Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment
Abstract
:1. Introduction
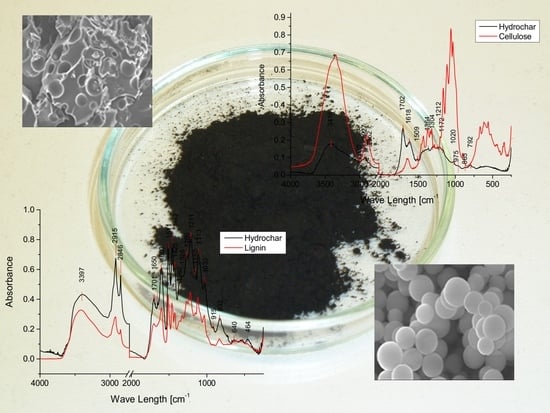
2. Results and Discussion
3. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204. [Google Scholar] [CrossRef]
- Titirici, M.-M. Hydrothermal Processes Sustainable Carbon Materials from Hydrothermal Processes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kruse, A.; Dahmen, N. Hydrothermal biomass conversion: Quo vadis? J. Supercrit. Fluids 2017. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Karayıldırım, T.; Sınağ, A.; Kruse, A. Char and Coke Formation as Unwanted Side Reaction of the Hydrothermal Biomass Gasification. Chem. Eng. Technol. 2008, 31, 1561–1568. [Google Scholar] [CrossRef]
- Funke, A.; Reebs, F.; Kruse, A. Experimental comparison of hydrothermal and vapothermal carbonization. Fuel Process. Technol. 2013, 115, 261–269. [Google Scholar] [CrossRef]
- Dinjus, E.; Kruse, A.; Tröger, N. Hydrothermal Carbonization—1. Influence of Lignin in Lignocelluloses. Chem. Eng. Technol. 2011, 34, 2037–2043. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740. [Google Scholar] [CrossRef]
- Leroy, V.; Cancellieri, D.; Leoni, E. Thermal degradation of ligno-cellulosic fuels: DSC and TGA studies. Thermochim. Acta 2006. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim. Acta 2013, 566, 36–43. [Google Scholar] [CrossRef]
- Islam, M.A.; Asif, M.; Hameed, B.H. Pyrolysis kinetics of raw and hydrothermally carbonized Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour. Technol. 2014, 179, 227–233. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrolysis 1997, 40–41, 171–186. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shimanouchi, T.; Kimura, Y. Characterization of the Residue and Liquid Products Produced from Husks of Nuts from Carya cathayensis Sarg by Hydrothermal Carbonization. ACS Sustain. Chem. Eng. 2015, 3, 591–598. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefin. 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Kruse, A.; Gawlik, A. Biomass Conversion in Water at 330−410 °C and 30−50 MPa. Identification of Key Compounds for Indicating Different Chemical Reaction Pathways. Ind. Eng. Chem. Res. 2003, 42, 267–279. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.-M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Kruse, A.; Kirchherr, M.; Gaag, S.; Zevaco, T.A. Hydrothermale Karbonisierung. 4. Thermische Eigenschaften der Produkte. Chem. Ing. Tech. 2015, 87, 1707–1712. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Ye, S.-C.; Sheen, H.-K. Hydrothermal carbonization of sugarcane bagasse via wet torrefaction in association with microwave heating. Bioresour. Technol. 2012, 118, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Glucose and Fructose Decomposition in Subcritical and Supercritical Water: Detailed Reaction Pathway, Mechanisms, and Kinetics. Ind. Eng. Chem. Res. 1999, 38, 2888–2895. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Bernardo, M.; Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Kruse, A. Evaluation of hydrothermal carbonization as a preliminary step for the production of functional materials from biogas digestate. J. Anal. Appl. Pyrolysis 2017, 124, 461–474. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse; Springe: Berlin/Heidelberg, Germany, 2009. [Google Scholar]














| Material | T [°C] | t [h] | DM [wt. %] | C [wt. %] | H [wt. %] | N [wt. %] | S [wt. %] | O [wt. %] | Ash [wt. %] |
|---|---|---|---|---|---|---|---|---|---|
| Beech Wood | |||||||||
| Biomass | 46 | 6.7 | 0.2 | 0.1 | 44.88 | 2.12 | |||
| Hydrochar | 180 | 2 | 20 | 50.9 | 6.2 | 0.2 | 0 | 41.24 | 1.46 |
| Hydrochar | 180 | 4 | 20 | 51.9 | 6 | 0.2 | 0 | 39.83 | 2.07 |
| Hydrochar | 220 | 2 | 20 | 57.4 | 5.4 | 0.4 | 0.1 | 34.5 | 2.2 |
| Hydrochar | 220 | 4 | 20 | 60.2 | 5 | 0.3 | 0.1 | 32.3 | 2.1 |
| Hydrochar | 250 | 2 | 10 | 63.4 | 5.2 | 0.3 | 0.1 | 27.99 | 3.01 |
| Hydrochar | 250 | 4 | 10 | 67.2 | 5.3 | 0.3 | 0.1 | 23.69 | 3.41 |
| Hydrochar | 220 | 2 | 10 | 54.5 | 6 | 0.2 | 0.1 | 37.99 | 1.21 |
| Hydrochar | 220 | 4 | 10 | 57.8 | 5.7 | 0.2 | 0.1 | 34.35 | 1.85 |
| Hydrochar | 220 | 17 | 10 | 66.5 | 5.5 | 0.3 | 0.1 | 24.98 | 2.62 |
| Straw | |||||||||
| Biomass | 43.3 | 6.4 | 0.4 | 0.2 | 44.65 | 5.05 | |||
| Hydrochar | 220 | 2 | 10 | 52.5 | 5.9 | 0.4 | 0.4 | 36.41 | 4.39 |
| Hydrochar | 220 | 4 | 10 | 57 | 5.8 | 0.4 | 0.3 | 32.01 | 4.49 |
| Hydrochar | 220 | 17 | 10 | 65.5 | 5.4 | 0.6 | 0.2 | 22.78 | 5.52 |
| Hydrochar | 250 | 2 | 10 | 63.6 | 5.5 | 0.5 | 0.2 | 24.3 | 5.9 |
| Hydrochar | 250 | 4 | 10 | 65.1 | 5.4 | 0.6 | 0.1 | 23.21 | 5.59 |
| Cauliflower | |||||||||
| Biomass, [8] | 45.3 1 | 5.6 1 | n.t. | n.t. | 46.8 1 | 9.95 | |||
| Hydrochar | 220 | 4 | 10 | 55.5 | 5.6 | 3.5 | 0.9 | n.t. | n.t. |
| Garden Garlic | |||||||||
| Hydrochar | 220 | 4 | 10 | 54.7 | 5.6 | 2.8 | 0.6 | n.t. | n.t. |
| Carrots/Potatoes | |||||||||
| Biomass, [19] | 40.2 1 | 6.3 1 | 0.74 1 | 0.15 1 | 52.7 1 | 5.7 | |||
| Hydrochar | 220 | 4 | 10 | 63.4 | 5.5 | 1.8 | 0.3 | n.t. | n.t. |
| Lignin | |||||||||
| Lignin, [8] | 66 1 | 6 1 | n.t. | n.t. | 26.9 1 | 1.33 | |||
| Hydrochar | 220 | 4 | 10 | 75.1 1 | 6.5 1 | n.t. | n.t. | 18.4 1 | n.t. |
| Cellulose | |||||||||
| Cellulose, [8] | 43.2 | 5.7 | n.t. | n.t. | 51.1 | b.d. | |||
| Hydrochar | 220 | 4 | 10 | 68.7 | 4.3 | n.t. | n.t. | 27 | b.d. |
| Biomass | Cellulose [wt. %] | Hemicellulose [wt. %] | Lignin [wt. %] | Others [wt. %] |
|---|---|---|---|---|
| Wheat Stroh | 38 | 29 | 15 | 18 |
| Beech Wood | 45 | 22 | 22 | 11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruse, A.; Zevaco, T.A. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. https://doi.org/10.3390/en11030674
Kruse A, Zevaco TA. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies. 2018; 11(3):674. https://doi.org/10.3390/en11030674
Chicago/Turabian StyleKruse, Andrea, and Thomas A. Zevaco. 2018. "Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment" Energies 11, no. 3: 674. https://doi.org/10.3390/en11030674