Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes
Abstract
:1. Introduction
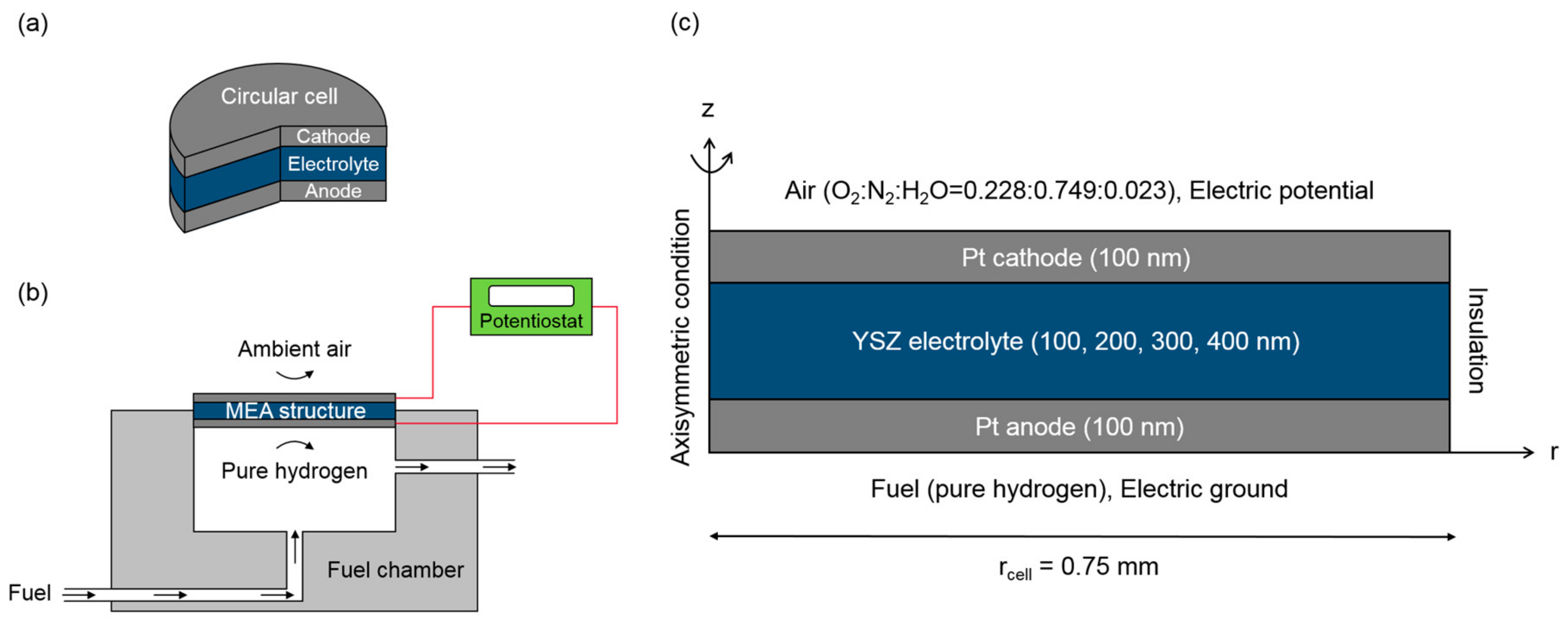
2. Model Description
2.1. Governing Equations
2.2. Boundary and Operating Conditions
2.3. Numerical Methods
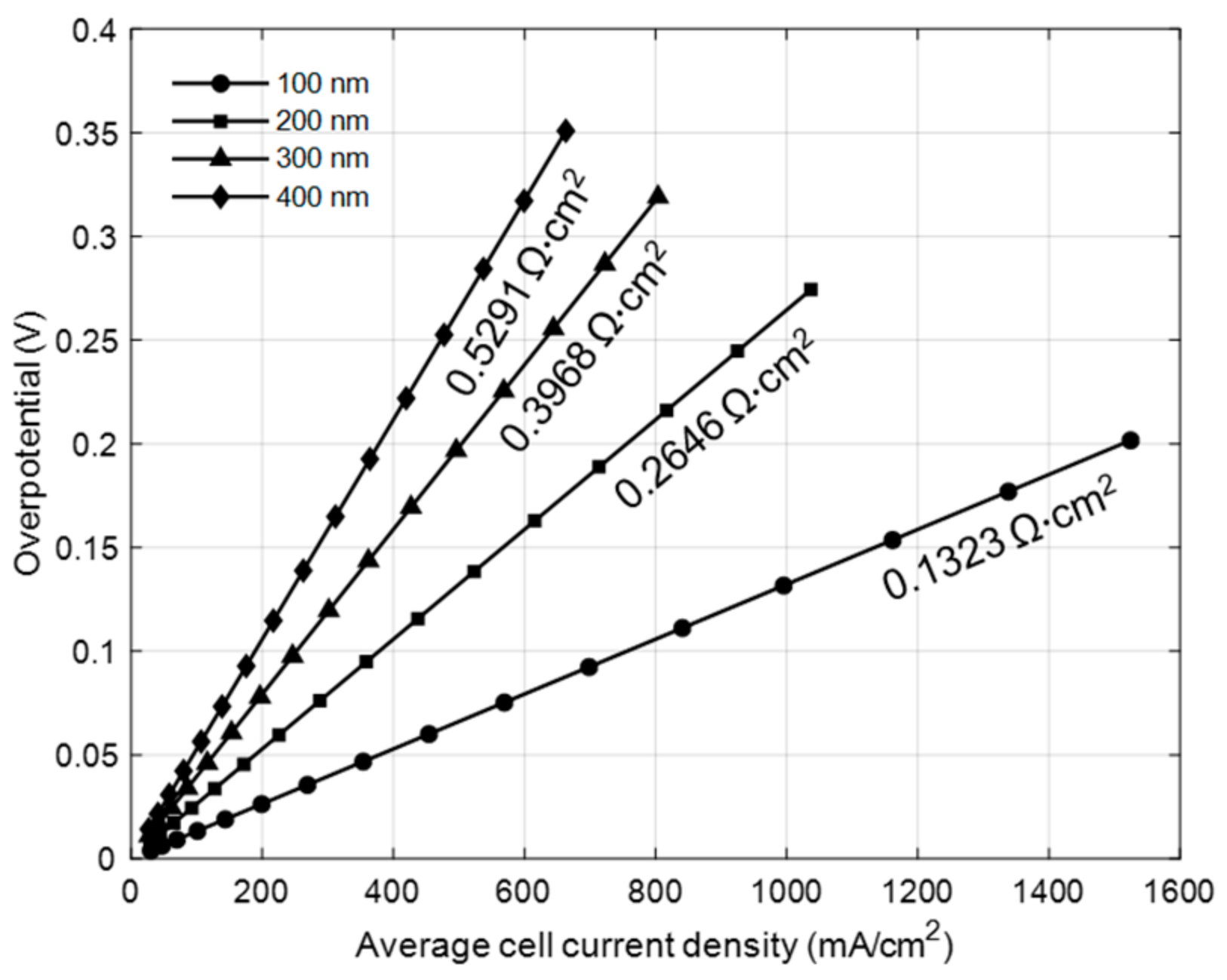
3. Results and Discussion
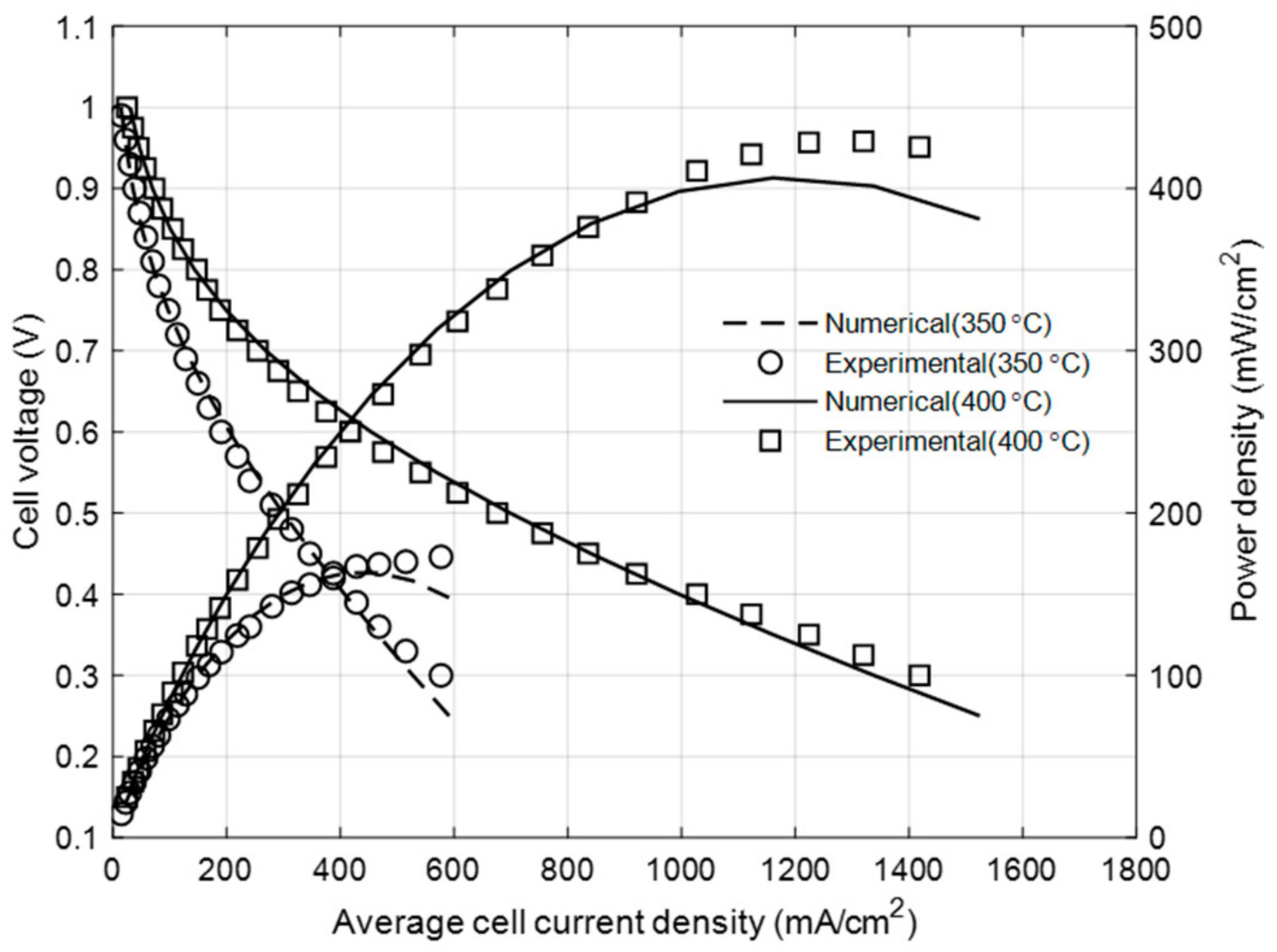
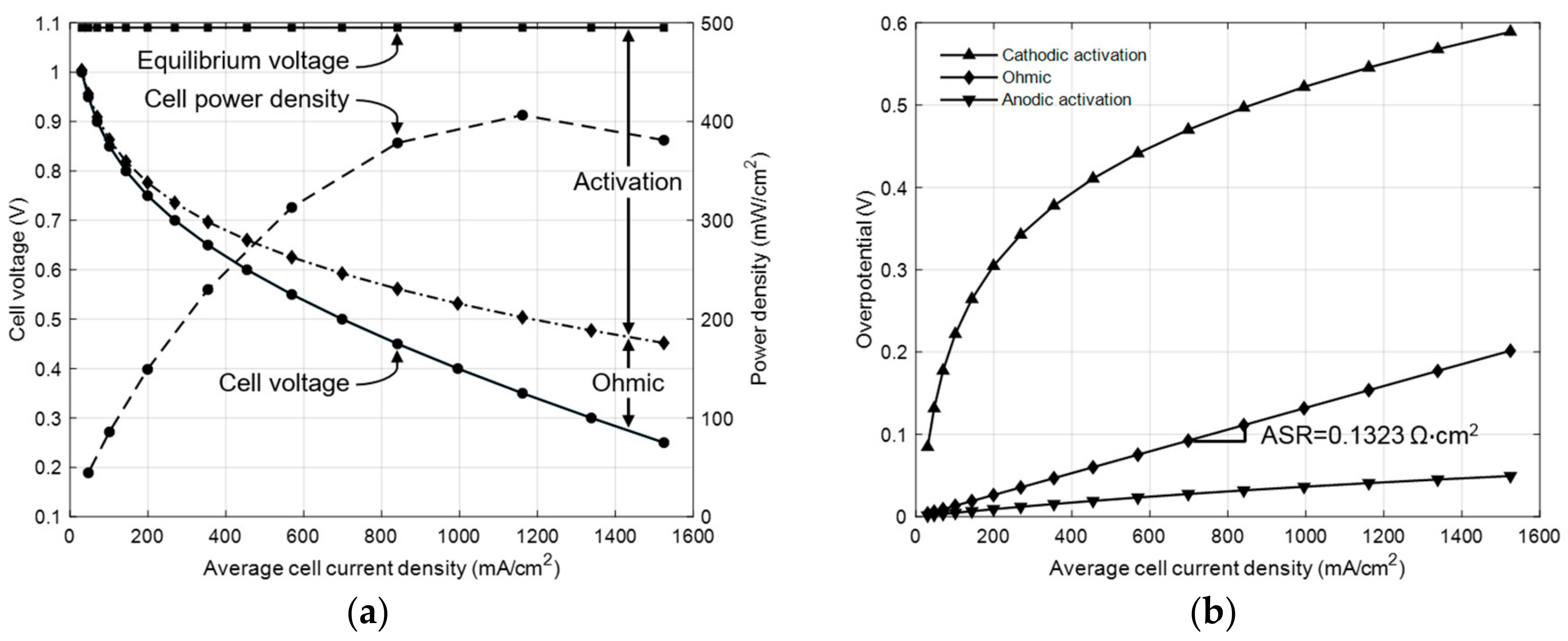
3.1. Model Calibration with Experimental Data
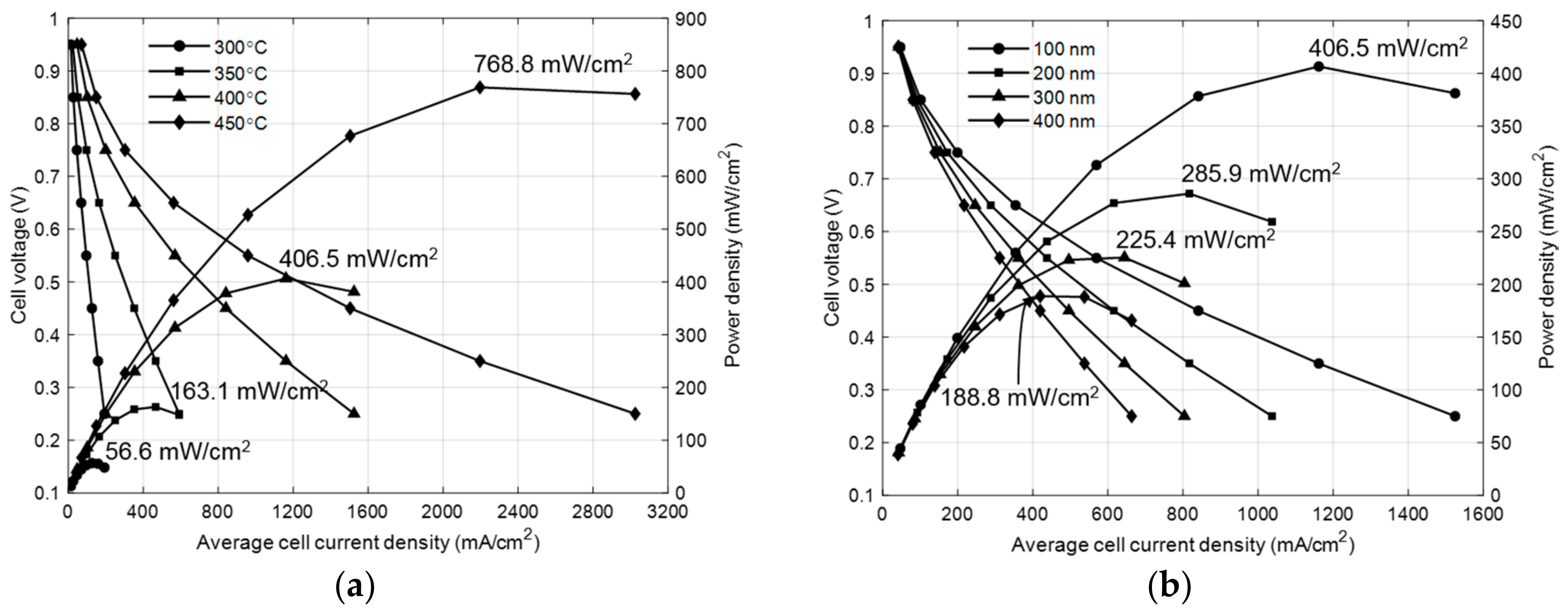
3.2. Effect of Operating Temperature and Electrolyte Thickness
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Evans, A.; Bieberle-Hutter, A.; Rupp, J.L.M.; Gauckler, L.J. Review on microfabricated micro-solid oxide fuel cell electrolytes. J. Power Sources 2009, 194, 119–129. [Google Scholar] [CrossRef]
- Liu, K.Y.; Yun, Y.J.; Lee, S.H.; Su, P.C. Sputtered nanoporous PtNi thin film cathodes with improved thermal stability for low temperature solid oxide fuel cells. J. Power Sources 2009, 194, 119–129. [Google Scholar] [CrossRef]
- Jung, W.; Kim, J.J; Tuller, H.L. Investigation of nanoporous platinum thin films fabricated by reactive sputtering: Application as micro-SOFC electrode. Electrochim. Acta 2017, 247, 558–563. [Google Scholar] [CrossRef]
- Hleig, J.; Tuller, H.L.; Maier, J. Electrodes and electrolytes in micro-SOFCs: A discussion of geometrical constraints. Solid State Ion. 2004, 174, 261–270. [Google Scholar]
- Huang, H.; Nakamura, M.; Su, P.; Fasching, R.; Saito, Y.; Frinz, F.B. High-performance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc. 2007, 154, B20–B24. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, D.Y.; Baek, J.D.; Yoon, Y.J.; Su, P.-C.; Lee, S.H. Effect of electrolyte thickness on electrochemical reaction and thermo-fluidic characteristics inside a SOFC unit cell. Energies 2018, 11, 473. [Google Scholar] [CrossRef]
- Hanna, J.; Lee, W.Y.; Shi, Y.; Ghoniem, A.F. Fundamentals of electro- and thermochemistry in the anode of solid oxide fuel cells with hydrocarbon and syngas fuels. Prog. Energy Combust. Sci. 2014, 40, 74–111. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Yoon, K.J.; Son, J.W.; Lee, J.H.; Kim, B.K.; Choi, W.; Hong, J. The effect of fuel utilization on heat and mass transfer within solid oxide fuel cells examined by three-dimensional numerical simulations. Int. J. Heat Mass Transf. 2016, 97, 77–93. [Google Scholar] [CrossRef]
- Andersson, M.; Paradis, H.; Yuan, J.; Sunden, B. Three dimensional modeling of a solid oxide fuel cell coupling charge transfer phenomena with transport processes and heat generation. Electrochim. Acta 2013, 109, 881–893. [Google Scholar] [CrossRef]
- Baek, J.D.; Liu, K.-Y.; Su, P.-C. A functional micro-solid oxide fuel cell with a 10 nm-thick freestanding electrolyte. J. Mater. Chem. A 2017, 5, 18414–18419. [Google Scholar] [CrossRef]
- He, Z.; Li, H.; Birgersson, E. Reduced model for the planar solid oxide fuel cell. Comput. Chem. Eng. 2013, 52, 155–167. [Google Scholar] [CrossRef]
- Baek, J.D.; Yoon, Y.; Lee, W.; Su, P.-C. A circular membrane for nano thin film micro solid oxide fuel cells enhanced mechanical stability. Energy Environ. Sci. 2015, 8, 3374–3380. [Google Scholar] [CrossRef]
- Lee, S.; Park, M.; Kim, H.; Yoon, K.J.; Son, J.W.; Lee, J.H.; Kim, B.K.; Choi, W.; Hong, J. Thermal conditions and heat transfer characteristics of high-temperature solid oxide fuel cells investigated by three-dimensional numerical simulations. Energy 2017, 120, 293–305. [Google Scholar] [CrossRef]
- Fatma, N.C.; Suryanarayana, R.P.; Francisco, E.-B.; Ismail, C. On modeling multi-component diffusion inside the porous anode of solid oxide fuel cells using Fick’s model. J. Power Sources 2009, 192, 467–474. [Google Scholar]
- Spiegel, C. PEM Fuel Cell Modeling and Simulation Using Matlab, 1st ed.; Elsevier: Cambridge, MA, USA, 2008; pp. 120–123. [Google Scholar]
- Chan, S.H.; Khor, K.A.; Xia, Z.T. A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness. J. Power Sources 2001, 93, 130–140. [Google Scholar] [CrossRef]
- Shi, J.; Xue, X. CFD analysis of a novel symmetrical planar SOFC design with micro-flow channels. Chem. Eng. J. 2010, 163, 119–125. [Google Scholar] [CrossRef]
- Current Density Distribution in Solid Oxide Fuel Cell. COMSOL Multiphysics Tutorial Guide. Available online: https://cn.comsol.com/model/current-density-distribution-in-a-solid-oxide-fuel-cell-514 (accessed on 18 July 2017).
- Aguiar, P.; Adjiman, C.S.; Brandon, N.P. Anode-supported intermediate temperature direct internal reforming solid oxide fuel cell. J. Power Sources 2004, 138, 120–136. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N.; Li, C. Numerical modeling of an anode-supported SOFC button cell considering anodic surface diffusion. J. Power Sources 2007, 164, 639–648. [Google Scholar] [CrossRef]
- Flynn, D.R.; O’Hagan, M.E. Measurements of the Thermal Conductivity and Electrical Resistivity of Platinum from 100 to 900 °C. J. Res. Natl. Bur. Stand. C Eng. Instrum. 1967, 71C, 255–284. [Google Scholar] [CrossRef]
- Ferguson, J.R.; Fiard, J.M.; Herbin, R. Three-dimensional numerical simulation for various geometries of solid oxide fuel cells. J. Power Sources 1996, 58, 109–122. [Google Scholar] [CrossRef]
- Naveed, A.; Stephen, P.D.; Daniel, L.; Kevin, K. A three-dimensional numerical model of a single-chamber solid oxide fuel cell. Int. J. Hydrogen Energy 2009, 34, 8645–8663. [Google Scholar]
- KaKac, S.; Pramuanjaroenkij, A.; Zhou, X.Y. A review of numerical modeling of solid oxide fuel cells. Int. J. Hydrogen Energy 2007, 32, 761–786. [Google Scholar] [CrossRef]
- Zhao, F.; Virkar, A.V. Dependence of polarization in adnode-supported solid oxide fuell cells on various cell parameters. J. Power Sources 2005, 141, 79–95. [Google Scholar] [CrossRef]
- Perng, S.; Chen, C. Numerical Investigation of Anode Thickness on the Performance and Heat/Mass Transport Phenomenon for an Anode-Supported SOFC Button Cell. Int. J. Nanomater. 2015, 16, 172876. [Google Scholar] [CrossRef]

| Parameter | Equation | Reference |
|---|---|---|
| (19) Electronic conductivity (S/m) | [22] | |
| (20) Effective Electronic conductivity (S/m) | [9] | |
| (21) Ionic conductivity (S/m) | [23] | |
| (22) Binary diffusivity (m2/s) | [9] | |
| (23) Mixture averaged diffusivity (m2/s) | [9] | |
| (24) Knudsen diffusivity (m2/s) | [9] | |
| (25) Effective diffusivity (m2/s) | [9] | |
| (26) Bruggeman correlration | [19] |
| Parameter | Value |
|---|---|
| Cathode thickness, Hc | 100 (nm) |
| Electrolyte thickness, Hel | 100, 200, 300, 400 (nm) |
| Anode thickness, Ha | 100 (nm) |
| Cell radius, rcell | 0.75 (mm) |
| Operating temperature, Top | 300, 350, 400, 450 (°C) |
| Operating pressure, Pop | 1 (atm) |
| Operating cell voltage, Vcell | 0.2~1.0 (V) |
| Cathode porosity, εc | 0.16 |
| Anode porosity, εa | 0.16 |
| Anodic charge transfer coefficient, cathode, , [24] | 0.375 |
| Cathodic charge transfer coefficient, cathode, , [24] | 0.125 |
| Anodic charge transfer coefficient, anode, , [24] | 1 |
| Cathodic charge transfer coefficient, anode, , [24] | 0.5 |
| Reaction order, cathode, , [18] | 0.5 |
| Reaction order, anode, , [18] | 0.5 |
| Pore radius, rpore | 10 (nm) |
| Number of charge, cathode, nc | 4 |
| Number of charge, anode, na | 2 |
| Boundary | Electronic Charge | Ionic Charge | Species |
|---|---|---|---|
| Ambient air/Cathode | Electric potential | Insulation | Mass fraction |
| Cathode/Electrolyte | Insulation | Insulation | Insulation |
| Anode/Electrolyte | Insulation | Insulation | Insulation |
| Hydrogen Fuel/Anode | Electric ground | Insulation | Mass fraction |
| Central axis | Symmetry | Symmetry | Symmetry |
| Side wall | Insulation | Insulation | Insulation |
| Parameter | Value | Unit |
|---|---|---|
| Ac | 4.0348 × 106 | (A/m2) |
| Ec | 3.7681 × 104 | (J/mol2) |
| Aa | 1.0719 × 106 | (A/m2) |
| Ea | 2.3850 × 103 | (J/mol2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.M.; Kim, D.Y.; Baek, J.D.; Yoon, Y.-J.; Su, P.-C.; Lee, S.H. Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes. Energies 2018, 11, 1204. https://doi.org/10.3390/en11051204
Park JM, Kim DY, Baek JD, Yoon Y-J, Su P-C, Lee SH. Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes. Energies. 2018; 11(5):1204. https://doi.org/10.3390/en11051204
Chicago/Turabian StylePark, Jee Min, Dae Yun Kim, Jong Dae Baek, Yong-Jin Yoon, Pei-Chen Su, and Seong Hyuk Lee. 2018. "Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes" Energies 11, no. 5: 1204. https://doi.org/10.3390/en11051204
APA StylePark, J. M., Kim, D. Y., Baek, J. D., Yoon, Y.-J., Su, P.-C., & Lee, S. H. (2018). Numerical Study on Electrochemical Performance of Low-Temperature Micro-Solid Oxide Fuel Cells with Submicron Platinum Electrodes. Energies, 11(5), 1204. https://doi.org/10.3390/en11051204





