Industrial-Scale Experimental Study on the Thermal Oxidation of Ventilation Air Methane and the Heat Recovery in a Multibed Thermal Flow-Reversal Reactor
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental System
2.2. Experimental Conditions
2.3. Energy Balance Analysis for the Multibed TFRR
3. Results and Discussion
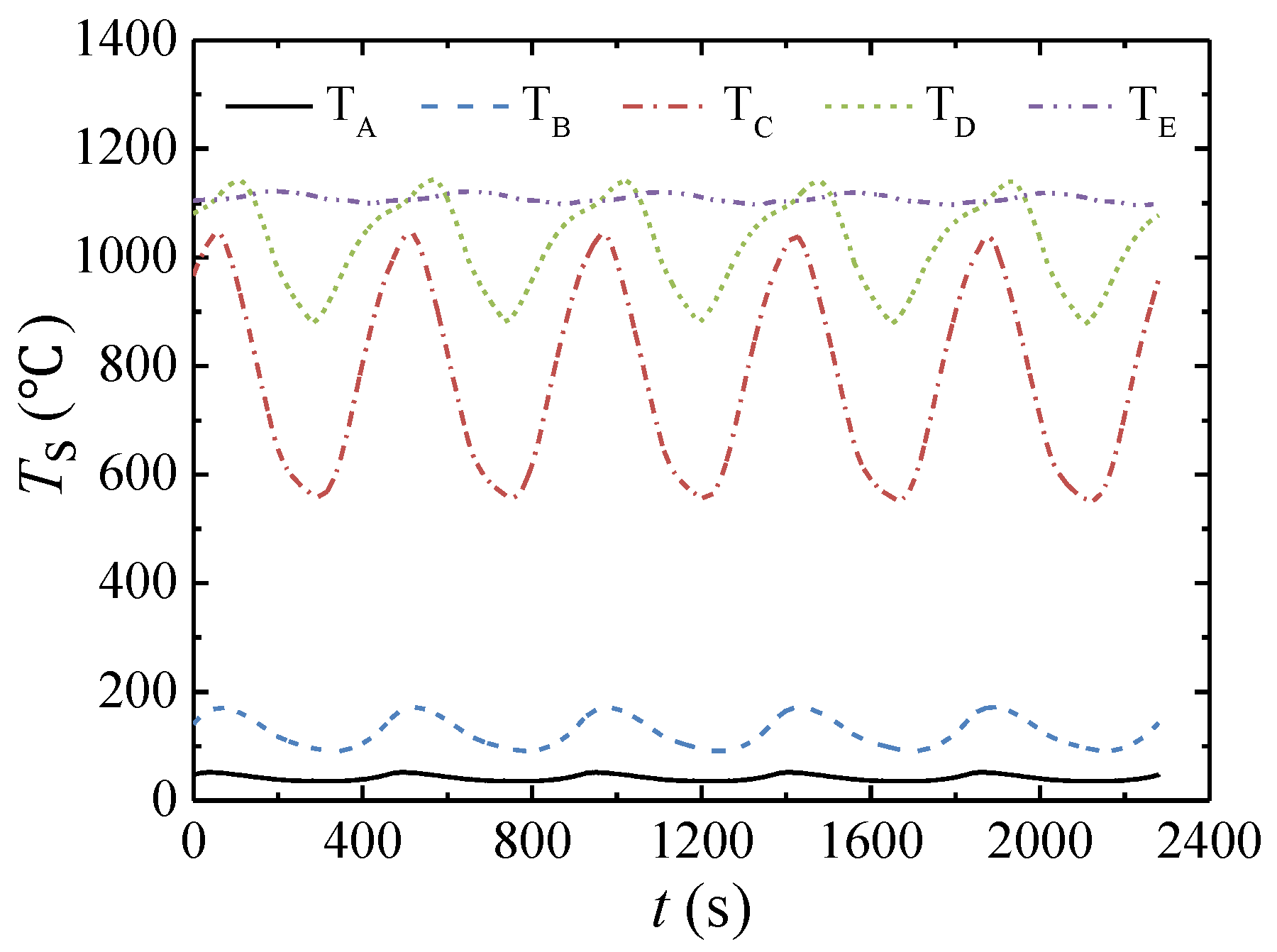
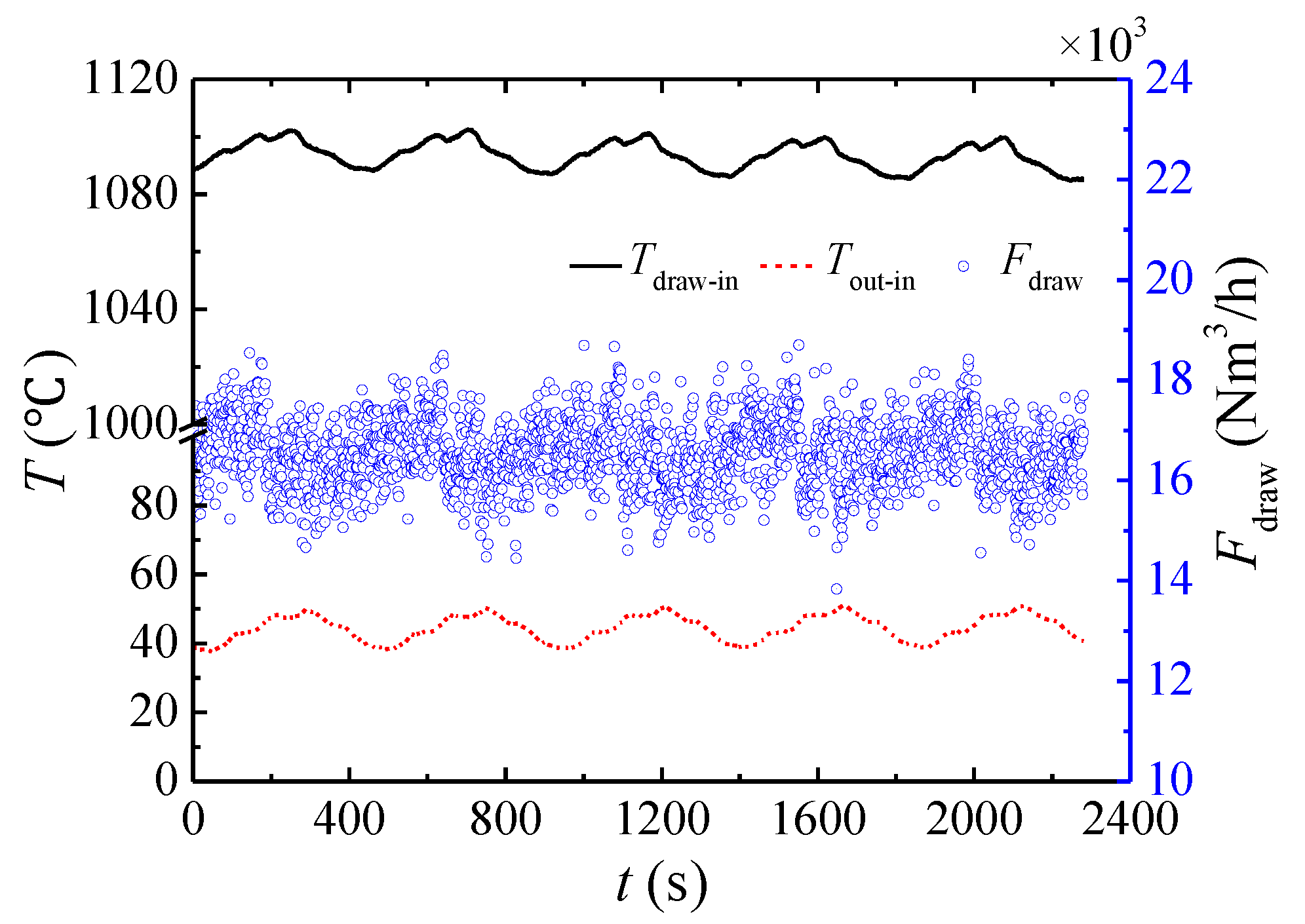
3.1. Performance of the Multibed TFRR
3.2. Critical Cases Concerning Automatic Thermal Maintenance
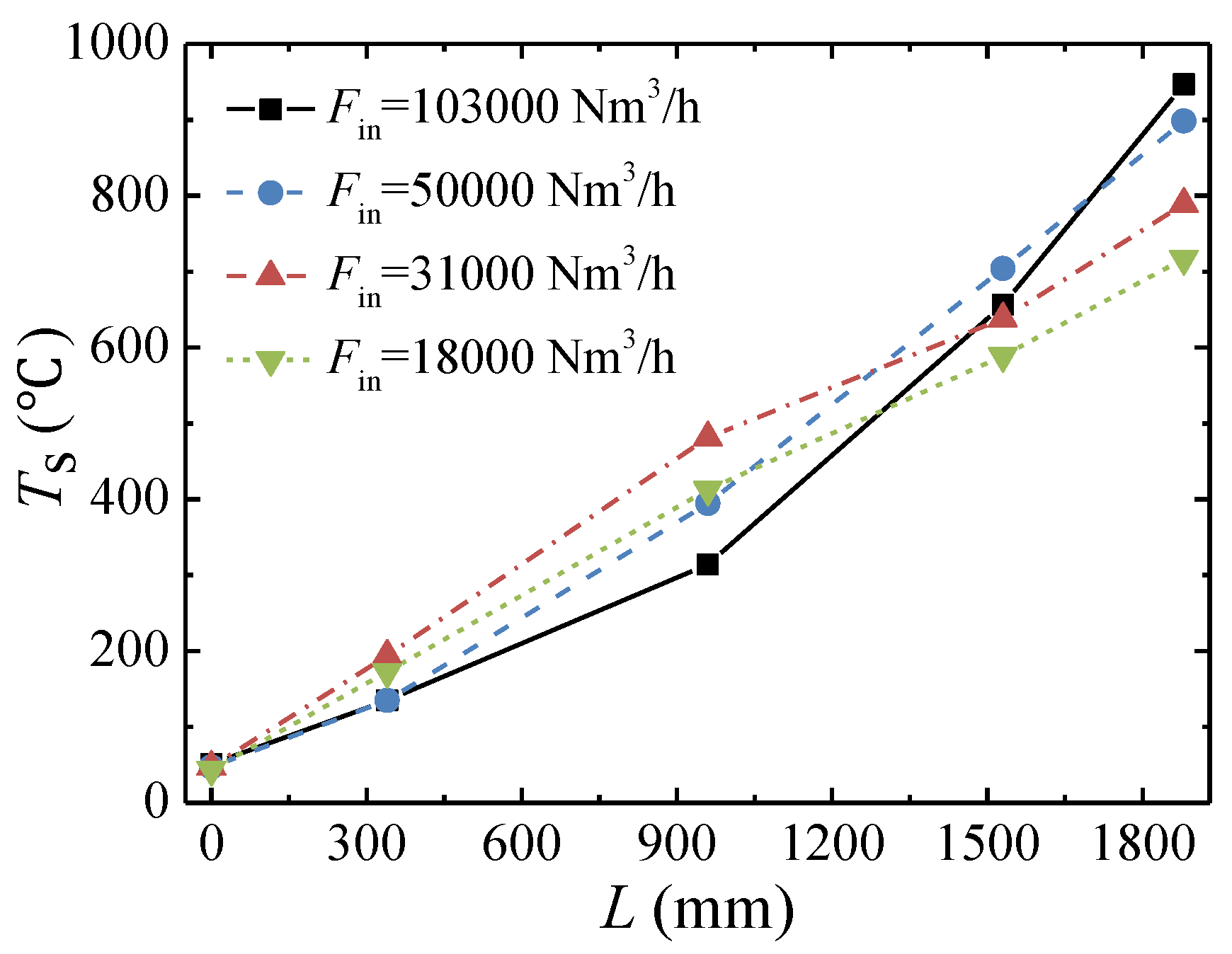
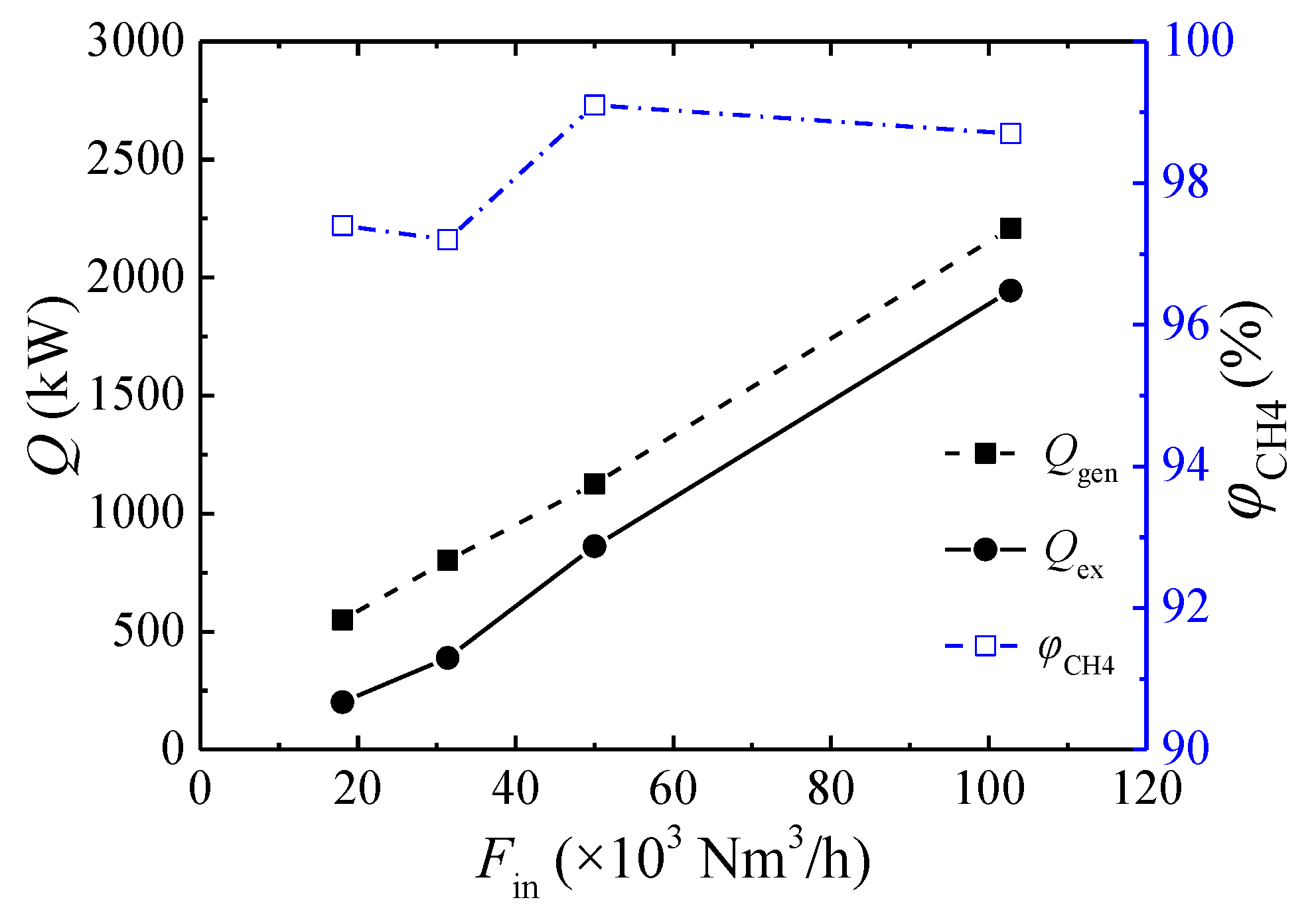
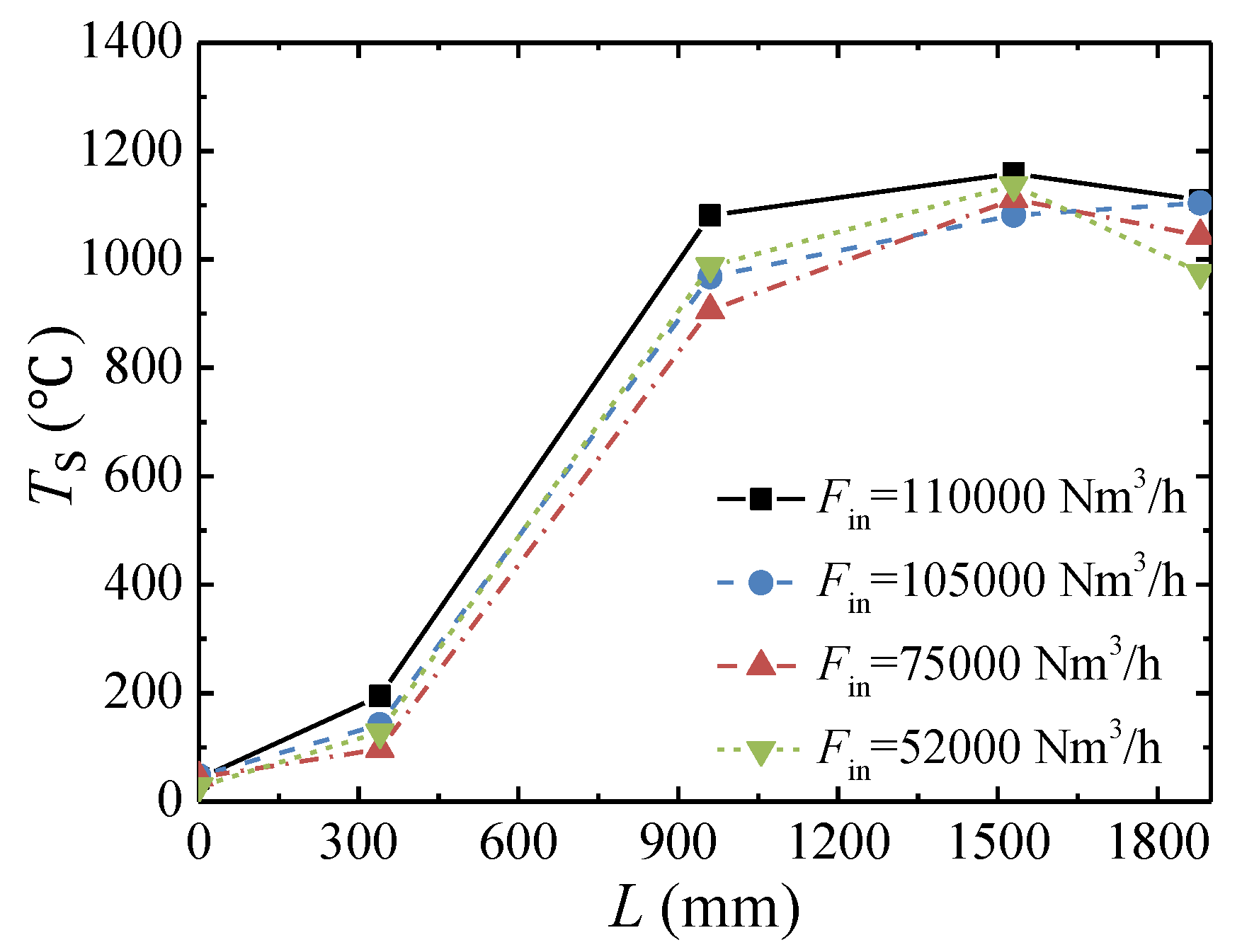
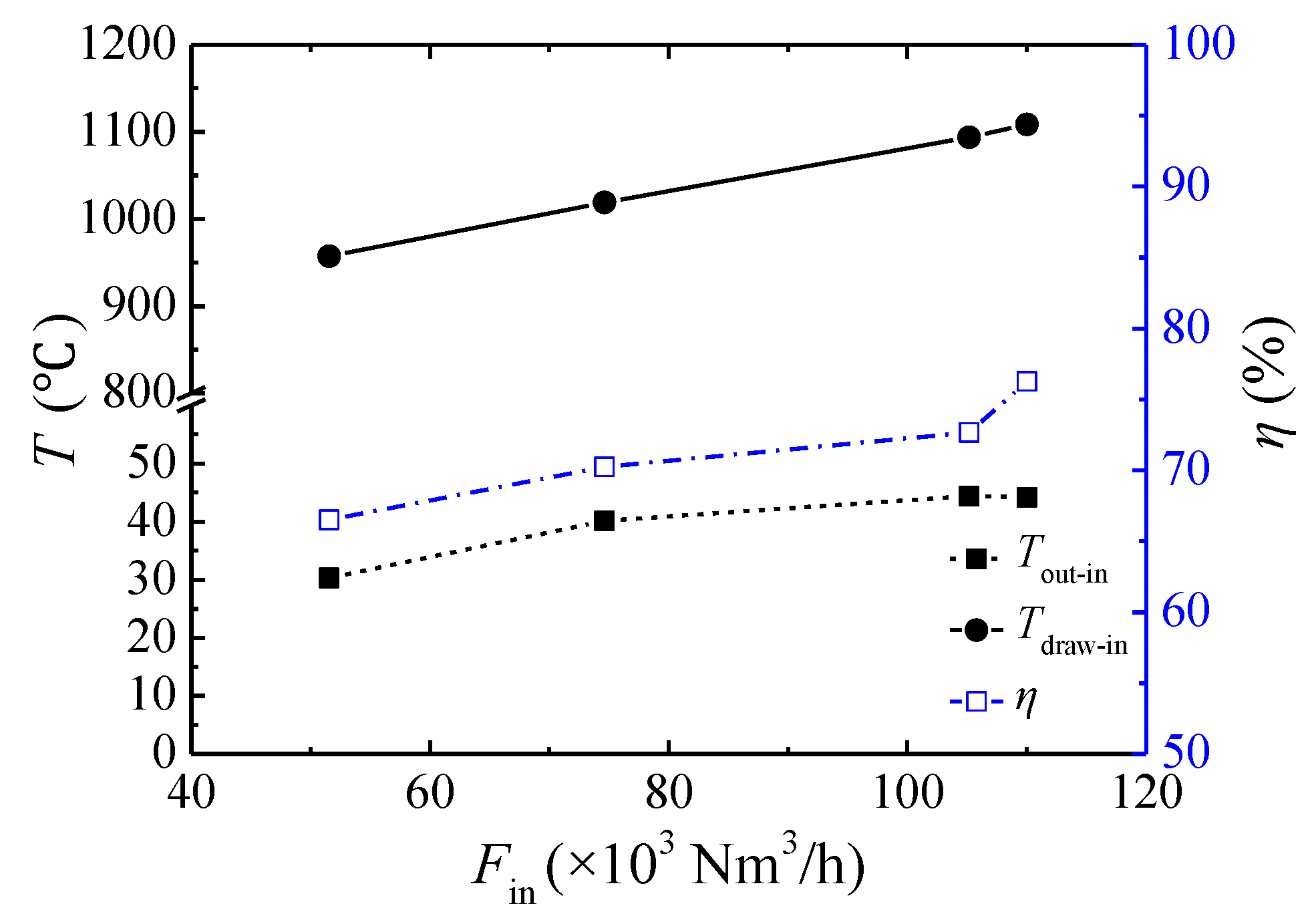
3.3. Effect of the Inlet Flow Rate
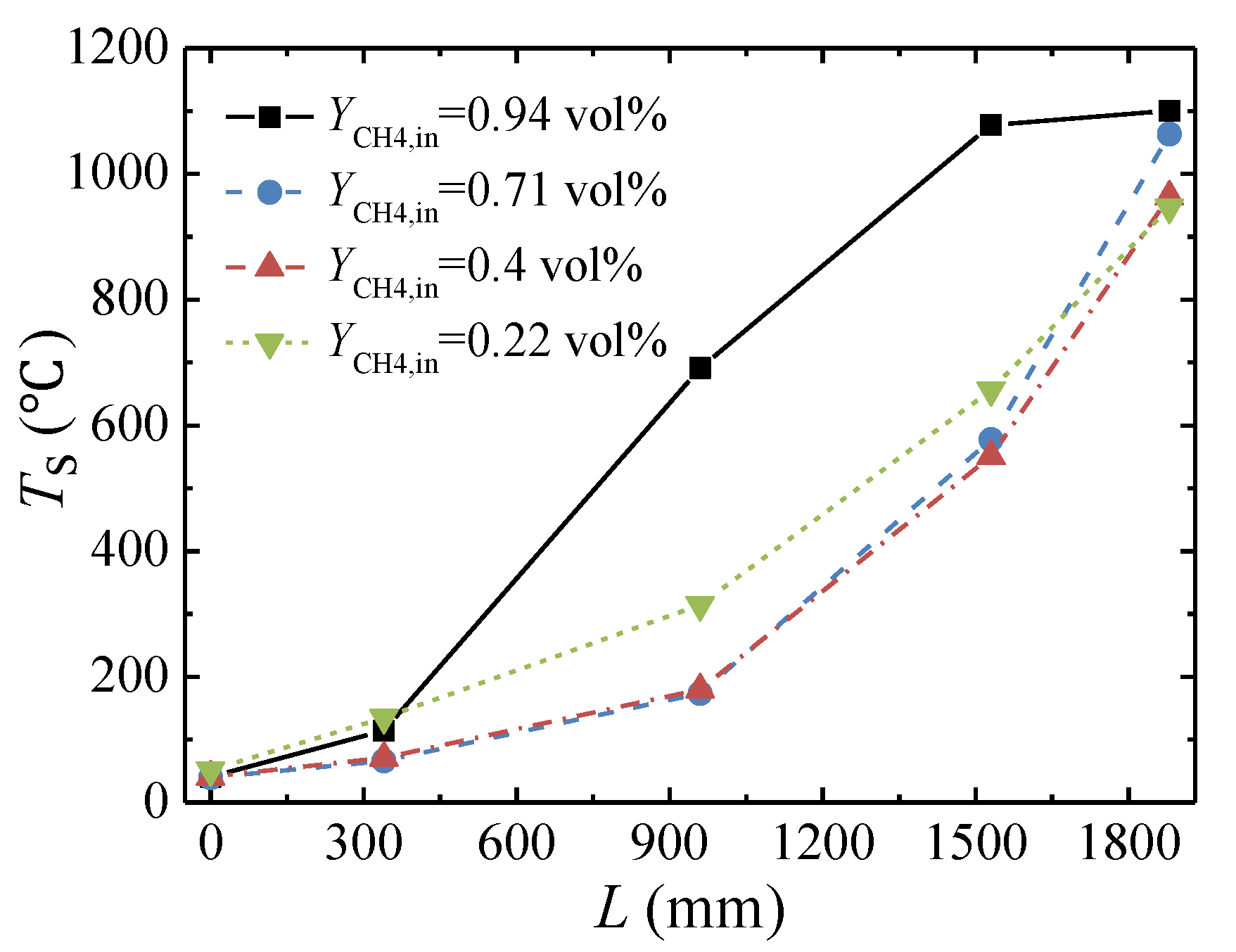
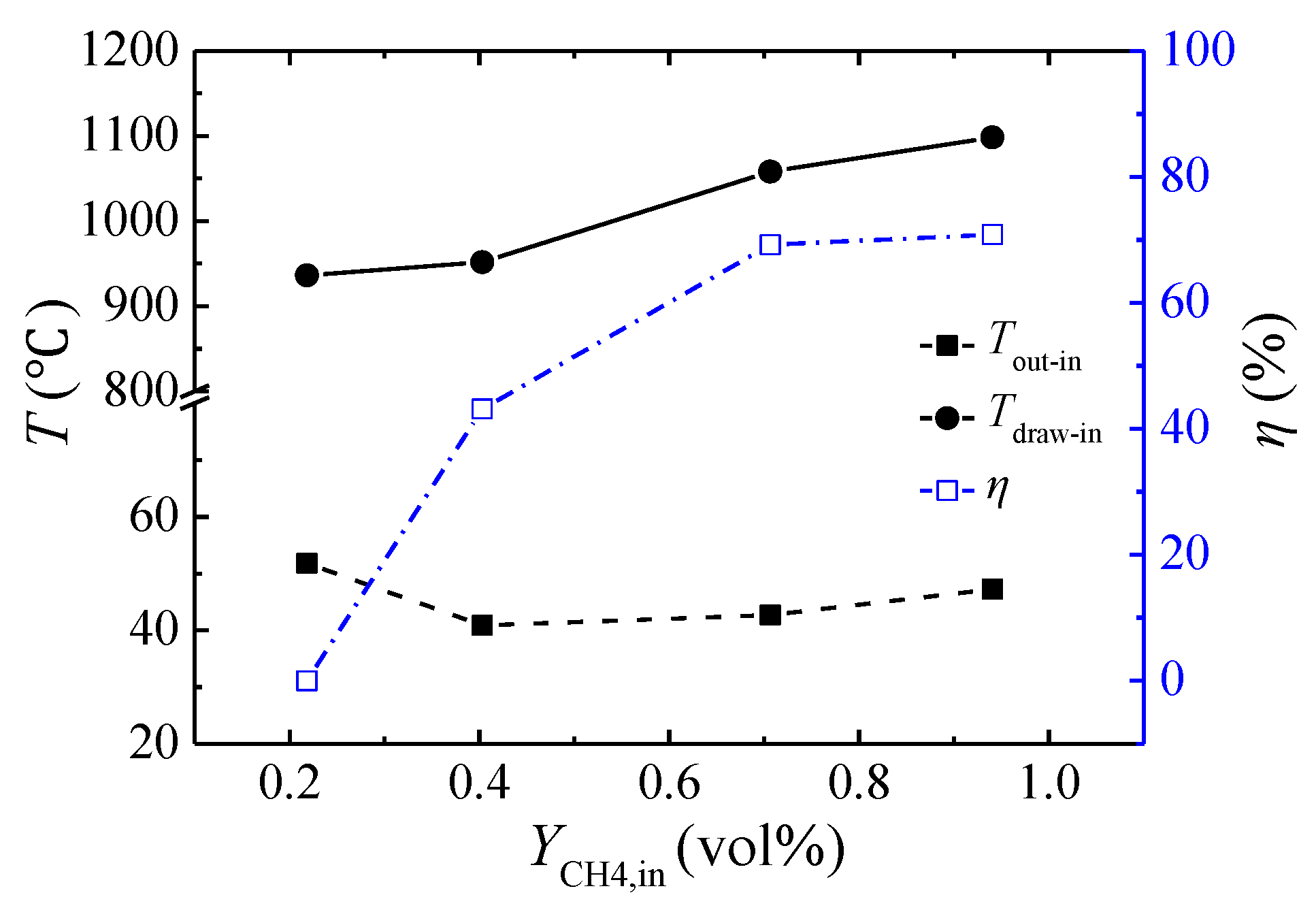
3.4. Effect of the Feed Methane Concentration
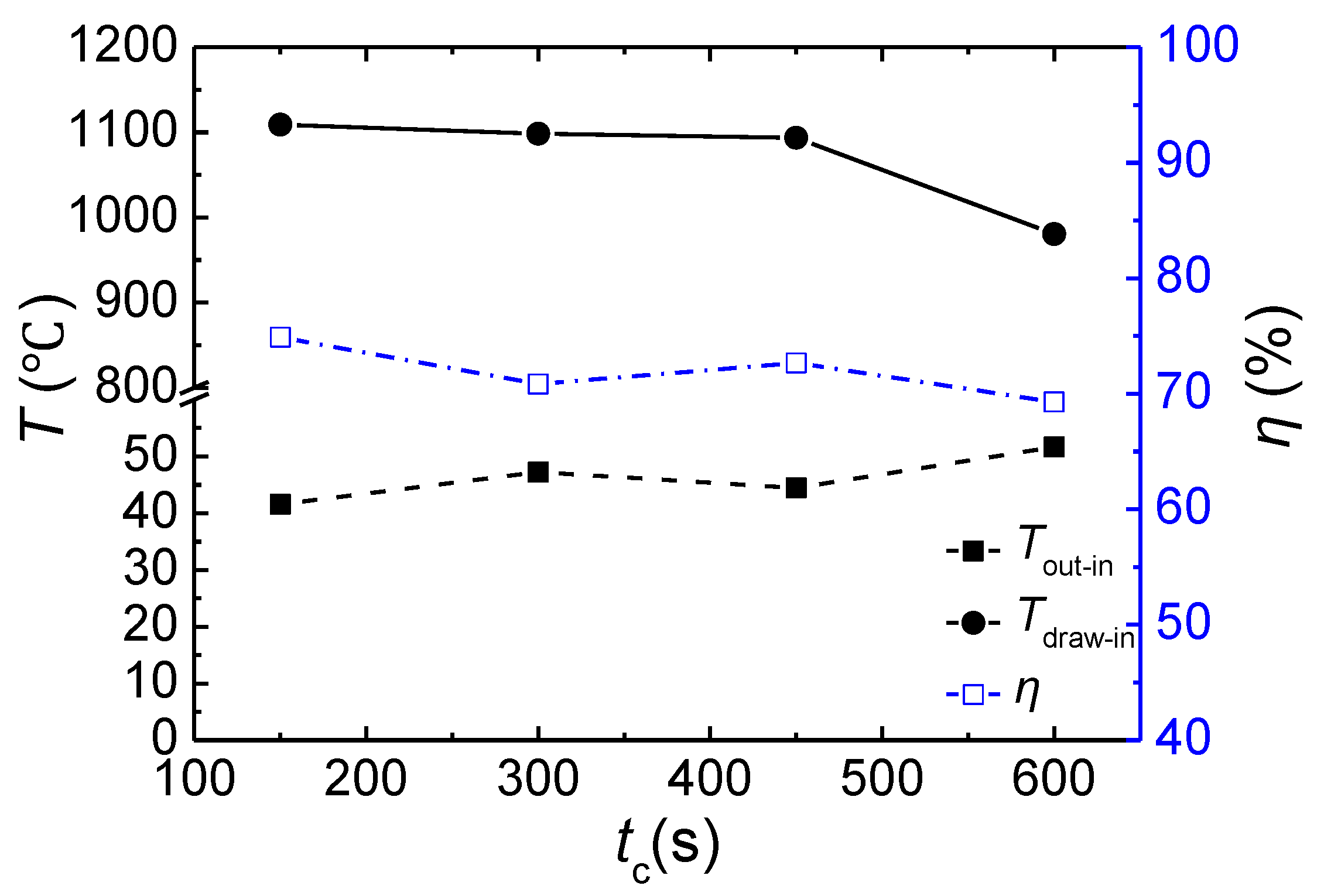
3.5. Effect of the Cycle Time
4. Conclusions
- (1)
- The CSS is asymmetrical due to the hot gas extracted from the combustion chamber. The multibed TFRR achieves a methane conversion higher than 97% for the thermal oxidation of VAM.
- (2)
- The lower concentration limit of automatic thermal maintenance decreases with increasing flow rate due to the heat dissipation from the reactor surface. At an inlet flow rate of 103,000 Nm3/h and a cycle time of 300 s, the lower concentration limit of automatic thermal maintenance is 0.22 vol %.
- (3)
- The peak temperature of the bed moves closer to the inlet side with increases in the feed methane concentration and decreases in the cycle time and is almost not affected by the change in inlet flow rate.
- (4)
- For this multibed TFRR, approximately 76.3% of the energy can be recovered at an inlet flow rate of 110,000 Nm3/h, a feed methane concentration of 1.02 vol %, and a cycle time of 450 s. The heat recovery efficiency increases linearly with increasing inlet flow rate, rises parabolically with an increasing feed methane concentration, and decreases weakly with increasing cycle time.
Author Contributions
Funding
Conflicts of Interest
References
- Dalianis, G.; Nanaki, E.; Xydis, G.; Zervas, E. New Aspects to greenhouse gas mitigation policies for low carbon cities. Energies 2016, 9, 128. [Google Scholar] [CrossRef]
- Su, S.; Beath, A.; Guo, H.; Mallett, C. An assessment of mine methane mitigation and utilization technologies. Prog. Energy Combust. Sci. 2005, 31, 123–170. [Google Scholar] [CrossRef]
- Liu, W.; Han, J.; Zhao, G. Potential and economic analysis of VAM utilization in China. China Coalbed Methane 2009, 6, 3–8. [Google Scholar]
- Wang, W.; Wang, H.; Li, H.; Li, D.; Li, H.; Li, Z. Experimental enrichment of low-concentration ventilation air methane in free diffusion conditions. Energies 2018, 11, 428. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, Z.; Zhang, C.; Xing, Y. Vacuum exhaust process in pilot-scale vacuum pressure swing adsorption for coal mine ventilation air methane enrichment. Energies 2018, 11, 1030. [Google Scholar] [CrossRef]
- Su, S.; Chen, H.; Teakle, P.; Xue, S. Characteristics of coal mine ventilation air flows. J. Environ. Manag. 2008, 86, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, I.; Aydin, G.; Aydiner, K. Mine ventilation air methane as a sustainable energy source. Renew. Sustain. Energy Rev. 2011, 15, 1042–1049. [Google Scholar] [CrossRef]
- Karacan, C.Ö.; Ruiz, F.A.; Cotè, M.; Phipps, S. Coal mine methane: A review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction. Int. J. Coal Geol. 2011, 86, 121–156. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Ran, J. Experiment and modeling of low concentration methane catalytic combustion in a fluidized bed reactor. Appl. Therm. Eng. 2016, 93, 660–667. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Liu, H.; Ji, S. Simulation of catalytic combustion of methane in a monolith honeycomb reactor. Chin. J. Chem. Eng. 2006, 14, 56–64. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Monoliths as suitable catalysts for reverse-flow combustors: Modeling and experimental validation. AIChE J. 2010, 56, 3162–3173. [Google Scholar]
- Wang, Y.; Man, C.; Che, D. Catalytic combustion of ventilation air methane in a reverse-flow reactor. Energy Fuels 2010, 24, 4841–4848. [Google Scholar] [CrossRef]
- Gosiewski, K. Efficiency of heat recovery versus maximum catalyst temperature in a reverse-flow combustion of methane. Chem. Eng. J. 2005, 107, 19–25. [Google Scholar] [CrossRef]
- Marín, P.; Ordóñez, S.; Díez, F.V. Procedures for heat recovery in the catalytic combustion of lean methane-air mixtures in a reverse flow reactor. Chem. Eng. J. 2009, 147, 356–365. [Google Scholar] [CrossRef]
- Wang, S.; Gao, D.; Wang, S. Steady and transient characteristics of catalytic flow reverse reactor integrated with central heat exchanger. Ind. Eng. Chem. Res. 2014, 53, 12644–12654. [Google Scholar] [CrossRef]
- Fernández, J.; Marín, P.; Díez, F.V.; Ordóñez, S. Experimental demonstration and modeling of an adsorption-enhanced reverse flow reactor for the catalytic combustion of coal mine ventilation air methane. Chem. Eng. J. 2015, 279, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Marín, P.; Díez, F.V.; Ordóñez, S. Combustion of coal mine ventilation air methane in a regenerative combustor with integrated adsorption: Reactor design and optimization. Appl. Therm. Eng. 2016, 102, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Gosiewski, K.; Pawlaczyk, A.; Warmuzinski, K.; Jaschik, M. A study on thermal combustion of lean methane–air mixtures: Simplified reaction mechanism and kinetic equations. Chem. Eng. J. 2009, 154, 9–16. [Google Scholar] [CrossRef]
- Pawlaczyk, A.; Gosiewski, K. Combustion of lean methane-air mixtures in monolith beds: Kinetic studies in low and high temperatures. Chem. Eng. J. 2015, 282, 29–36. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Xu, H.; Liu, Z.; Liu, R. Modeling thermal oxidation of coal mine methane in a non-catalytic reverse-flow reactor. J. Mech. Eng. 2014, 60, 495–505. [Google Scholar] [CrossRef]
- Lan, B.; Li, Y.R. Numerical simulation of thermodynamic performance in a honeycomb ceramic channel. In Proceedings of the 3rd International Symposium on Mine Safety Science and Engineering, Montreal, QC, Canada, 13–19 August 2016. [Google Scholar]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Thermal combustion of lean methane–air mixtures: Flow reversal research and demonstration reactor model and its validation. Chem. Eng. J. 2012, 207–208, 76–84. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Energy recovery from ventilation air methane via reverse-flow reactors. Energy 2015, 92, 13–23. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Yuan, D.; Chen, G. Demonstration and its validation for ventilation air methane (VAM) thermal oxidation and energy recovery project. Appl. Therm. Eng. 2015, 90, 75–85. [Google Scholar] [CrossRef]
- Gosiewski, K.; Matros, Y.S.; Warmuzinski, K.; Jaschik, M.; Tanczyk, M. Homogeneous vs. catalytic combustion of lean methane-air mixtures in reverse-flow reactors. Chem. Eng. Sci. 2008, 63, 5010–5019. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A. Catalytic or thermal reversed flow combustion of coal mine ventilation air methane: What is better choice and when? Chem. Eng. J. 2014, 238, 78–85. [Google Scholar] [CrossRef]
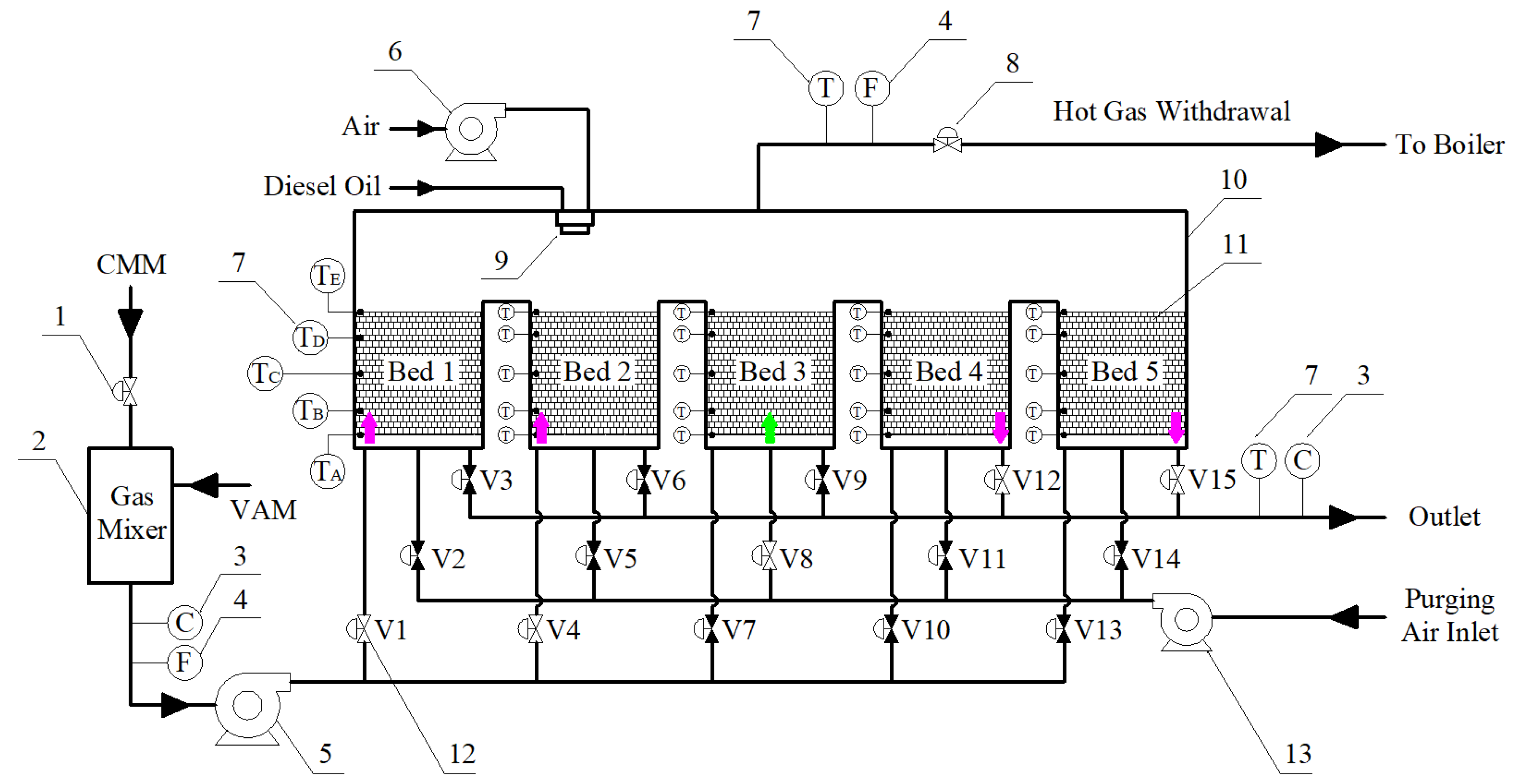
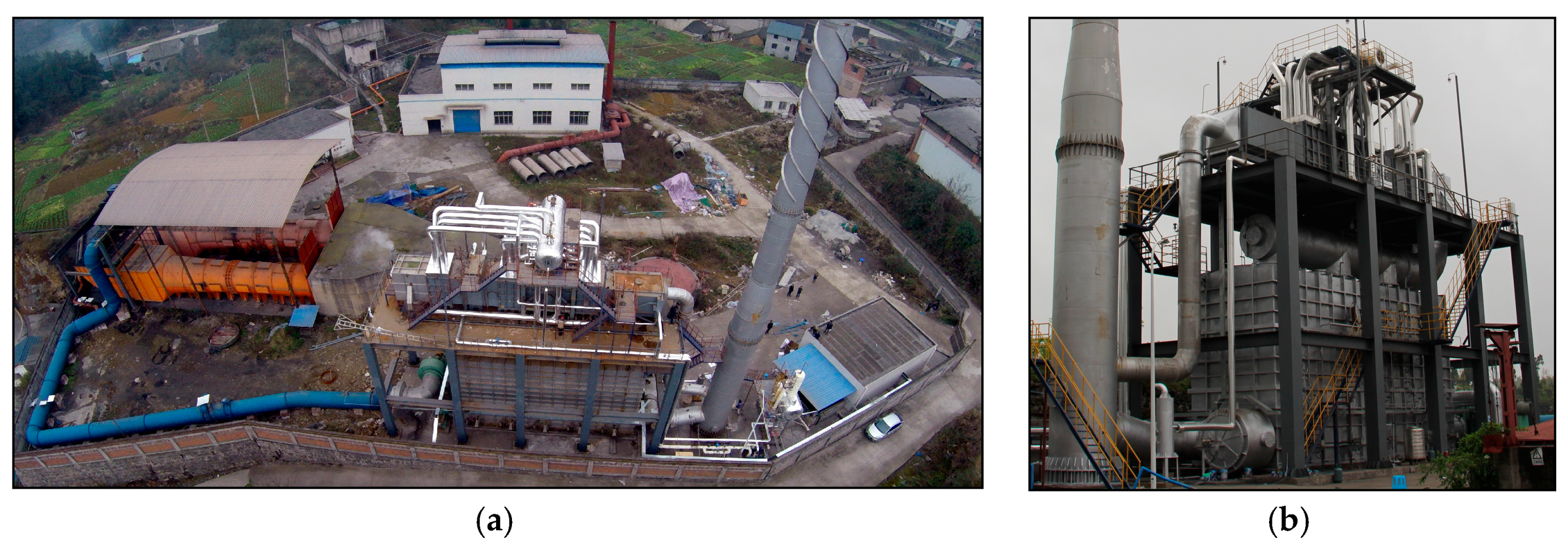
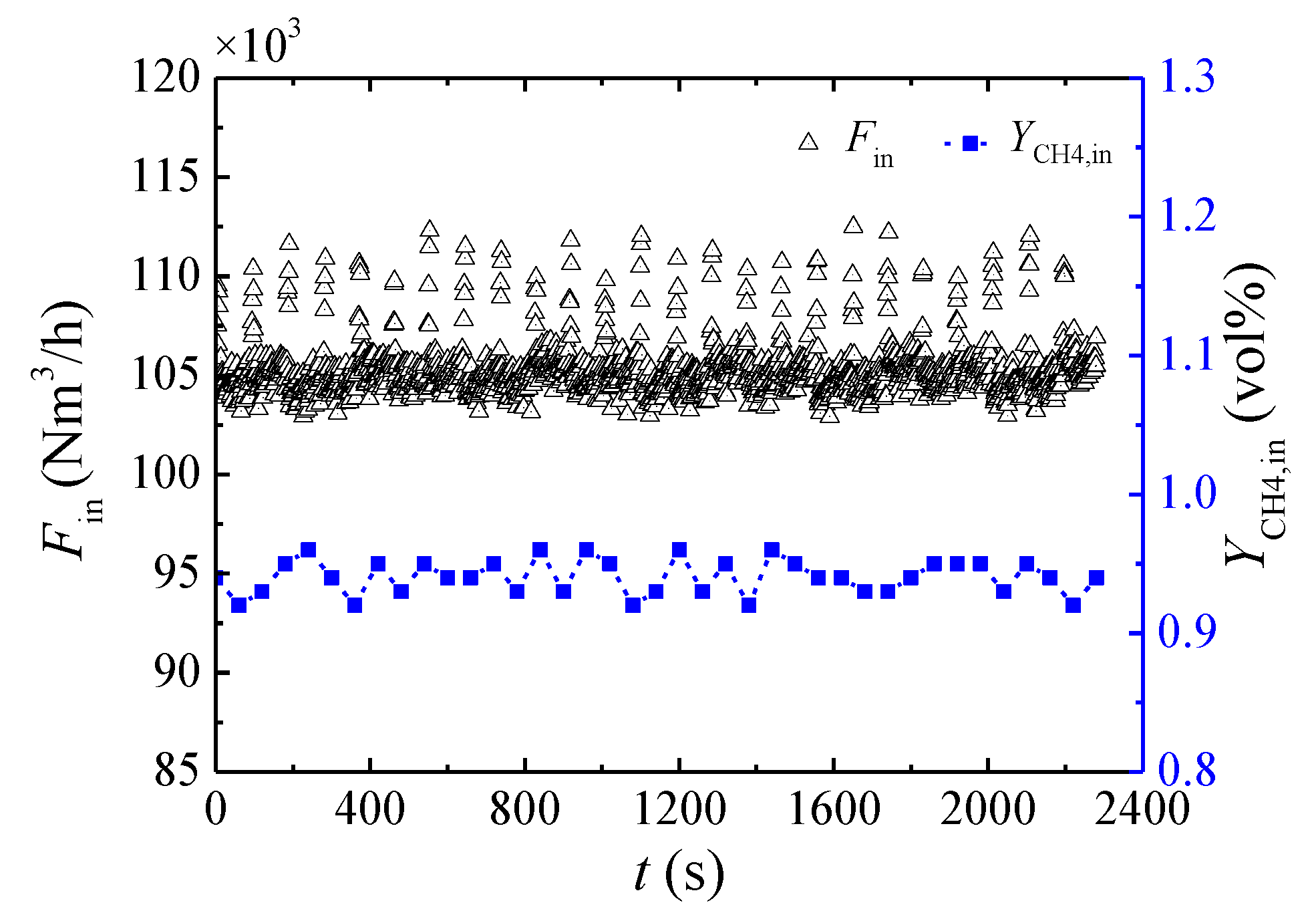
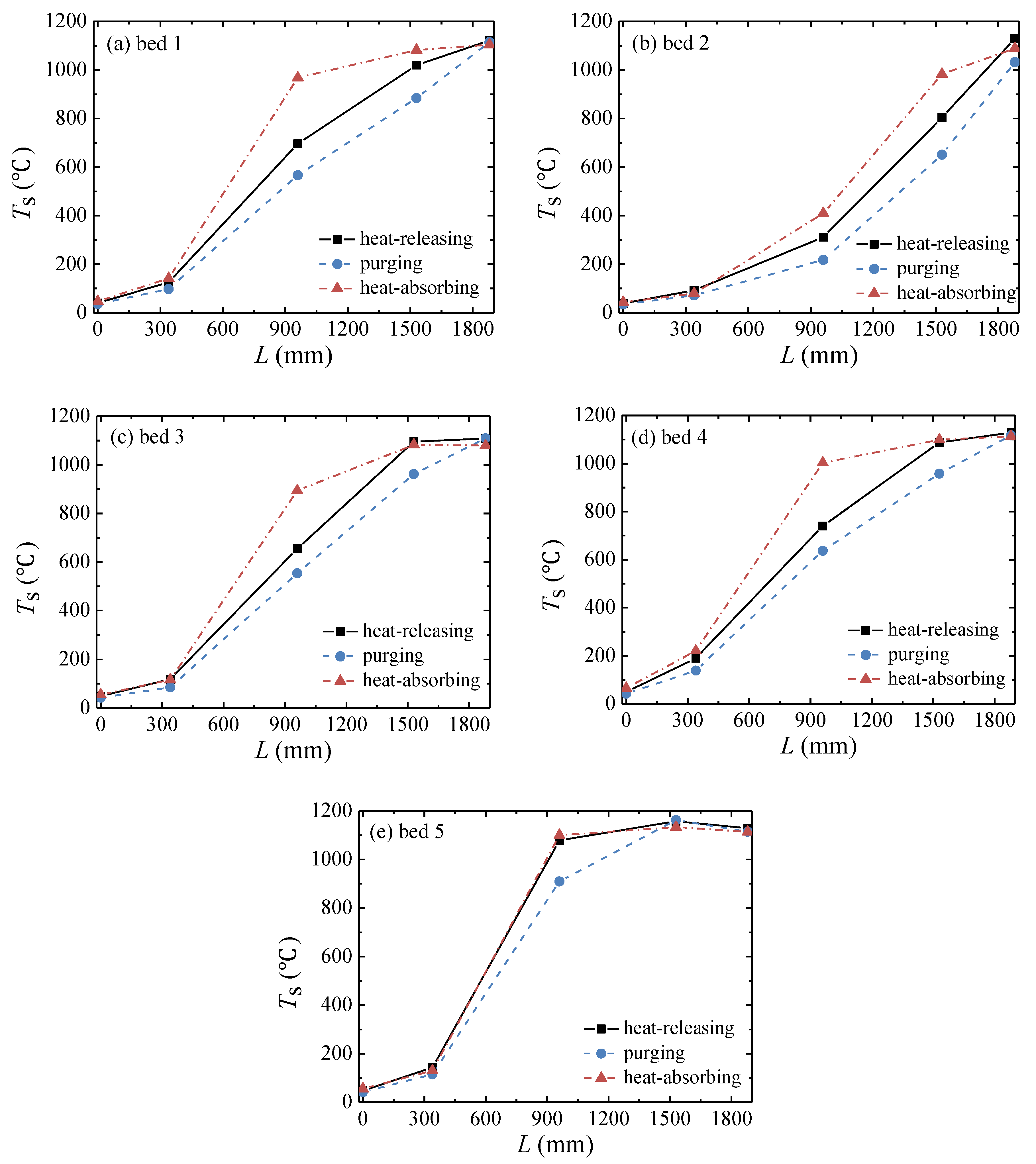


| Cycle | Valves of Bed 1 | Valves of Bed 2 | Valves of Bed 3 | Valves of Bed 4 | Valves of Bed 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V10 | V11 | V12 | V13 | V14 | V15 | |
| 1/5 cycle | O | × | × | O | × | × | × | O | × | × | × | O | × | × | O |
| 2/5 cycle | O | × | × | × | O | × | × | × | O | × | × | O | O | × | × |
| 3/5 cycle | × | O | × | × | × | O | × | × | O | O | × | × | O | × | × |
| 4/5 cycle | × | × | O | × | × | O | O | × | × | O | × | × | × | O | × |
| 5/5 cycle | × | × | O | O | × | × | O | × | × | × | O | × | × | × | O |
| Case | Fin (×103 Nm3/h) | YCH4,in (vol %) | tc (s) | φCH4 (%) | η (%) |
|---|---|---|---|---|---|
| Case 1 | 105 | 0.94 | 450 | 99.1 | 72.7 |
| Case 2 | 103 | 0.22 | 300 | 98.7 | 0 |
| Case 3 | 50 | 0.23 | 450 | 99.1 | 0 |
| Case 4 | 31 | 0.26 | 450 | 97.2 | 0 |
| Case 5 | 18 | 0.32 | 450 | 97.4 | 0 |
| Case 6 | 110 | 1.02 | 450 | 99.8 | 76.3 |
| Case 7 | 75 | 0.98 | 450 | 97.5 | 70.2 |
| Case 8 | 52 | 1.06 | 450 | 99.7 | 66.5 |
| Case 9 | 105 | 0.94 | 300 | 97.7 | 70.8 |
| Case 10 | 106 | 0.71 | 300 | 99 | 69.2 |
| Case 11 | 107 | 0.40 | 300 | 99 | 43.1 |
| Case 12 | 103 | 0.95 | 300 | 97 | 74.9 |
| Case 13 | 106 | 0.95 | 600 | 99.8 | 69.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, B.; Li, Y.-R.; Zhao, X.-S.; Kang, J.-D. Industrial-Scale Experimental Study on the Thermal Oxidation of Ventilation Air Methane and the Heat Recovery in a Multibed Thermal Flow-Reversal Reactor. Energies 2018, 11, 1578. https://doi.org/10.3390/en11061578
Lan B, Li Y-R, Zhao X-S, Kang J-D. Industrial-Scale Experimental Study on the Thermal Oxidation of Ventilation Air Methane and the Heat Recovery in a Multibed Thermal Flow-Reversal Reactor. Energies. 2018; 11(6):1578. https://doi.org/10.3390/en11061578
Chicago/Turabian StyleLan, Bo, You-Rong Li, Xu-Sheng Zhao, and Jian-Dong Kang. 2018. "Industrial-Scale Experimental Study on the Thermal Oxidation of Ventilation Air Methane and the Heat Recovery in a Multibed Thermal Flow-Reversal Reactor" Energies 11, no. 6: 1578. https://doi.org/10.3390/en11061578




