NO Removal from Simulated Flue Gas with a NaClO2 Mist Generated Using the Ultrasonic Atomization Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Apparatus
2.2. Experimental Procedures
2.3. Data Processing
3. Results and Discussion
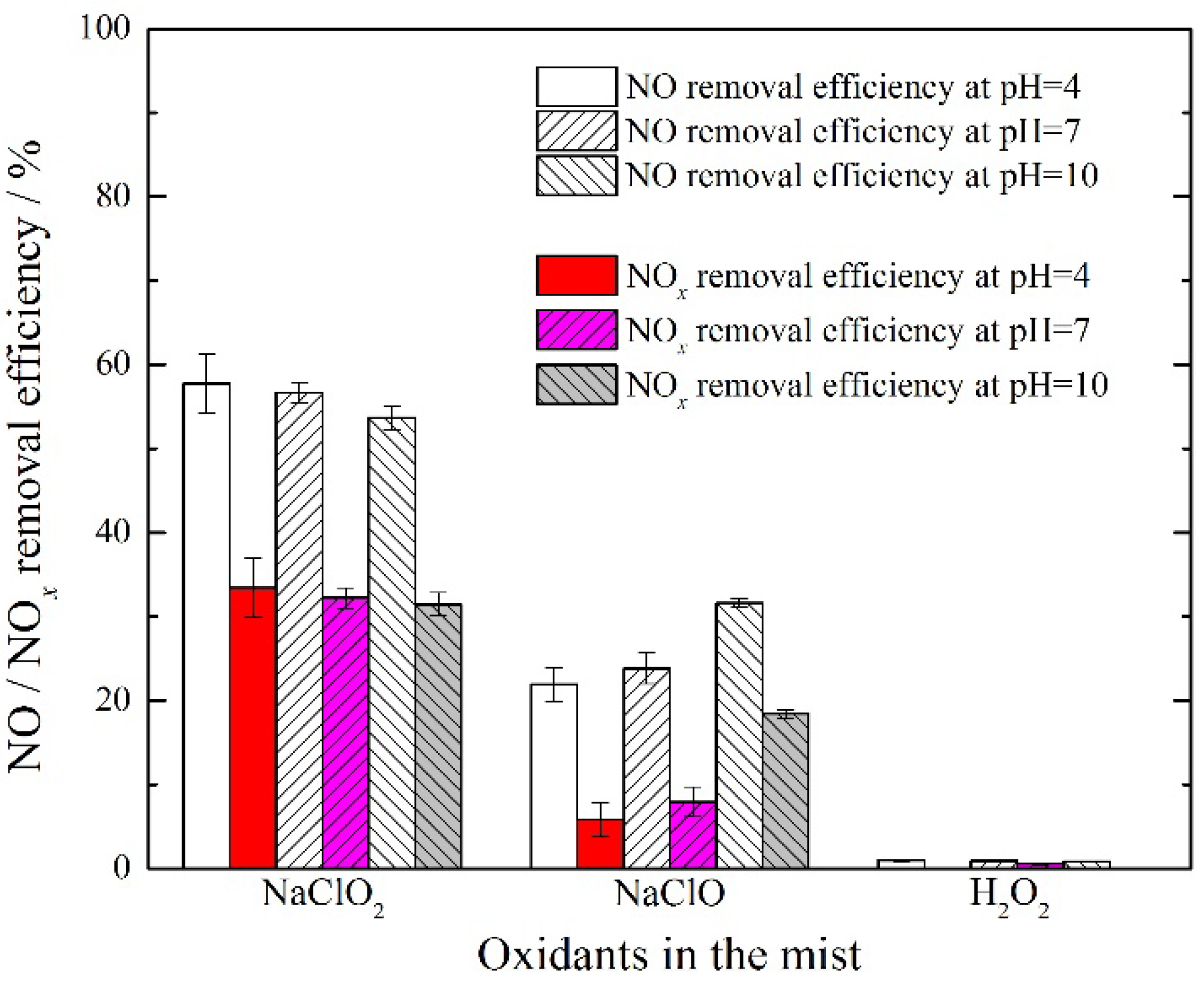
3.1. Comparison of Different Oxidants in Mist
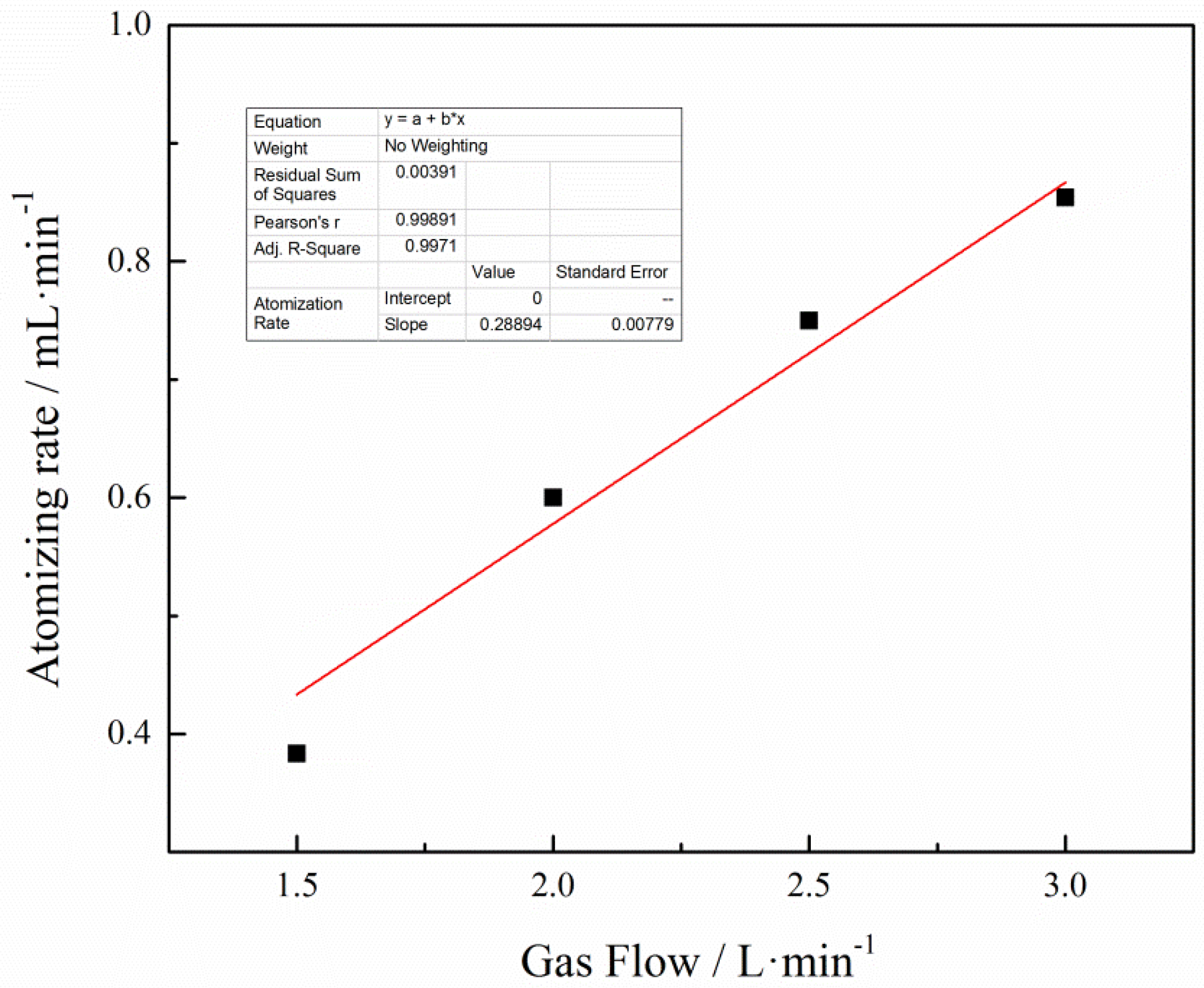
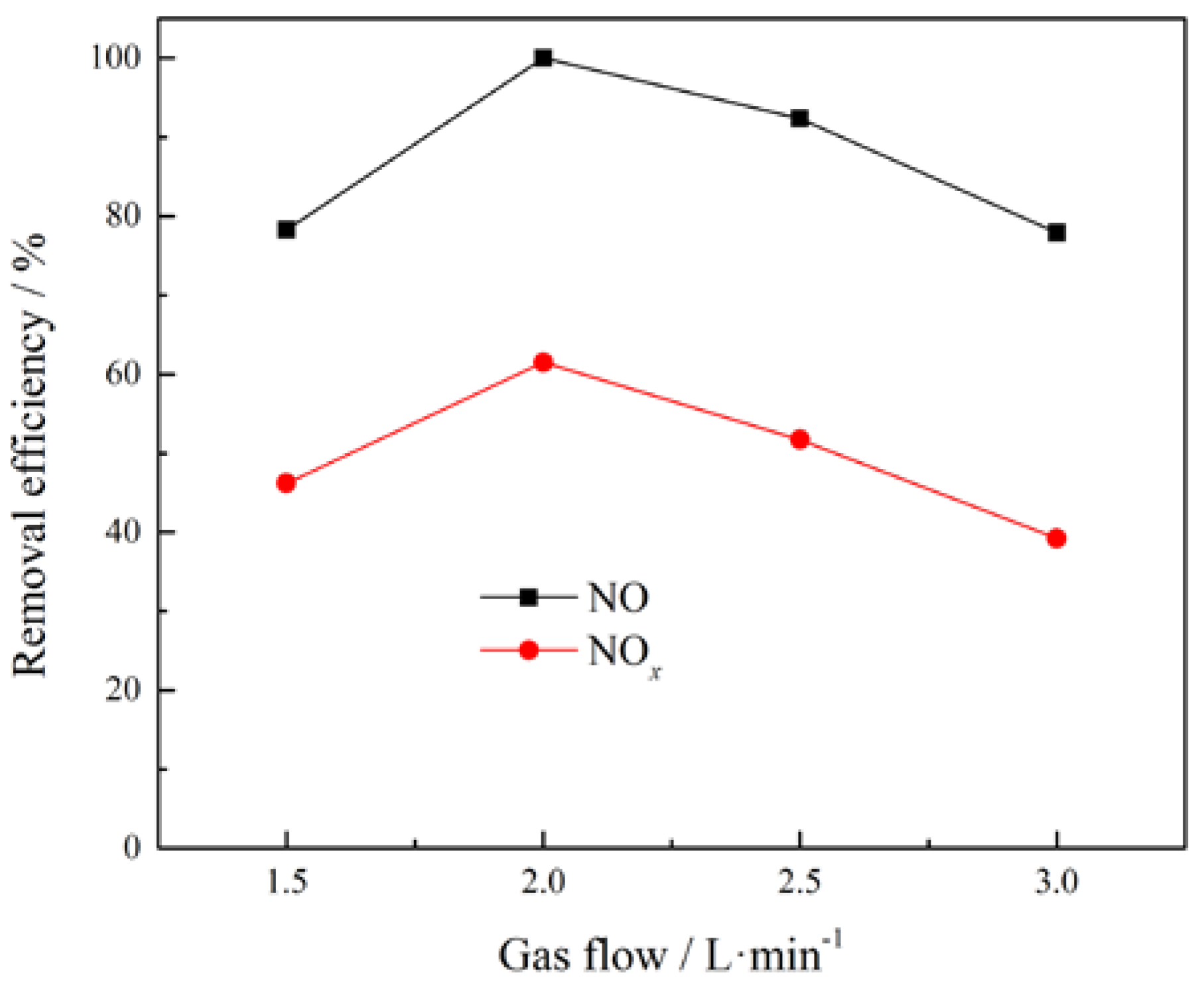
3.2. Effect of Gas Flow Rate
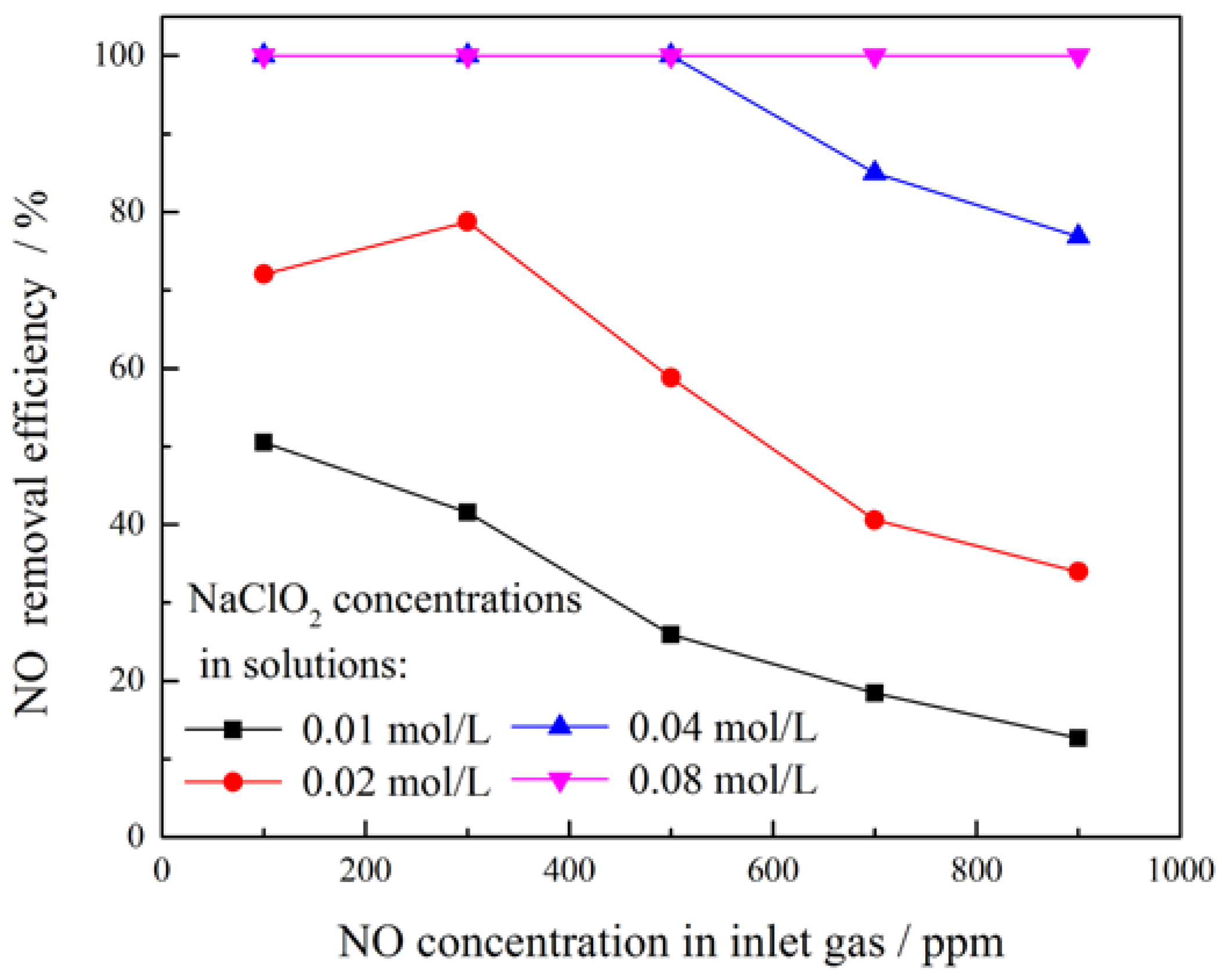
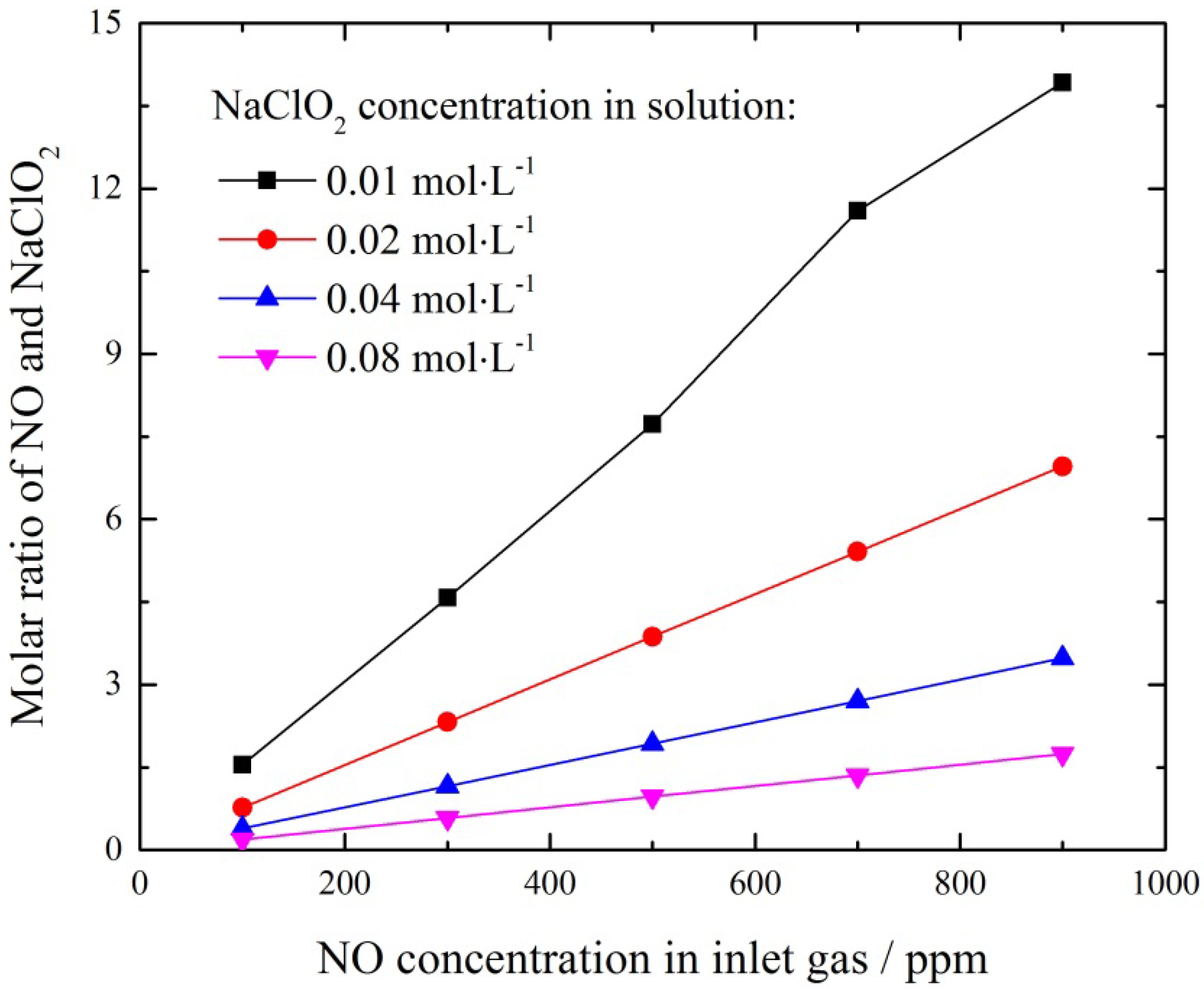
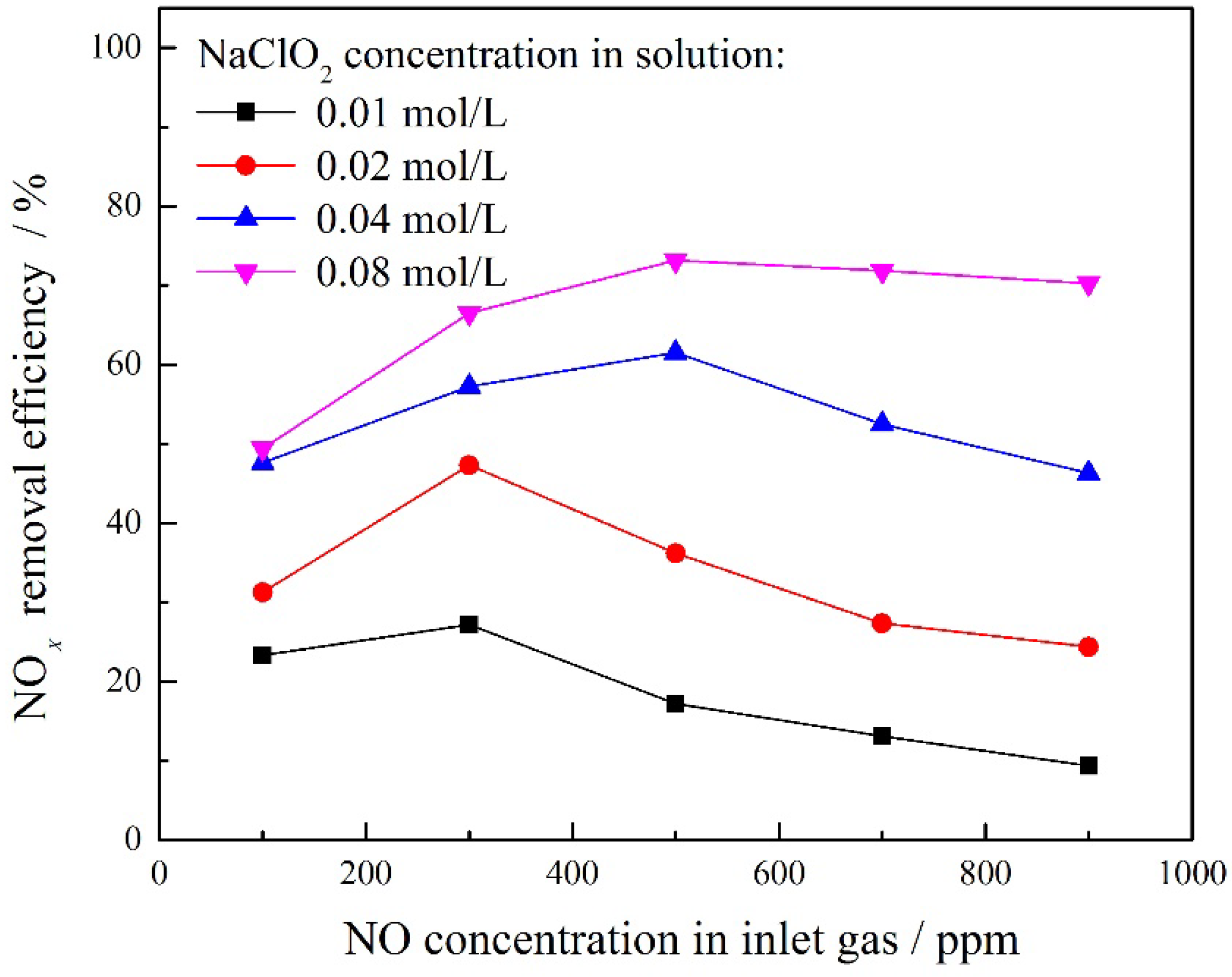
3.3. Effect of NaClO2 Concentration
3.4. Effect of NO Concentration
3.5. Effect of Solution pH
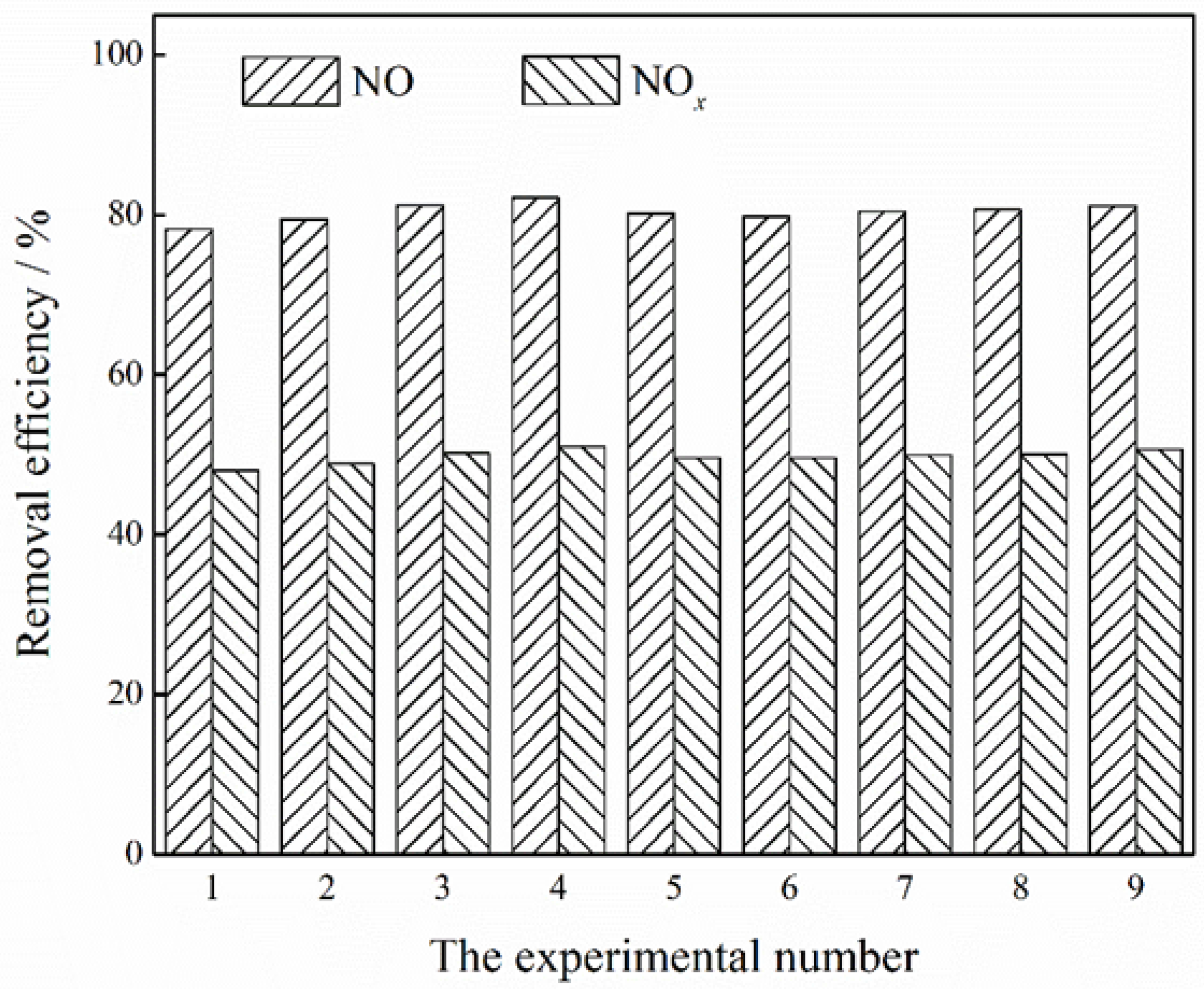
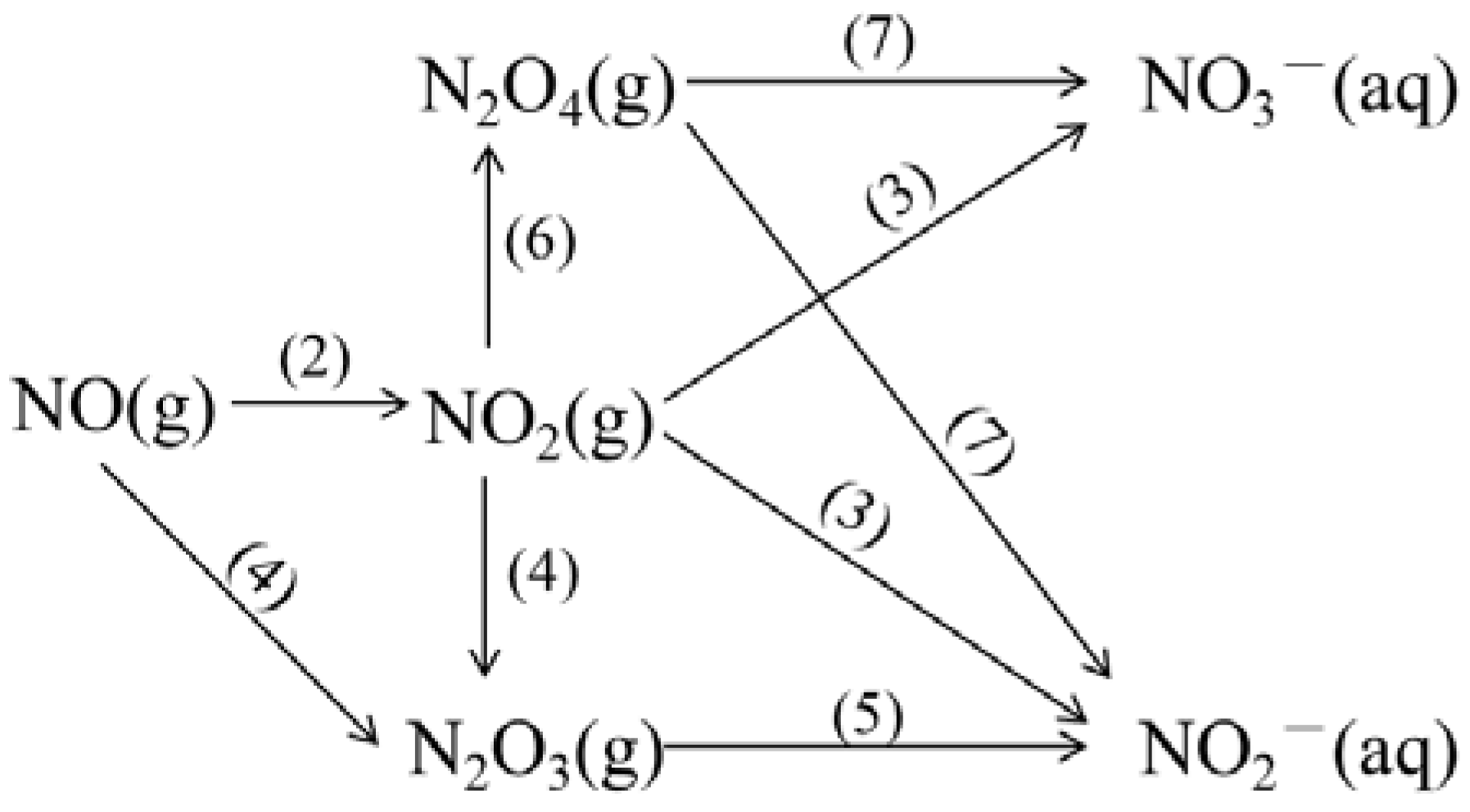
3.6. Parallel Tests and Reaction Chemistry
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kan, H.; Chen, R.; Tong, S. Ambient air pollution, climate change, and population health in China. Environ. Int. 2012, 42, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Wang, P.; Chen, Q.; Jiang, W.; Chu, Y.-H.; Chiang, P.-C. Development of high-gravity technology for removing particulate and gaseous pollutant emissions: Principles and applications. J. Clean. Prod. 2017, 149, 540–556. [Google Scholar] [CrossRef]
- Yang, L.; Bao, J.; Yan, J.; Liu, J.; Song, S.; Fan, F. Removal of fine particles in wet flue gas desulfurization system by heterogeneous condensation. Chem. Eng. J. 2010, 156, 25–32. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S.; Zhu, Y. Characterization of particle and gaseous emissions from marine diesel engines with different fuels and impact of after-treatment technology. Energies 2017, 10, 1110. [Google Scholar] [CrossRef]
- Sun, W.-Y.; Ding, S.-L.; Zeng, S.-S.; Su, S.-J.; Jiang, W.-J. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of no using ozone. J. Hazard. Mater. 2011, 192, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Jiang, Y.; Xing, Z.; Wang, X.; Huang, S.; Liu, Q.; Yang, J. MoO3 modified CeO2/TiO2 catalyst prepared by a single step sol–gel method for selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2015, 29, 43–47. [Google Scholar] [CrossRef]
- Gou, X.; Wang, Y.; Wu, C.; Liu, S.; Zhao, D.; Li, Y.; Iram, S. Low temperature selective catalytic reduction using molding catalysts Mn-Ce/FA and Mn-Ce/FA-30% TiO2. Energies 2017, 10, 2084. [Google Scholar] [CrossRef]
- Farcy, B.; Abou-Taouk, A.; Vervisch, L.; Domingo, P.; Perret, N. Two approaches of chemistry downsizing for simulating selective non catalytic reduction DeNOx process. Fuel 2014, 118, 291–299. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S.; Liu, D.; Zhang, H. Study on removing SO2 and NOx simultaneously in ships emissions by wet scrubbing based on natrium-alkali method. J. Chem. Eng. Jpn. 2015, 48, 834–840. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, T.; Wang, Y.; He, Y.; Jia, J. Spray absorption and electrochemical reduction of nitrogen oxides from flue gas. Environ. Sci. Technol. 2013, 47, 9514–9522. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G.; Khan, M.A. Nitric oxide removal by combined persulfate and ferrous–EDTA reaction systems. Chem. Eng. J. 2015, 281, 575–587. [Google Scholar] [CrossRef]
- Guo, R.-T.; Hao, J.-K.; Pan, W.-G.; Yu, Y.-L. Liquid phase oxidation and absorption of NO from flue gas: A review. Sep. Sci. Technol. 2015, 50, 310–321. [Google Scholar] [CrossRef]
- Xie, D.; Sun, Y.; Zhu, T.; Ding, L. Removal of NO in mist by the combination of plasma oxidation and chemical absorption. Energy Fuels 2016, 30, 5071–5076. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Feng, Y.; Zhu, Y. Marine emission pollution abatement using ozone oxidation by a wet scrubbing method. Ind. Eng. Chem. Res. 2016, 55, 5825–5831. [Google Scholar] [CrossRef]
- Fang, P.; Cen, C.-P.; Wang, X.-M.; Tang, Z.-J.; Tang, Z.-X.; Chen, D.-S. Simultaneous removal of SO2, NO and Hg0 by wet scrubbing using urea + kMnO4 solution. Fuel Process. Technol. 2013, 106, 645–653. [Google Scholar] [CrossRef]
- Bhanarkar, A.; Gupta, R.; Biniwale, R.; Tamhane, S. Nitric oxide absorption by hydrogen peroxide in airlift reactor: A study using response surface methodology. Int. J. Environ. Sci. Technol. 2014, 11, 1537–1548. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Owusu, S.O. Aqueous absorption and oxidation of nitric oxide with oxone for the treatment of tail gases: Process feasibility, stoichiometry, reaction pathways, and absorption rate. Ind. Eng. Chem. Res. 2003, 42, 4084–4100. [Google Scholar] [CrossRef]
- Khan, N.E.; Adewuyi, Y.G. Absorption and oxidation of nitric oxide (NO) by aqueous solutions of sodium persulfate in a bubble column reactor. Ind. Eng. Chem. Res. 2010, 49, 8749–8760. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Sakyi, N.Y. Removal of nitric oxide by aqueous sodium persulfate simultaneously activated by temperature and Fe2+ in a lab-scale bubble reactor. Ind. Eng. Chem. Res. 2013, 52, 14687–14697. [Google Scholar] [CrossRef]
- Mondal, M.K.; Chelluboyana, V.R. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem. Eng. J. 2013, 217, 48–53. [Google Scholar] [CrossRef]
- Guo, R.-T.; Pan, W.-G.; Zhang, X.-B.; Xu, H.-J.; Jin, Q.; Ding, C.-G.; Guo, S.-Y. The absorption kinetics of NO into weakly acidic NaClO solution. Sep. Sci. Technol. 2013, 48, 2871–2875. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Kundu, N. Reaction kinetics of oxidative absorption of nitric oxide into sodium hypochlorite solution. Int. J. Adv. Res. Sci. Technol. 2015, 4, 313–318. [Google Scholar]
- Adewuyi, Y.G.; He, X.; Shaw, H.; Lolertpihop, W. Simultaneous absorption and oxidation of NO and SO2 by aqueous solutions of sodium chlorite. Chem. Eng. Commun. 1999, 174, 21–51. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Lee, S.H.; Jung, J.H.; Shon, B.H.; Lee, H.K. Study on the removal of NOx from simulated flue gas using acidic NaClO2 solution. J. Environ. Sci. 2008, 20, 33–38. [Google Scholar] [CrossRef]
- Guo, R.-T.; Pan, W.-G.; Ren, J.-X.; Zhang, X.-B.; Jin, Q. Absorption of NO from simulated flue gas by using NaClO2/(NH4)2CO3 solutions in a stirred tank reactor. Korean J. Chem. Eng. 2013, 30, 101–104. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Jin, D.S.; Lee, S.H.; Moon, S.H.; Jung, J.H.; Lee, H.K. Removal of NO from flue gas by aqueous chlorine-dioxide scrubbing solution in a lab-scale bubbling reactor. J. Hazard. Mater. 2008, 150, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Zhang, Y.; Wang, Z.; Li, Y.; Yuan, B.; Mao, X.; Zhao, Y. An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent. Chem. Eng. J. 2017, 307, 562–571. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, X.; Liu, S.; Yao, J. Experiments on and mechanism of simultaneous removal of Hg0, SO2 and NO from flus gas using NaClO2 solution. Environ. Technol. 2009, 30, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-W.; Choi, S.; Park, D.-W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-W.; Park, D.-W. Removal kinetics for gaseous NO and SO2 by an aqueous NaClO2 solution mist in a wet electrostatic precipitator. Environ. Technol. 2017, 38, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, R.; Zhang, P.; Zhou, S. Integrative process for simultaneous removal of SO2 and NO utilizing a vaporized H2O2/Na2S2P8. Energy Fuels 2014, 28, 6502–6510. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Pan, J. Novel process of simultaneous removal of nitric oxide and sulfur dioxide using a vacuum ultraviolet (VUV)-activated O2/H2O/H2O2 system in a wet VUV–spraying reactor. Environ. Sci. Technol. 2016, 50, 12966–12975. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhong, Q.; Zhang, S.; Song, F.; Bu, Y. Simultaneous removal of NOx and SO2 from coal-fired flue gas by catalytic oxidation-removal process with H2O2. Chem. Eng. J. 2014, 243, 176–182. [Google Scholar] [CrossRef]
- Han, Z.; Yang, S.; Pan, X.; Zhao, D.; Yu, J.; Zhou, Y.; Xia, P.; Zheng, D.; Song, Y.; Yan, Z. New experimental results of NO removal from simulated flue gas by wet scrubbing using NaClO solution. Energy Fuels 2017, 31, 3047–3054. [Google Scholar] [CrossRef]
- Yang, S.-L.; Han, Z.-T.; Dong, J.-M.; Zheng, Z.-S.; Pan, X.-X. UV-enhanced NaClO oxidation of nitric oxide from simulated flue gas. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Fang, P.; Tang, Z.; Chen, X.; Huang, J.; Chen, D.; Tang, Z.; Cen, C. Split, partial oxidation and mixed absorption: A novel process for synergistic removal of multiple pollutants from simulated flue gas. Ind. Eng. Chem. Res. 2017, 56, 5116–5126. [Google Scholar] [CrossRef]
- Yang, S.; Pan, X.; Han, Z.; Zheng, D.; Yu, J.; Xia, P.; Liu, B.; Yan, Z. Nitrogen oxide removal from simulated flue gas by UV-irradiated sodium chlorite solution in a bench-scale scrubbing reactor. Ind. Eng. Chem. Res. 2017, 56, 3671–3678. [Google Scholar] [CrossRef]
- Wu, H.G.; Jin, M.; Wang, F.; Yu, G.X.; Lu, P. Performance of sodium chlorite/urea on simultaneous desulfurization and denitrification in a rotating packed bed. Adv. Mater. Res. 2014, 908, 183–186. [Google Scholar] [CrossRef]
- Chien, T.-W.; Chu, H.; Hsueh, H.-T. Spray scrubbing of the nitrogen oxides into NaClO2 solution under acidic conditions. J. Environ. Sci. Health Part A 2001, 36, 403–414. [Google Scholar] [CrossRef]
- Lang, R.J. Ultrasonic atomization of liquids. J. Acoust. Soc. Am. 1962, 34, 6–8. [Google Scholar] [CrossRef]
- Barreras, F.; Amaveda, H.; Lozano, A. Transient high-frequency ultrasonic water atomization. Exp. Fluids 2002, 33, 405–413. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Zhao, D.; Zheng, D.; Pan, X.; Liu, B.; Han, Z.; Gao, Y.; Wang, J.; Yan, Z. NO Removal from Simulated Flue Gas with a NaClO2 Mist Generated Using the Ultrasonic Atomization Method. Energies 2018, 11, 1043. https://doi.org/10.3390/en11051043
Han Z, Zhao D, Zheng D, Pan X, Liu B, Han Z, Gao Y, Wang J, Yan Z. NO Removal from Simulated Flue Gas with a NaClO2 Mist Generated Using the Ultrasonic Atomization Method. Energies. 2018; 11(5):1043. https://doi.org/10.3390/en11051043
Chicago/Turabian StyleHan, Zhitao, Dongsheng Zhao, Dekang Zheng, Xinxiang Pan, Bojun Liu, Zhiwei Han, Yu Gao, Junming Wang, and Zhijun Yan. 2018. "NO Removal from Simulated Flue Gas with a NaClO2 Mist Generated Using the Ultrasonic Atomization Method" Energies 11, no. 5: 1043. https://doi.org/10.3390/en11051043





