Experimental Study of the Pore Structure and Permeability of Coal by Acidizing
Abstract
:1. Introduction
2. Experimental Devices, Coal Specimens, Acids
2.1. Theory
2.2. Experiment Devices
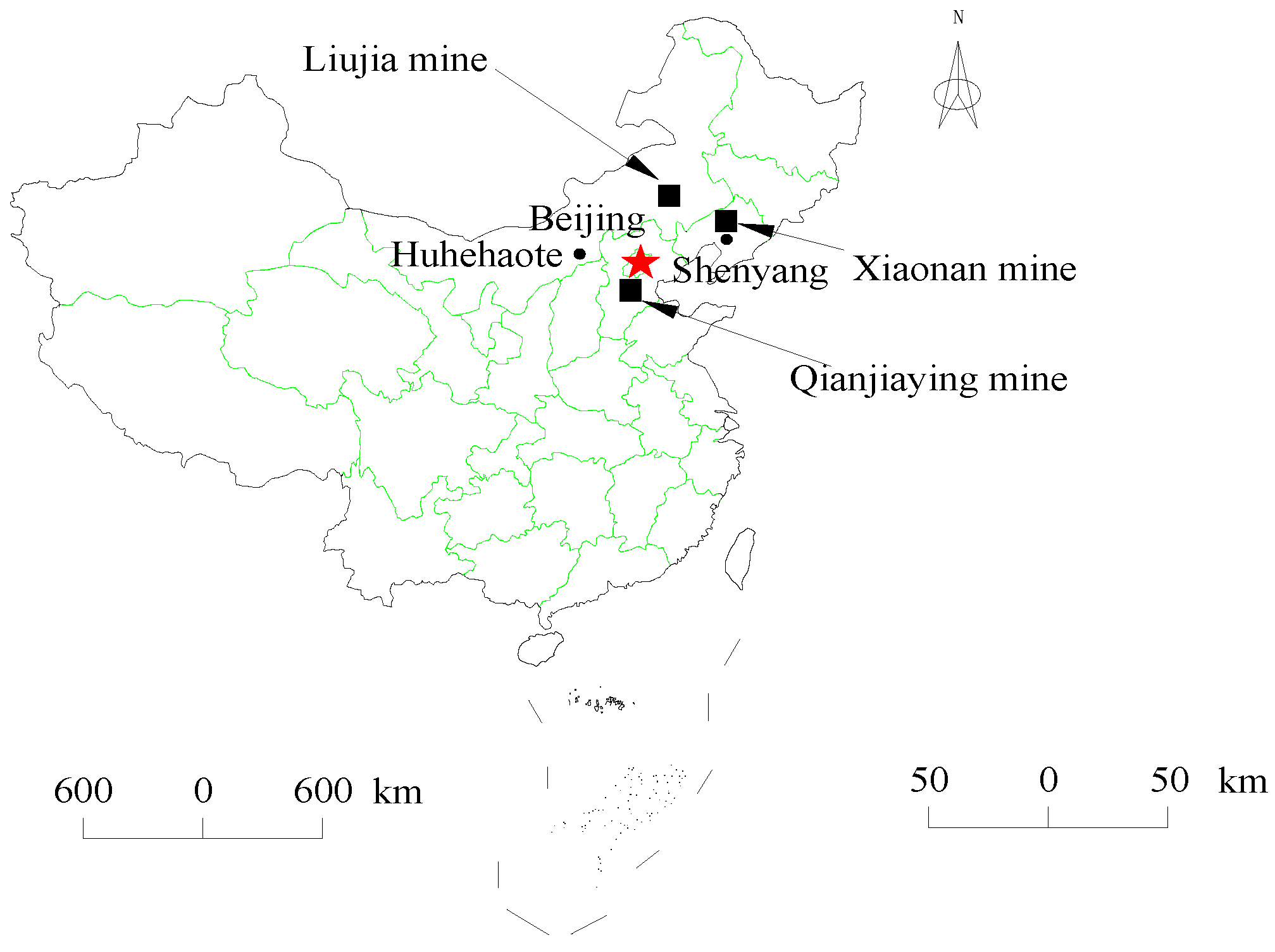
2.3. Coal Specimens
2.4. Acids
3. Experiment Design
3.1. Low Temperature Nitrogen Adsorption Experiment and Brunauer–Emmett–Teller Method
3.2. Seepage Experiment
4. Result and Discussion
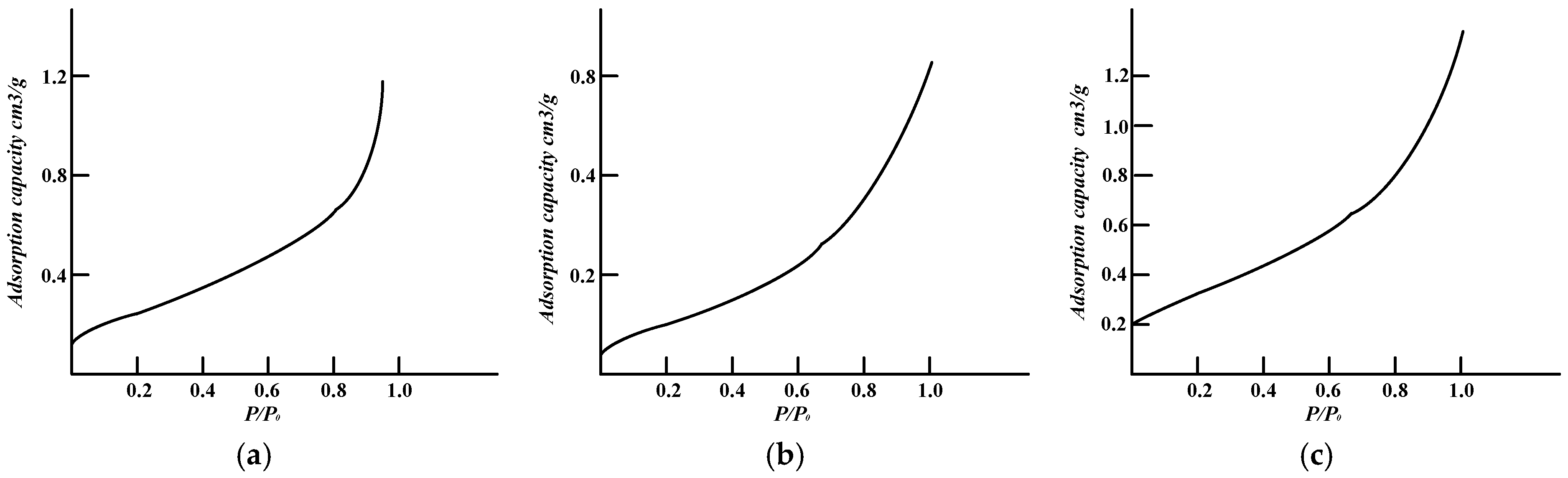
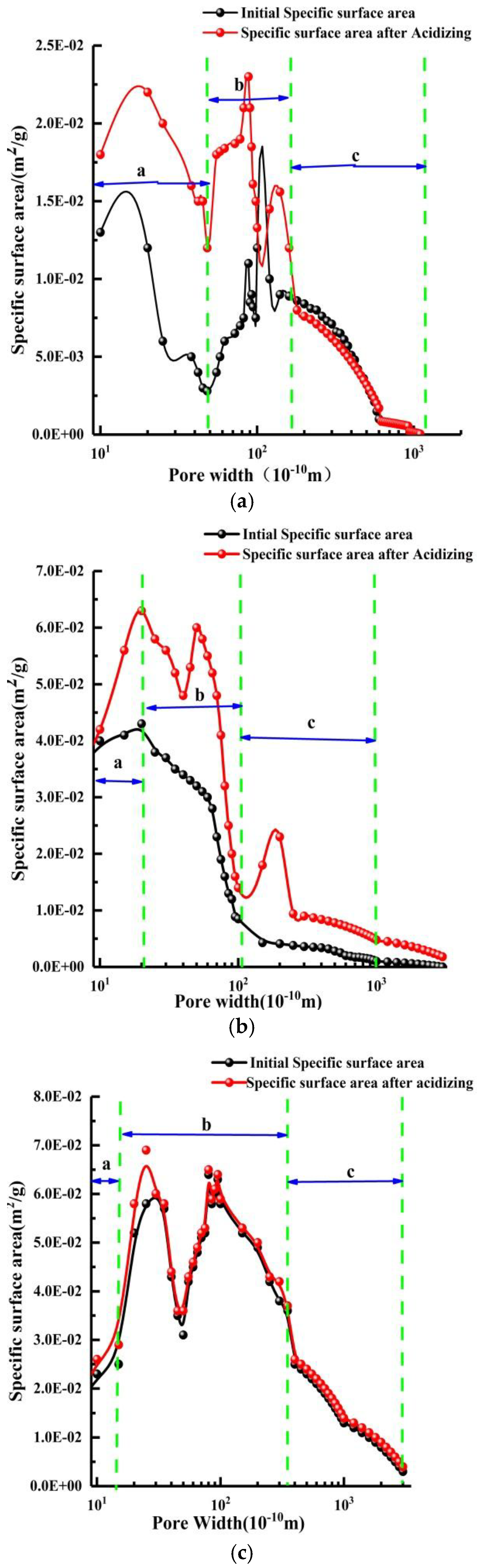
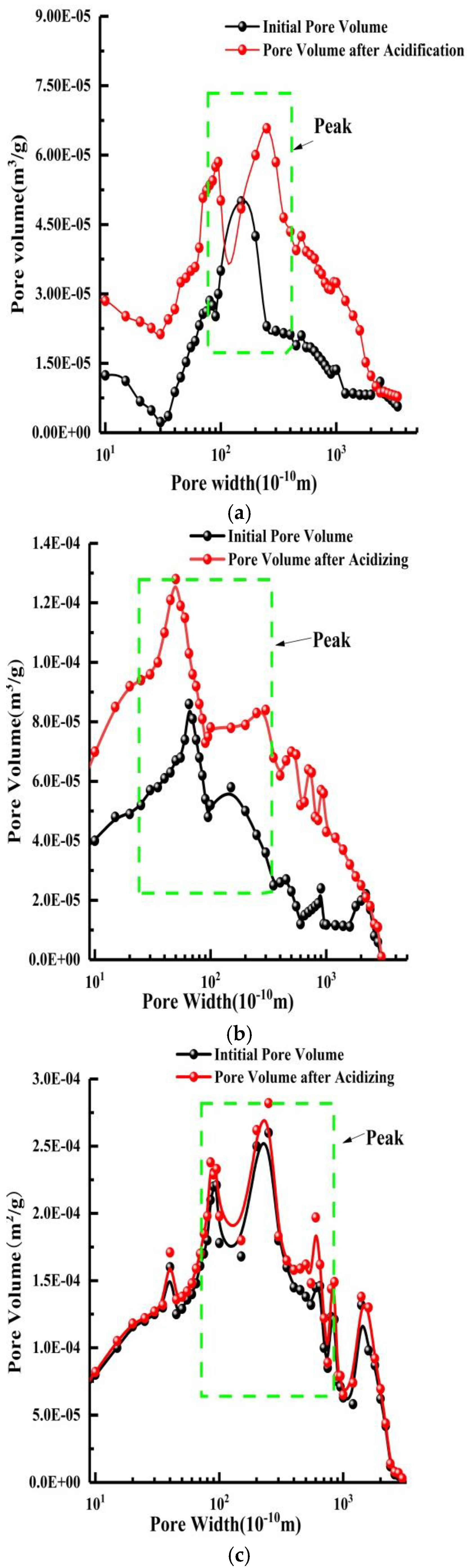
4.1. Pore Structure
4.2. Permeability Laws
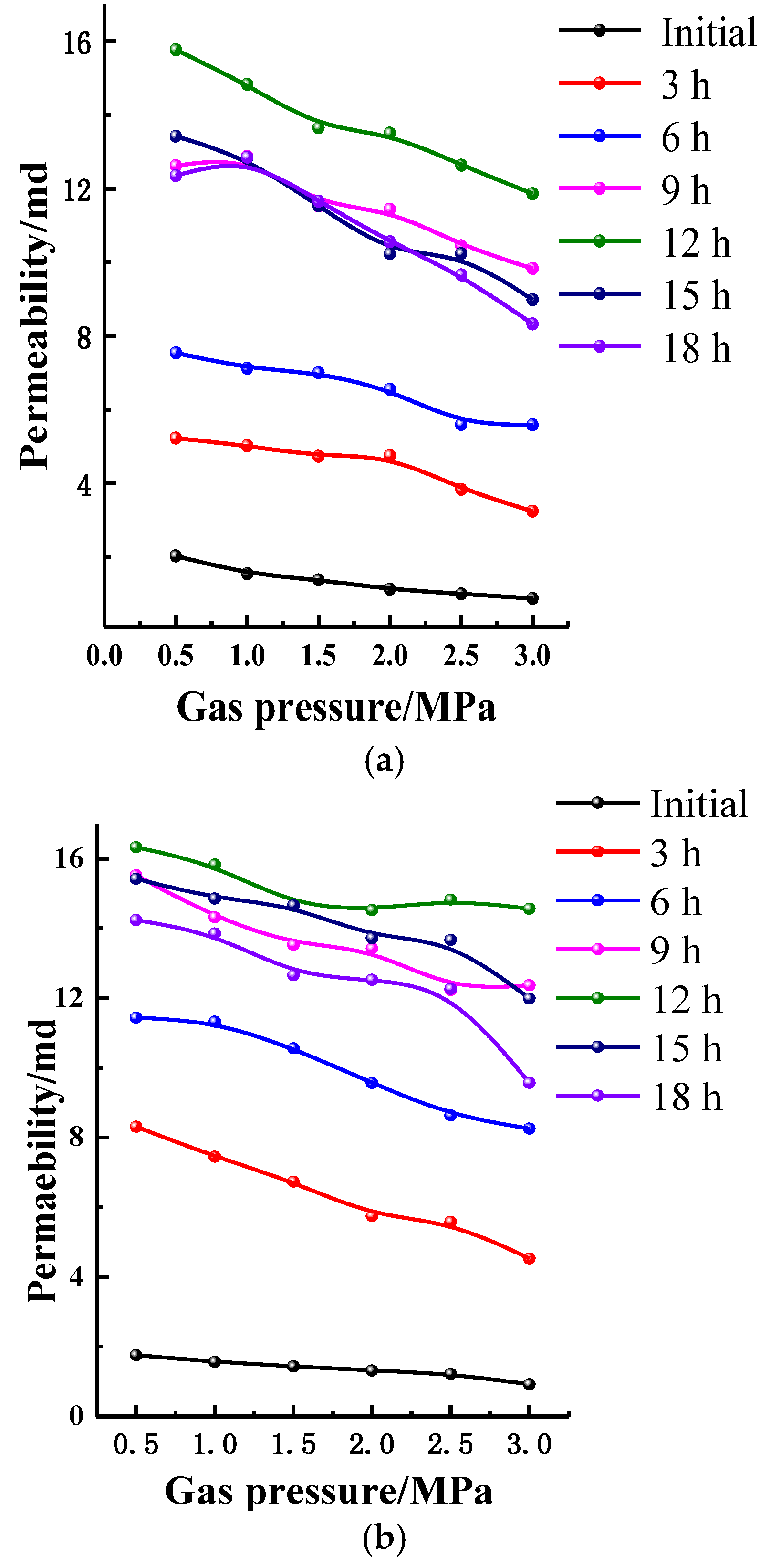
4.2.1. Permeability Laws under Constant Confining Pressure
4.2.2. Permeability Laws under Constant Confining Pressure and Gas Pressure
5. Conclusions
- (1)
- Acidizing is an effective method to increase the permeability of coal reservoirs by injecting one or several kinds of acid solution into the cleats in the coal reservoirs. We propose an acidizing combination, which consists of hydrochloric acid (HCl), 2% hydrofluoric acid (HF), and 2% ammonium chloride solution (NH4Cl).
- (2)
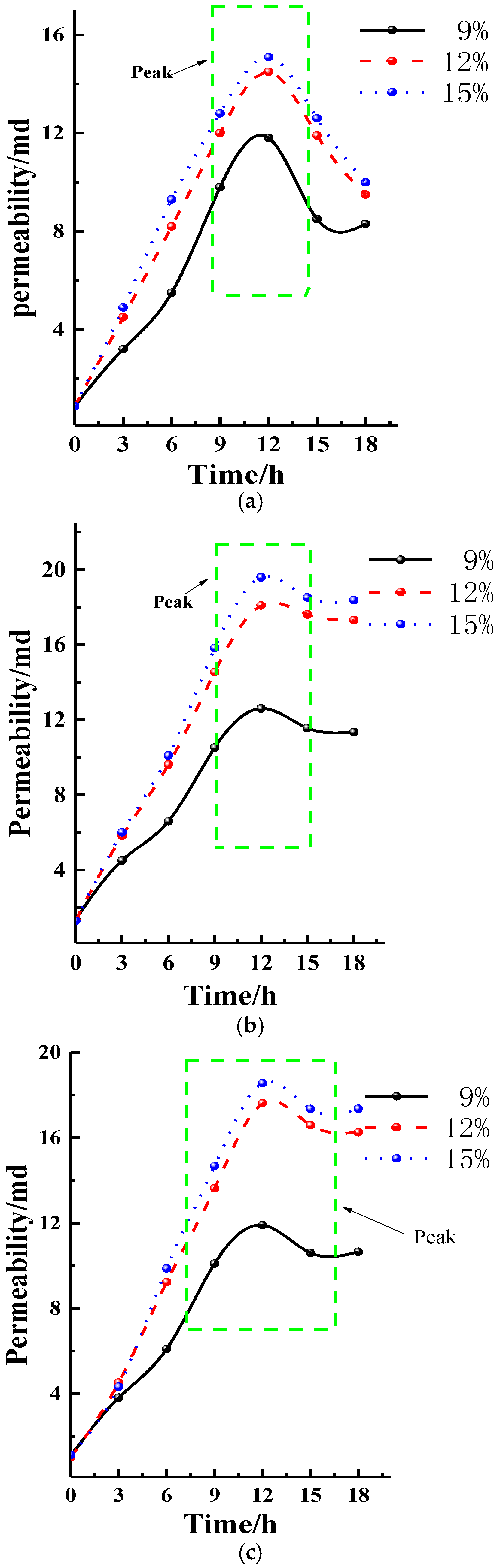
- After acidizing, BET and pore volume increases, mainly concentrating at about 10 nm, but for high-rank coal this was not obvious. The permeability of the specimens increases, stabilizes, and then retreats during acidizing. The mass fraction for the test coal specimens is usually about 12~15%.
- (3)
- The acid reaction is divided into three stages according to the progress of the reaction. In the first stage (0~12 h), the permeability of specimens increases rapidly. In the second stage (12~15 h), the permeability of coal specimens becomes stable. In the third stage (15~18 h), the permeability of coal specimens becomes slightly fall.
- (4)
- The chemical reactions of the acid and the minerals in the coal are summarized. The reaction kinetics of the acid and the minerals in the coal and the influencing factors of the acid-coal reactions are analyzed. On account of the dynamic equation, the composition of coal, the surface area of the acid-coal reaction, the mass fraction of HF, and the environmental conditions of the reaction system will all impact the process of reaction.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lama, R.D.; Bodziony, J. Management of outburst in underground coal mines. Int. J. Coal Geol. 1998, 35, 83–115. [Google Scholar] [CrossRef]
- Bustin, R.M.; Clarkson, C.R. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Chen, D.; Pan, Z.J.; Liu, J.S.; Connell, L.D. An improved relative permeability model for coal reservoirs. Int. J. Coal Geol. 2013, 109, 45–57. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.P.; Lai, F.P. Experimental study on porosity and permeability of anthracite coal under different stresses. J. Pet. Sci. Eng. 2015, 133, 810–817. [Google Scholar] [CrossRef]
- Lv, Y.M.; Tang, D.Z.; Xu, H.; Luo, H.H. Production characteristics and the key factors in high-rank coalbed methane fields: A case study on the Fanzhuang Block, Southern Qinshui Basin, China. Int. J. Coal Geol. 2012, 96–97, 93–108. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.W.; Tian, H.J.; Chen, W.G.; Peng, X.D.; Zhang, H. The pilot appraisal of acid fracturing of coalbed methane reservoir in southeast Qinshui Basin, China. Earth Sci. Res. J. 2016, 20, C1–C6. [Google Scholar] [CrossRef]
- Niu, S.W.; Zhao, Y.S.; Hu, Y.Q. Experimental Ivestigation of the Temperature and Pore Pressure Effect on Permeability of Lignite Under the In Situ Condition. Transp. Porous Media 2014, 101, 137–148. [Google Scholar] [CrossRef]
- Hou, P.; Gao, F.; Ju, Y.; Yang, Y.G.; Gao, Y.N.; Liu, J. Effect of water and nitrogen fracturing fluids on initiation and extension of fracture in hydraulic fracturing of porous rock. J. Nat. Gas Sci. Eng. 2017, 45, 38–52. [Google Scholar] [CrossRef]
- Gao, J.F.; Xing, H.L.; Turner, L.; Steel, K.; Sedek, M.; Golding, S.D.; Rudolph, V. Pore-Scale Numerical Investigation on Chemical Stimulation in Coal and Permeability Enhancement for Coal Seam Gas Production. Transp. Porous Media 2017, 116, 335–351. [Google Scholar] [CrossRef]
- Kumar, H.; Elsworth, D.; Liu, J.S.; Pone, D.; Mathews, J.P. Permeability evolution of propped artificial fractures in coal on injection of CO2. J. Pet. Sci. Eng. 2015, 133, 695–704. [Google Scholar] [CrossRef]
- Keshavarz, A.; Badalyan, A.; Carageorgos, T.; Bedrikovetsky, P.; Johnson, R. Stimulation of coal seam permeability by micro-sized graded proppant placement using selective fluid properties. Fuel 2015, 144, 228–236. [Google Scholar] [CrossRef]
- Li, Q.G.; Lin, B.Q.; Zhai, C. The effect of pulse frequency on the fracture extension during hydraulic fracturing. J. Nat. Gas Sci. Eng. 2014, 21, 296–303. [Google Scholar] [CrossRef]
- Colmenares, L.B.; Zoback, M.D. Hydraulic fracturing and wellbore completion of coalbed methane wells in the Powder River Basin, Wyoming: Implications for water and gas production. AAPG Bull. 2007, 91, 51–67. [Google Scholar] [CrossRef]
- Fujioka, M.; Yamaguchi, S.; Nako, M. CO2-ECBM field tests in the Ishikari Coal Basin of Japan. Int. J. Coal Geol. 2010, 82, 287–298. [Google Scholar] [CrossRef]
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Potential for enhanced gas recovery and CO2 storage in the Marcellus Shale in the Eastern United States. Int. J. Coal Geol. 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Jiao, Z.S.; Surdam, R.C.; Zhou, L.F.; Stauffer, P.H.; Luo, T.T. A feasibility study of geological CO2 sequestration in the Ordos Basin, China. In Proceedings of the 10th International Conference on Greenhouse Gas Control Technologies, Amsterdam, The Netherlands, 19–23 September 2010; Gale, J., Hendriks, C., Turkenberg, W., Eds.; Elsevier Science Bv: Amsterdam, The Netherlands, 2011; Volume 4, pp. 5982–5989. [Google Scholar]
- Wu, Y.; Liu, J.S.; Elsworth, D.; Chen, Z.W.; Connell, L.; Pan, Z.J. Dual poroelastic response of a coal seam to CO2 injection. Int. J. Greenh. Gas Control 2010, 4, 668–678. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Yan, F.Z.; Lin, B.Q.; Zhu, C.J.; Shen, C.M.; Zou, Q.L.; Guo, C.; Liu, T. A novel ECBM extraction technology based on the integration of hydraulic slotting and hydraulic fracturing. J. Nat. Gas Sci. Eng. 2015, 22, 571–579. [Google Scholar] [CrossRef]
- Lu, C.P.; Dou, L.M.; Zhang, N.; Xue, J.H.; Liu, G.J. Microseismic and acoustic emission effect on gas outburst hazard triggered by shock wave: A case study. Nat. Hazards 2014, 73, 1715–1731. [Google Scholar] [CrossRef]
- Terry, R.C. Methane Produced from Wet Coal Deposits—By Injecting Oxygen-Contg. Gas through Well-Bore, Removing Formation Water, and Producing Free and Desorbed Methane. U.S. Patent US4544037-A, 1 October 1985. [Google Scholar]
- Steel, K.M.; Patrick, J.W. The production of ultra clean coal by chemical demineralisation. Fuel 2001, 80, 2019–2023. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takeda, S.; Tsurue, T. Influence of mineral and chemical-composition of coal ashes on their fusibility. Fuel Process. Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Balucan, R.D.; Turner, L.G.; Steel, K.M. Acid-induced mineral alteration and its influence on the permeability and compressibility of coal. J. Nat. Gas Sci. Eng. 2016, 33, 973–987. [Google Scholar] [CrossRef]
- Turner, L.G.; Steel, K.M. A study into the effect of cleat demineralisation by hydrochloric acid on the permeability of coal. J. Nat. Gas Sci. Eng. 2016, 36, 931–942. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Lan, S.; Li, F.; Qiao, Q.; Sun, C.; Zhang, X. Anti-Swelling Agent Is Obtained by Mixing and Stirring, Clay Stabilizing Agent, Water and Cationic Polymer Which Is Copolymer of Dimethyl Diallyl Ammonium Chloride and Acrylamide. China Patent CN102786924-A, CN102786924-B, 21 November 2012. [Google Scholar]
- Day, R.E.; Parfitt, G.D. Characterization of surface of rutile by nitrogen and water vapour adsorption. Trans. Faraday Soc. 1967, 63, 708–716. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]







| Materials | Calcite | Dolomite | Kaolin | Quartz | Amorphous | |
|---|---|---|---|---|---|---|
| Specimens | ||||||
| Qian Jiaying | 11% | 3% | 8% | 4% | 74% | |
| Xiaonan | 18% | 6% | 4% | 3% | 69% | |
| Liujia | 12% | 2% | 4% | 3% | 79% | |
| Specimens | Initial SSA () | SSA after Acidizing () | Initial Pore Volume () | Pore Volume after Acidizing () | Coal Rank |
|---|---|---|---|---|---|
| Qian Jiaying | 0.8258 | 1.3457 | 0.002891 | 0.008245 | Long flame coal |
| Xiaonan | 3.5151 | 6.2326 | 0.004258 | 0.018690 | Non-caking coal |
| Liujia | 6.4672 | 8.8345 | 0.013270 | 0.014250 | Coking coal |
| Specimen | Mass Fraction/% | Permeability/×10−15 m2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Origin | 3 h | 6 h | 9 h | 12 h | 15 h | 18 h | ||
| Qian 1# | 9 | 0.875 | 3.243 | 5.589 | 9.834 | 11.863 | 8.987 | 8.325 |
| Qian 2# | 12 | 0.923 | 4.532 | 8.256 | 12.368 | 14.561 | 11.987 | 9.568 |
| Qian 3# | 15 | 0.821 | 4.952 | 9.311 | 12.886 | 15.122 | 12.635 | 10.007 |
| Xiao1# | 9 | 1.381 | 4.513 | 5.835 | 10.524 | 12.607 | 11.565 | 11.347 |
| Xiao 2# | 12 | 1.321 | 5.825 | 9.624 | 14.551 | 18.185 | 17.624 | 17.314 |
| Xiao3# | 15 | 1.287 | 6.005 | 10.104 | 15.831 | 19.624 | 18.523 | 18.387 |
| Liu 1# | 9 | 1.101 | 3.815 | 5.721 | 10.102 | 11.897 | 10.987 | 10.654 |
| Liu 2# | 12 | 1.023 | 4.532 | 9.235 | 13.627 | 17.263 | 16.587 | 16.254 |
| Liu 3# | 15 | 1.135 | 4.234 | 9.871 | 14.681 | 18.563 | 17.355 | 17.361 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Wen, G.; Sun, H.; Zhao, X. Experimental Study of the Pore Structure and Permeability of Coal by Acidizing. Energies 2018, 11, 1162. https://doi.org/10.3390/en11051162
Zhao B, Wen G, Sun H, Zhao X. Experimental Study of the Pore Structure and Permeability of Coal by Acidizing. Energies. 2018; 11(5):1162. https://doi.org/10.3390/en11051162
Chicago/Turabian StyleZhao, Bo, Guangcai Wen, Haitao Sun, and Xusheng Zhao. 2018. "Experimental Study of the Pore Structure and Permeability of Coal by Acidizing" Energies 11, no. 5: 1162. https://doi.org/10.3390/en11051162




