Technol. 2012, 41, 699–703. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Dai, W.; Wang, S.; Rao, Y.; Zhou, S. Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation. Energies 2018, 11, 1756. https://doi.org/10.3390/en11071756
Yan S, Dai W, Wang S, Rao Y, Zhou S. Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation. Energies. 2018; 11(7):1756. https://doi.org/10.3390/en11071756
Chicago/Turabian StyleYan, Shuo, Wenjie Dai, Shuli Wang, Yongchao Rao, and Shidong Zhou. 2018. "Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation" Energies 11, no. 7: 1756. https://doi.org/10.3390/en11071756




