Improving the Energy Efficiency of Direct Formate Fuel Cells with a Pd/C-CeO2 Anode Catalyst and Anion Exchange Ionomer in the Catalyst Layer
Abstract
:1. Introduction
2. Results and Discussion
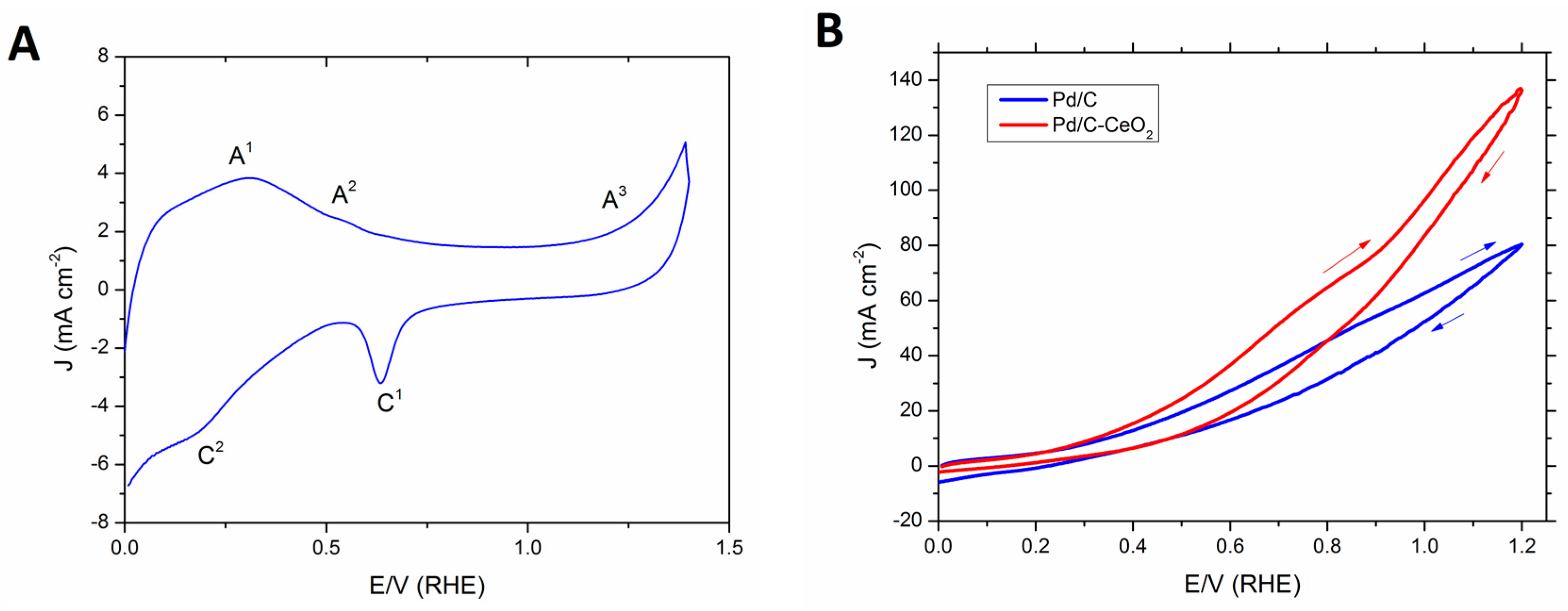
2.1. Pd/C-CeO2 Catalyst FOR Activity
2.2. DFFC Performance
2.3. Ionomer Synthesis and Performance
2.4. Ionomer Performance in DFFCs
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of Pd/C (10 wt %)
4.3. Synthesis of C-CeO2 Support (50:50)
4.4. Synthesis of Pd/C-CeO2 (10 wt % Pd)
4.5. Electrochemical and Physical Characterization
4.6. Polymer Synthesis
4.6.1. Preparation of pVBC
4.6.2. Preparation of pVBC-DABCO
4.7. Fuel Cell MEAs
4.8. Fuel Cell Tests
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- An, L.; Chen, R. Direct formate fuel cells: A review. J. Power Sources 2016, 320, 127–139. [Google Scholar] [CrossRef]
- Vo, T.; Purohit, K.; Nguyen, C.; Biggs, B.; Mayoral, S.; Haan, J.L. Formate: An Energy Storage and Transport Bridge between Carbon Dioxide and a Formate Fuel Cell in a Single Device. ChemSusChem 2015, 8, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.P.; Prakash, G.K.S.; Olah, G.A. Electrochemical CO2 Reduction: Recent Advances and Current Trends. Isr. J. Chem. 2014, 54, 1451–1466. [Google Scholar] [CrossRef]
- Spinner, N.S.; Vega, J.A.; Mustain, W.E. Recent progress in the electrochemical conversion and utilization of CO2. Catal. Sci. Technol. 2012, 2, 19–28. [Google Scholar] [CrossRef]
- Qiao, J.L.; Liu, Y.Y.; Hong, F.; Zhang, J.J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Gunasekar, G.H.; Park, K.; Jung, K.D.; Yoon, S. Recent developments in the catalytic hydrogenation of CO2 to formic acid/formate using heterogeneous catalysts. Inorg. Chem. Front. 2016, 3, 882–895. [Google Scholar] [CrossRef]
- Xu, Z.; McNamara, N.D.; Neumann, G.T.; Schneider, W.F.; Hicks, J.C. Catalytic Hydrogenation of CO2 to Formic Acid with Silica-Tethered Iridium Catalysts. ChemCatChem 2013, 5, 1769–1771. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, Y.E.; Dong, T.; Yuan, X.F.; Lv, L.L.; Wei, X.B.; Wang, J. Formate: A Possible Replacement for Formic Acid in Fuel Cells. Aust. J. Chem. 2017, 70, 757–763. [Google Scholar] [CrossRef]
- Li, Y.S.; Sun, X.D.; Feng, Y. Hydroxide Self-Feeding High-Temperature Alkaline Direct Formate Fuel Cells. ChemSusChem 2017, 10, 2135–2139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Feng, Y.; Sun, X.D.; He, Y.L. A Sodium-Ion-Conducting Direct Formate Fuel Cell: Generating Electricity and Producing Base. Angew. Chem. Int. Ed. 2017, 56, 5734–5737. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.G.; Silva, J.C.M.; Buzzo, G.S.; Neto, A.O.; Assumpcao, M.H.M.T. Use of PtAu/C electrocatalysts toward formate oxidation: Electrochemical and fuel cell considerations. Mater. Renew. Sustain. 2016, 5, 15. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Minami, D.; Hua, C.; Miller, A.; Tran, K.; Haan, J.L. Ambient Temperature Operation of a Platinum-Free Direct Formate Fuel Cell. J. Fuel Cell Sci. Technol. 2015, 12, 014501. [Google Scholar] [CrossRef]
- Da Silva, S.G.; Silva, J.C.M.; Buzzo, G.S.; Spinace, E.V.; Neto, A.O.; Assumpcao, M.H.M.T. PdAu/C Electrocatalysts as Anodes for Direct Formate Fuel Cell. Electrocatalysis 2015, 6, 442–446. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, Z.K.; Zhao, T.S. A high-performance alkaline exchange membrane direct formate fuel cell. Appl. Energy 2014, 115, 405–410. [Google Scholar] [CrossRef]
- Wang, L.Q.; Bellini, M.; Filippi, J.; Folliero, M.; Lavacchi, A.; Innocenti, M.; Marchionni, A.; Miller, H.A.; Vizza, F. Energy efficiency of platinum-free alkaline direct formate fuel cells. Appl. Energy 2016, 175, 479–487. [Google Scholar] [CrossRef]
- Yan, B.; Concannon, N.M.; Milshtein, J.D.; Brushett, F.R.; Surendranath, Y. A Membrane-Free Neutral pH Formate Fuel Cell Enabled by a Selective Nickel Sulfide Oxygen Reduction Catalyst. Angew. Chem. Int. Ed. 2017, 56, 7496–7499. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S. A liquid-electrolyte-free anion-exchange membrane direct formate-peroxide fuel cell. Int. J. Hydrog. Energy 2016, 41, 3600–3604. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Bartrom, A.M.; Tran, K.; Haan, J.L. Operation of the Alkaline Direct Formate Fuel Cell in the Absence of Added Hydroxide. Fuel Cells 2013, 13, 922–926. [Google Scholar] [CrossRef]
- Wang, L.Q.; Lavacchi, A.; Bevilacqua, M.; Bellini, M.; Fornasiero, P.; Filippi, J.; Innocenti, M.; Marchionni, A.; Miller, H.A.; Vizza, F. Energy Efficiency of Alkaline Direct Ethanol Fuel Cells Employing Nanostructured Palladium Electrocatalysts. ChemCatChem 2015, 7, 2214–2221. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Chen, Y.X.; Filippi, J.; Fornasiero, P.; Innocenti, M.; Lavacchi, A.; Marchionni, A.; Oberhauser, W.; Vizza, F. Energy Efficiency Enhancement of Ethanol Electrooxidation on Pd-CeO2/C in Passive and Active Polymer Electrolyte-Membrane Fuel Cells. ChemSusChem 2012, 5, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Lavacchi, A.; Miller, H.A.; Bevilacqua, M.; Filippi, J.; Innocenti, M.; Marchionni, A.; Oberhauser, W.; Wang, L.; Vizza, F. Nanotechnology makes biomass electrolysis more energy efficient than water electrolysis. Nat. Commun. 2014, 5, 4036. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Piana, M.; Boccia, M.; Filpi, A.; Flammia, E.; Miller, H.A.; Orsini, M.; Salusti, F.; Santiccioli, S.; Ciardelli, F.; Pucci, A. H-2/air alkaline membrane fuel cell performance and durability, using novel ionomer and non-platinum group metal cathode catalyst. J. Power Sources 2010, 195, 5875–5881. [Google Scholar] [CrossRef]
- MIller, H.A.; Vizza, F.; Lavacchi, A. Direct Alcohol Fuel Cells: Nanostructured Materials for the Electrooxidation of Alcohols in Alkaline Media. In Nanomaterials for Fuel Cell Catalysts; Ozoemena, K.I., Chen, S., Eds.; Springer: Basel, Switzerland, 2016; pp. 447–516. [Google Scholar]
- Miller, H.A.; Vizza, F.; Marelli, M.; Zadick, A.; Dubau, L.; Chatenet, M.; Geiger, S.; Cherevko, S.; Doan, H.; Pavlicek, R.K.; et al. Highly active nanostructured palladium-ceria electrocatalysts for the hydrogen oxidation reaction in alkaline medium. Nano Energy 2017, 33, 293–305. [Google Scholar] [CrossRef]
- Miller, H.A.; Lavacchi, A.; Vizza, F.; Marelli, M.; Di Benedetto, F.; Acapito, F.D.I.; Paska, Y.; Page, M.; Dekel, D.R. A Pd/C-CeO2 Anode Catalyst for High-Performance Platinum-Free Anion Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 6004–6007. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, N.; Chudacoff, M.; Dauriat, A.; Dinkell, F.; Doka, G.; Emmenegger, M.C.F.; Gnansounou, E.; Kljun, N.; Schleiss, K.; Spielmann, M.; et al. Life Cycle Inventories of Bioenergy. Ecoinvent Report No. 17; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- Jiang, J.H.; Wieckowski, A. Prospective direct formate fuel cell. Electrochem. Commun. 2012, 18, 41–43. [Google Scholar] [CrossRef]
- Faraj, M.; Boccia, M.; Miller, H.; Martini, F.; Borsacchi, S.; Geppi, M.; Pucci, A. New LDPE based anion-exchange membranes for alkaline solid polymeric electrolyte water electrolysis. Int. J. Hydrogen Energy 2012, 37, 14992–15002. [Google Scholar] [CrossRef]
- Faraj, M.; Elia, E.; Boccia, M.; Filpi, A.; Pucci, A.; Ciardelli, F. New Anion Conducting Membranes Based on Functionalized Styrene-Butadiene-Styrene Triblock Copolymer for Fuel Cells Applications. J. Polym. Sci. Polym. Chem. 2011, 49, 3437–3447. [Google Scholar] [CrossRef]
- Vengatesan, S.; Santhi, S.; Sozhan, G.; Ravichandran, S.; Davidson, D.J.; Vasudevan, S. Novel cross-linked anion exchange membrane based on hexaminium functionalized poly(vinylbenzyl chloride). RSC Adv. 2015, 5, 27365–27371. [Google Scholar] [CrossRef]
- Filpi, A.; Boccia, M.; Pucci, A.; Ciardelli, F. Modulation of the electrochemical properties of SBS-based anionic membranes by the amine molecular structure. E-Polymers 2013, 13. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Filippi, J.; Lavacchi, A.; Oberhauser, W.; Marchionni, A.; Moneti, S.; Vizza, F.; Psaro, R.; Dal Santo, V.; et al. Single-site and nanosized Fe-Co electrocatalysts for oxygen reduction: Synthesis, characterization and catalytic performance. J. Power Sources 2011, 196, 2519–2529. [Google Scholar] [CrossRef]
- Miller, H.A.; Bevilacqua, M.; Filippi, J.; Lavacchi, A.; Marchionni, A.; Marelli, M.; Moneti, S.; Oberhauser, W.; Vesselli, E.; Innocenti, M.; et al. Nanostructured Fe-Ag electrocatalysts for the oxygen reduction reaction in alkaline media. J. Mater. Chem. A 2013, 1, 13337–13347. [Google Scholar] [CrossRef]

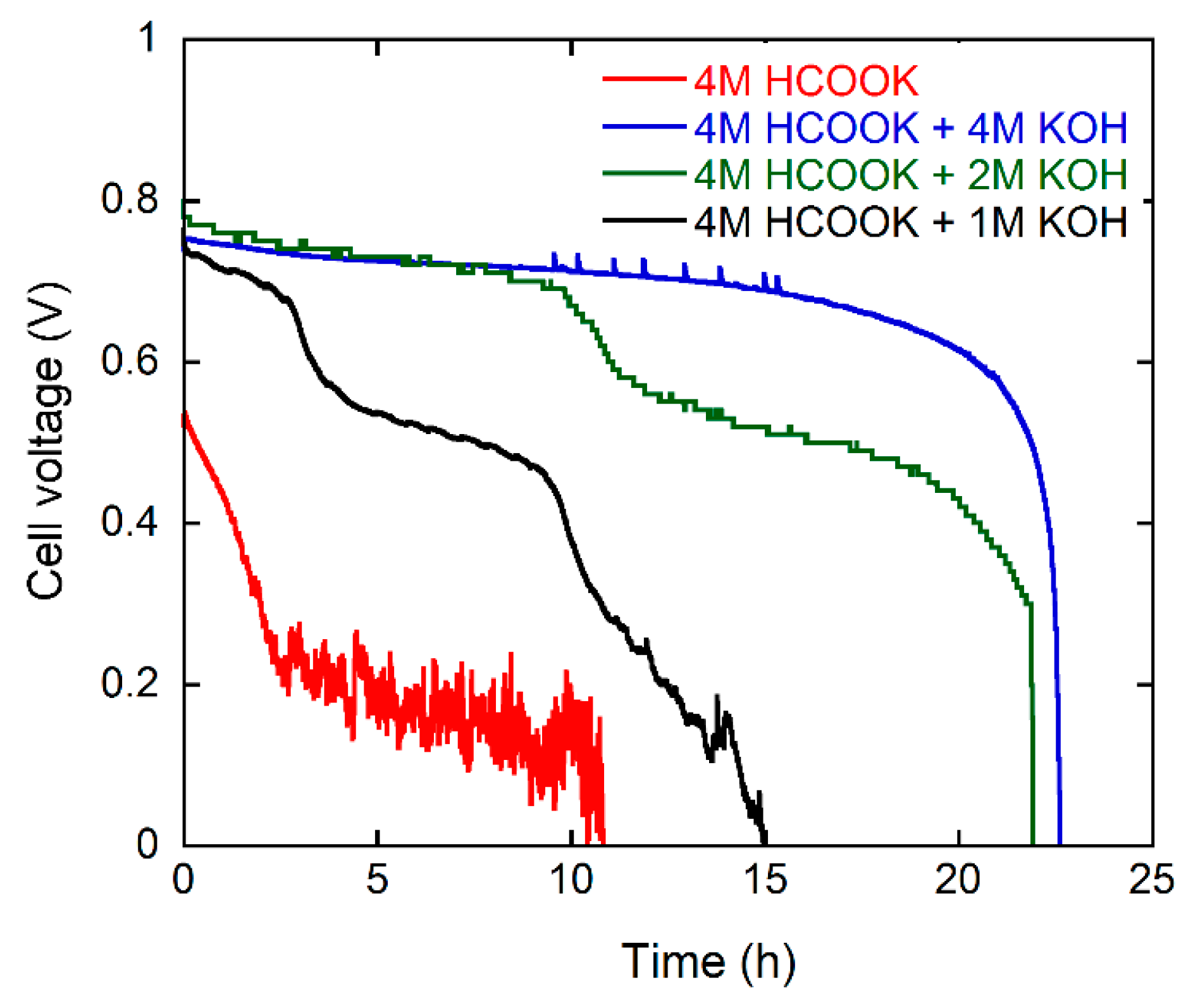


| Fuel Cell Data | Fuel Solution (30 mL) | ||||
|---|---|---|---|---|---|
| 4 M Formate 4 M KOH | 4 M Formate 2 M KOH | 4 M Formate 1 M KOH | 4 M Formate | 4 M Formate (with Ionomer) | |
| Open Circuit Voltage (V) | 0.99 | 0.92 | 1.01 | 0.84 | 0.89 |
| Maximum Power Density (mW·cm−2) | 243 | 193 | 160 | 89 | 110 |
| Discharge Energy (kJ) | 14 | 11 | 6 | 2 | 4 |
| Faradic Efficiency (%) | 89 | 85 | 58 | 51 | 87 |
| Energy Efficiency (%) | 46 | 36 | 20 | 7 | 12 |
| Fuel Composition | 4/4 | 4/2 | 4/1 | 4/0 | 4/0 * |
| Discharge Energy (kJ) | 14 | 11 | 6 | 2 | 4 |
| Cost of KOH consumed (kJ) | 160 | 80 | 40 | 0 | 0 |
| Net energy (kJ) | −146 | −69 | −34 | +2 | +4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, H.A.; Ruggeri, J.; Marchionni, A.; Bellini, M.; Pagliaro, M.V.; Bartoli, C.; Pucci, A.; Passaglia, E.; Vizza, F. Improving the Energy Efficiency of Direct Formate Fuel Cells with a Pd/C-CeO2 Anode Catalyst and Anion Exchange Ionomer in the Catalyst Layer. Energies 2018, 11, 369. https://doi.org/10.3390/en11020369
Miller HA, Ruggeri J, Marchionni A, Bellini M, Pagliaro MV, Bartoli C, Pucci A, Passaglia E, Vizza F. Improving the Energy Efficiency of Direct Formate Fuel Cells with a Pd/C-CeO2 Anode Catalyst and Anion Exchange Ionomer in the Catalyst Layer. Energies. 2018; 11(2):369. https://doi.org/10.3390/en11020369
Chicago/Turabian StyleMiller, Hamish Andrew, Jacopo Ruggeri, Andrea Marchionni, Marco Bellini, Maria Vincenza Pagliaro, Carlo Bartoli, Andrea Pucci, Elisa Passaglia, and Francesco Vizza. 2018. "Improving the Energy Efficiency of Direct Formate Fuel Cells with a Pd/C-CeO2 Anode Catalyst and Anion Exchange Ionomer in the Catalyst Layer" Energies 11, no. 2: 369. https://doi.org/10.3390/en11020369
APA StyleMiller, H. A., Ruggeri, J., Marchionni, A., Bellini, M., Pagliaro, M. V., Bartoli, C., Pucci, A., Passaglia, E., & Vizza, F. (2018). Improving the Energy Efficiency of Direct Formate Fuel Cells with a Pd/C-CeO2 Anode Catalyst and Anion Exchange Ionomer in the Catalyst Layer. Energies, 11(2), 369. https://doi.org/10.3390/en11020369










