Biochemical Conversion and Microbial Community in Response to Ternary pH Buffer System during Anaerobic Digestion of Swine Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inocula
2.2. Experimental Setup
2.3. DNA Extraction
2.4. Analytical Method
2.4.1. Physicochemical Analyses
2.4.2. Microbial Community Analyses
2.5. Calculations and Statistical Analyses
2.5.1. Extent of Solubilization and Acidification
2.5.2. Efficiency of Biochemical Conversion Data Analysis
3. Results and Discussion
3.1. Overall Performance of AD Reactors
3.1.1. Efficiency of Biochemical Conversion
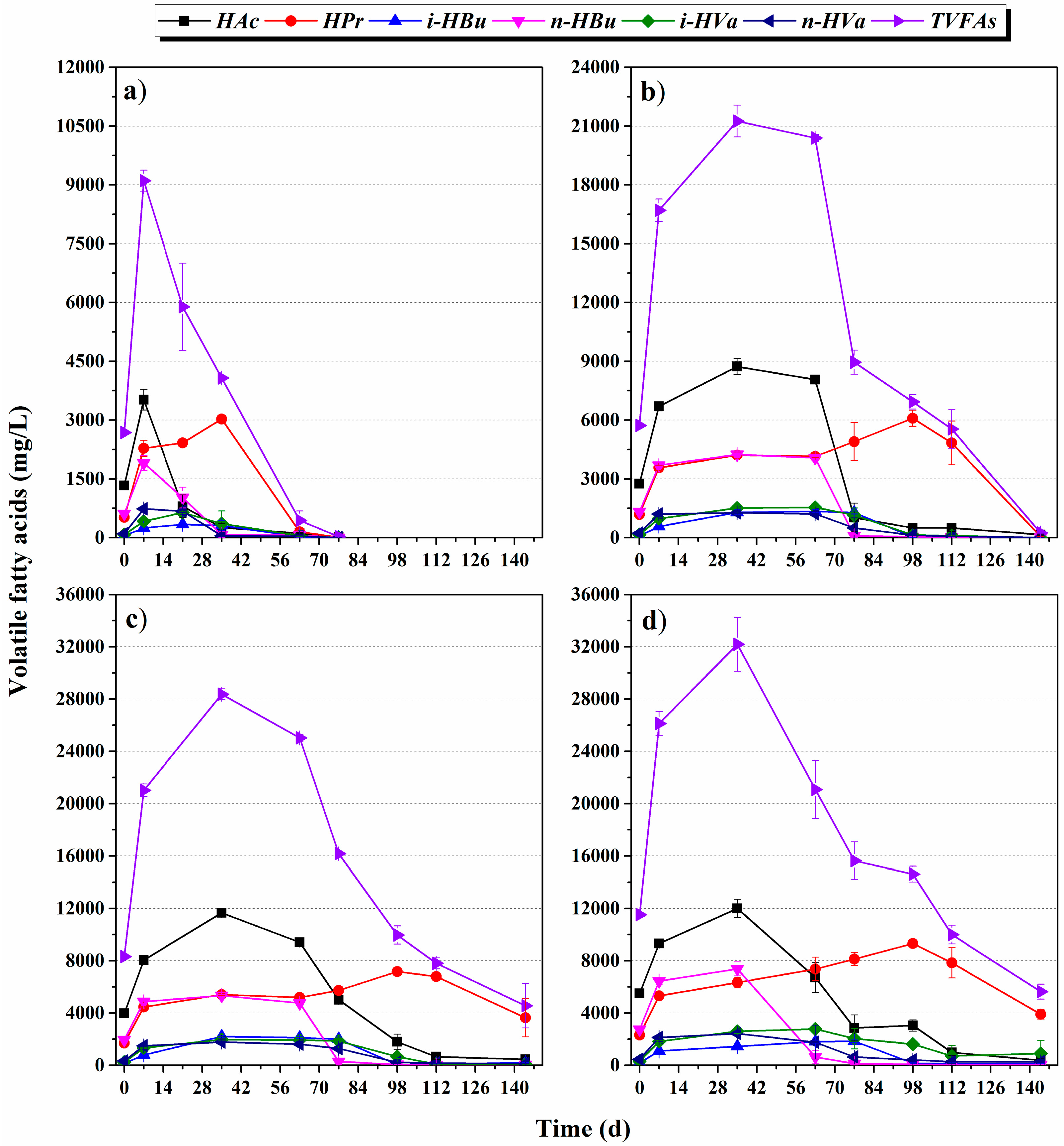
3.1.2. Evolution of VFA Composition
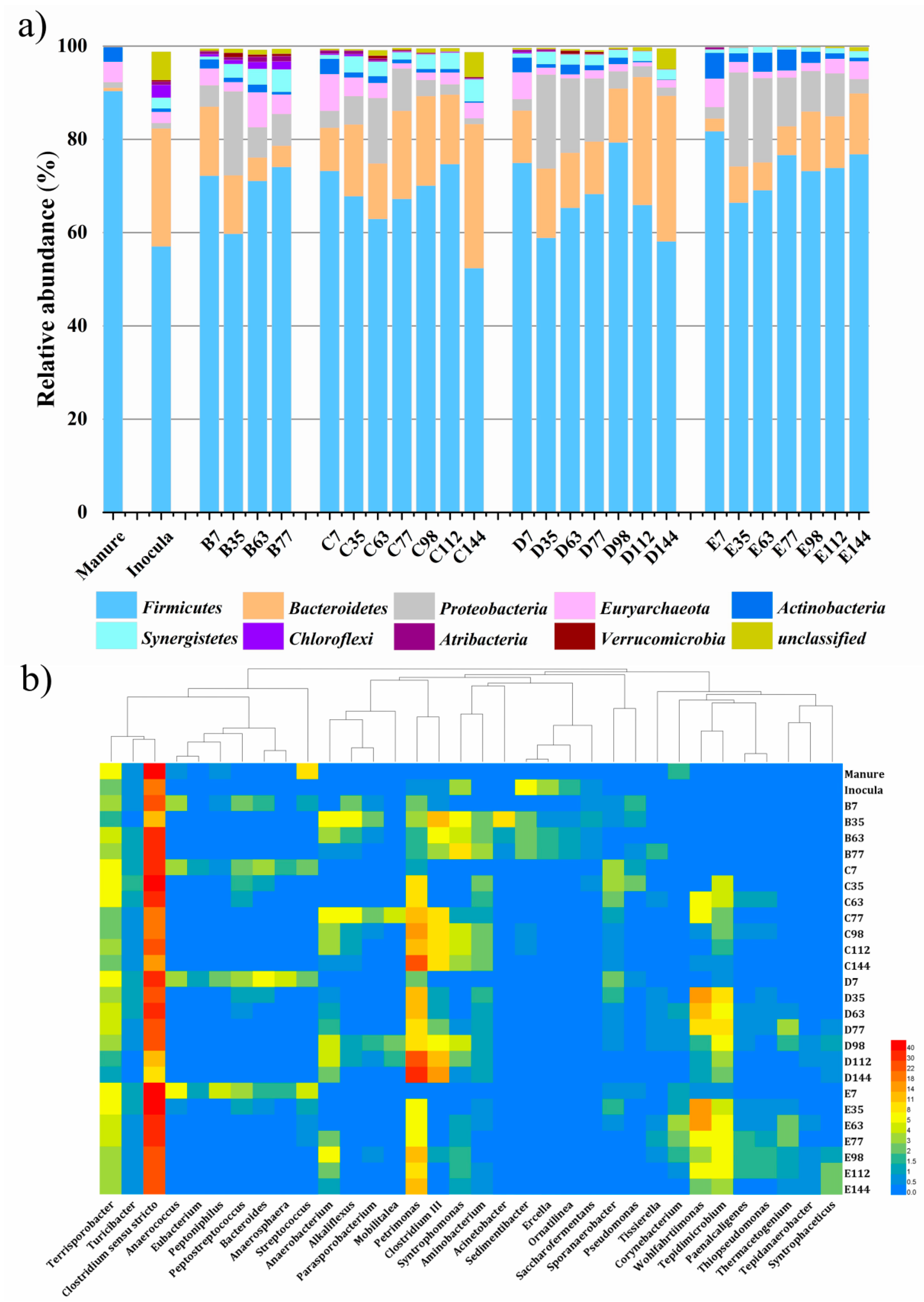
3.2. Microbial Community Dynamics
3.2.1. Bacterial Community
3.2.2. Archaeal Community
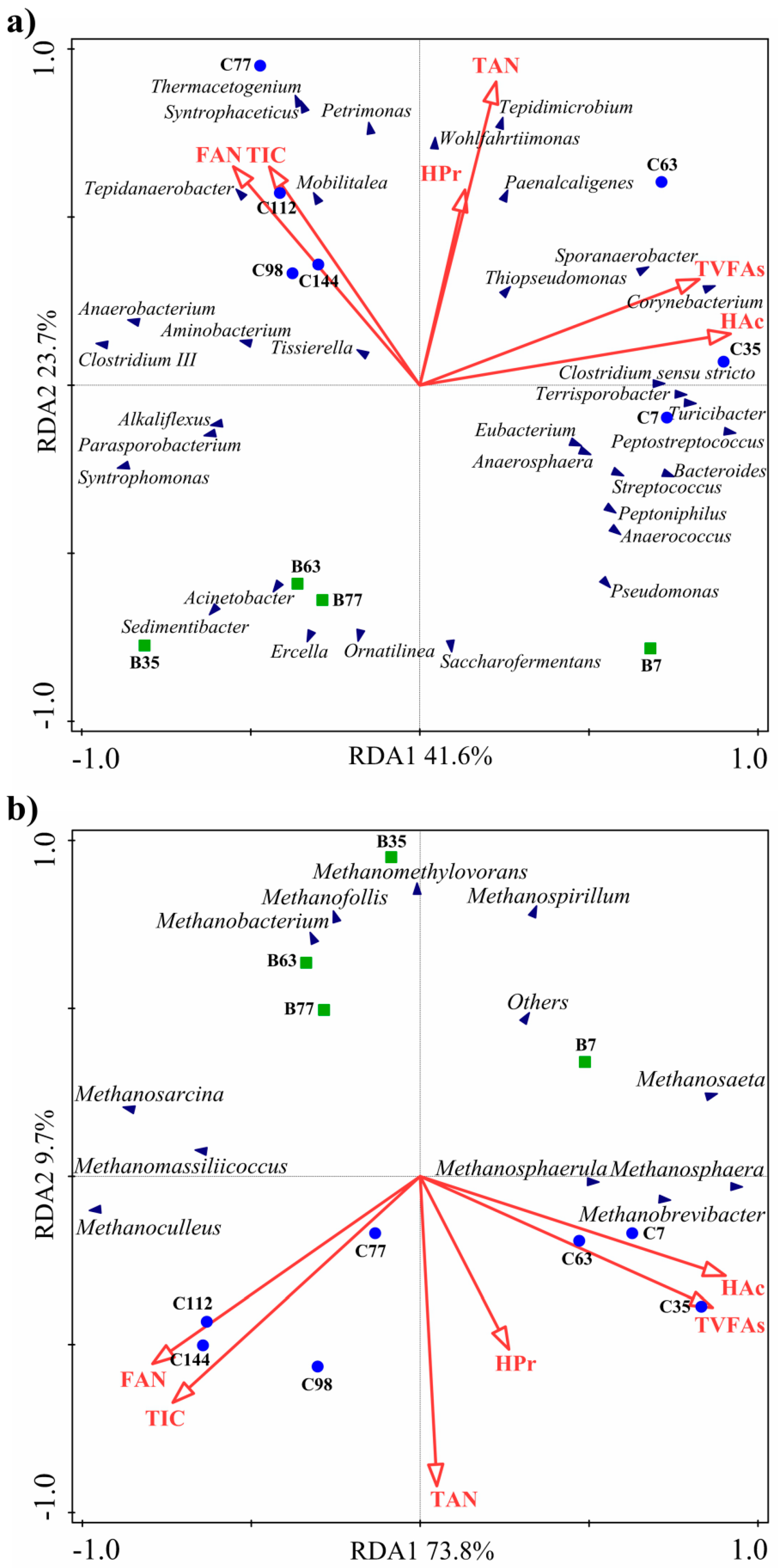
3.3. Relationship with Ternary pH Buffer System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaneeckhaute, C.; Styles, D.; Prade, T.; Adams, P.; Thelin, G.; Rodhe, L.; Gunnarsson, I.; D’Hertefeldt, T. Closing nutrient loops through decentralized anaerobic digestion of organic residues in agricultural regions: A multi-dimensional sustainability assessment. Resour. Conserv. Recycl. 2018, 136, 110–117. [Google Scholar] [CrossRef]
- Wu, J.; Hu, Y.Y.; Wang, S.F.; Cao, Z.P.; Li, H.Z.; Fu, X.M.; Wang, K.J.; Zuo, J.E. Effects of thermal treatment on high solid anaerobic digestion of swine manure: Enhancement assessment and kinetic analysis. Waste Manag. 2017, 62, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, J.; Sui, Q.; Wei, Y. Biogas-pH automation control strategy for optimizing organic loading rate of anaerobic membrane bioreactor treating high COD wastewater. Bioresour. Technol. 2016, 203, 62–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Meng, X.; Liu, J.; Dian, L.; Sui, Q.; Zhang, J.; Zhong, H.; Wei, Y. Formation and characteristics of a ternary pH buffer system for in-situ biogas upgrading in two-phase anaerobic membrane bioreactor treating starch wastewater. Bioresour. Technol. 2018, 269, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yu, D.; Wei, Y.; Zhang, Y.; Zhang, Q.; Wang, Z.; Liu, J.; Wang, Y. Endogenous ternary pH buffer system with ammonia-carbonates-VFAs in high solid anaerobic digestion of swine manure: An alternative for alleviating ammonia inhibition? Process Biochem. 2018, 69, 144–152. [Google Scholar] [CrossRef]
- Duan, N.; Ran, X.; Li, R.; Kougias, P.; Zhang, Y.; Lin, C.; Liu, H. Performance evaluation of mesophilic anaerobic digestion of chicken manure with algal digestate. Energies 2018, 11, 1829. [Google Scholar] [CrossRef]
- Keucken, A.; Habagil, M.; Batstone, D.; Jeppsson, U.; Arnell, M. Anaerobic co-digestion of sludge and organic food waste—Performance, inhibition, and impact on the microbial community. Energies 2018, 11, 2325. [Google Scholar] [CrossRef]
- Shi, X.; Lin, J.; Zuo, J.; Li, P.; Li, X.; Guo, X. Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J. Environ. Sci. China 2017, 55, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, W.; Li, R. Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Bioresour. Technol. 2018, 265, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Qiao, W.; Qiang, H.; Li, Y.Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Fotidis, I.A.; Wang, H.; Fiedel, N.R.; Luo, G.; Karakashev, D.B.; Angelidaki, I. Bioaugmentation as a solution to increase methane production from an ammonia-rich substrate. Environ. Sci. Technol. 2014, 48, 7669–7676. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling ammonia inhibition for efficient biogas production from chicken manure: Status and technical trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; Gonzalez-Fernandez, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sanchez, J.; Campanaro, S.; Guivernau, M.; Fernandez, B.; Prenafeta-Boldu, F.X. Effect of ammonia on the active microbiome and metagenome from stable full-scale digesters. Bioresour. Technol. 2017, 250, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Sui, Q.; Meng, X.; Wang, R.; Zhang, J.; Yu, D.; Chen, M.; Wang, Y.; Wei, Y. Effects of endogenous inhibitors on the evolution of antibiotic resistance genes during high solid anaerobic digestion of swine manure. Bioresour. Technol. 2018, 270, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Maspolim, Y.; Guo, C.; Xiao, K.; Zhou, Y.; Ng, W.J. Performance and microbial community analysis in alkaline two-stage enhanced anaerobic sludge digestion system. Biochem. Eng. J. 2016, 105, 296–305. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Wu, J.; Chen, H.; Yu, P.; Liu, J.; Li, Y.-Y. Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: Performance, energy balance and reaction process. Energy Convers. Manag. 2018, 156, 215–223. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, Q.; Pan, Z.; Jiang, Y.; Liebetrau, J.; Nelles, M.; Dong, H.; Dong, R. Performance evaluation of a novel anaerobic digestion operation process for treating high-solids content chicken manure: Effect of reduction of the hydraulic retention time at a constant organic loading rate. Waste Manag. 2017, 64, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A comprehensive model of anaerobic bioconversion of complex substrates to biogas. Biotechnol. Bioeng. 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Ciccoli, R.; Sperandei, M.; Petrazzuolo, F.; Broglia, M.; Chiarini, L.; Correnti, A.; Farneti, A.; Pignatelli, V.; Tabacchioni, S. Anaerobic digestion of the above ground biomass of Jerusalem Artichoke in a pilot plant: Impact of the preservation method on the biogas yield and microbial community. Biomass Bioenergy 2018, 108, 190–197. [Google Scholar] [CrossRef]
- Dohrmann, A.B.; Walz, M.; Lowen, A.; Tebbe, C.C. Clostridium cluster I and their pathogenic members in a full-scale operating biogas plant. Appl. Microbiol. Biotechnol. 2015, 99, 3585–3598. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wan, C.; Liu, X.; Lee, D.-J.; Lei, Z.; Zhang, Y.; Tay, J.H. Effect of initial pH on mesophilic hydrolysis and acidification of swine manure. Bioresour. Technol. 2013, 136, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, M.; de Souza Oliveira Filho, J.; Perez Murcia, M.D.; Bustamante, M.A.; Moral, R.; Coll, M.D.; Lopez Santisima-Trinidad, A.B.; Pascual, J.A. Mesophilic anaerobic digestion of pig slurry and fruit and vegetable waste: Dissection of the microbial community structure. J. Clean. Prod. 2017, 156, 757–765. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Kong, X.; Li, L.; Yuan, Z.; Dong, R.; Sun, Y. Effects of ammonia on propionate degradation and microbial community in digesters using propionate as a sole carbon source. J. Chem. Technol. Biotechnol. 2017, 92, 2538–2545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.; Wang, Y.; Zhong, H.; Sui, Q.; Zhang, C.; Wei, Y. Effects of graphene oxide on the performance, microbial community dynamics and antibiotic resistance genes reduction during anaerobic digestion of swine manure. Bioresour. Technol. 2017, 245, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Zhou, J.; Yuan, Y.; Dai, Y.; Li, D.; Li, Z.; Liu, X.; Yan, Z. The dynamic changes and interactional networks of prokaryotic community between co-digestion and mono-digestions of corn stalk and pig manure. Bioresour. Technol. 2017, 225, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, O.; Ben Hania, W.; Postec, A.; Bouallagui, H.; Hamdi, M.; Bonin, P.; Ollivier, B.; Fardeau, M.-L. Aminobacterium thunnarium sp. nov. a mesophilic, amino acid-degrading bacterium isolated from an anaerobic sludge digester, pertaining to the phylum Synergistetes. Int. J. Syst. Evol. Microbiol. 2015, 65, 609–614. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Units | TS-4 | TS-8 | TS-11 | TS-14 |
|---|---|---|---|---|---|
| VSin | % | 3.00 ± 0.08 | 6.11 ± 0.03 | 8.32 ± 0.06 | 10.85 ± 0.10 |
| VSout | % | 1.08 ± 0.01 | 2.17 ± 0.07 | 3.41 ± 0.11 | 5.70 ± 0.16 |
| VS removal | % | 75.6 ± 1.2 | 76.0 ± 1.4 | 69.7 ± 1.9 | 55.8 ± 1.3 |
| TS removal | % | 63.0 ± 0.2 | 62.9 ± 0.1 | 56.7 ± 1.2 | 43.9 ± 1.3 |
| Cumulative Methane | NmL | 2480 ± 9 | 5741 ± 339 | 6955 ± 593 | 7287 ± 376 |
| Methane Potential | NmL/g-VS | 271 ± 1 | 314 ± 19 | 277 ± 24 | 214 ± 11 |
| TCOD reduction | % | 74.2 ± 2.9 | 76.7 ± 1.5 | 68.5 ± 3.2 | 65.4 ± 2.8 |
| T80a | days | 59 | 104 | 118 | NQ b |
| T80*c | days | 56 | 59 | 56 | - |
| Samples | pH Value | TAN (mg·L−1) | TAN Increment b | FAN (mg·L−1) | FAN Increment c | Extent of Acidification (%) | Extent of Solubilization (%) | |
|---|---|---|---|---|---|---|---|---|
| Manure | 7.08 ± 0.08 a | 3974 ± 87 | - | - | - | - | - | |
| Inoculum | 8.02 ± 0.04 | 2281 ± 36 | - | 280 ± 4 | - | - | - | |
| TS-4 | B0 | 7.21 ± 0.01 | 928 ± 22 | 0 | 20 ± 0 | 0 | 7.35 ± 0.06 | 20.49 ± 0.37 |
| B7 | 7.08 ± 0.09 | 1392 ± 62 | 0.50 | 20 ± 0 | 0.00 | 32.52 ± 0.91 | 38.10 ± 0.20 | |
| B35 | 7.30 ± 0.04 | 1570 ± 20 | 0.69 | 43 ± 5 | 1.15 | 38.39 ± 0.48 | 45.88 ± 0.06 | |
| B63 | 7.79 ± 0.01 | 1608 ± 47 | 0.73 | 125 ± 6 | 5.25 | 38.72 ± 0.61 | 45.92 ± 0.20 | |
| B77 | 7.75 ± 0.04 | 1659 ± 86 | 0.79 | 114 ± 15 | 4.70 | 39.59 ± 0.49 | 46.19 ± 1.17 | |
| TS-8 | C0 | 6.95 ± 0.01 | 1825 ± 44 | 0 | 22 ± 0 | 0 | 7.54 ± 0.05 | 18.71 ± 1.30 |
| C7 | 7.00 ± 0.03 | 3060 ± 158 | 0.68 | 38 ± 0 | 0.73 | 25.45 ± 0.95 | 34.87 ± 0.76 | |
| C35 | 6.42 ± 0.01 | 3569 ± 81 | 0.96 | 13 ± 1 | −0.41 | 31.92 ± 1.25 | 39.01 ± 1.45 | |
| C77 | 7.73 ± 0.02 | 3232 ± 52 | 0.77 | 223 ± 10 | 9.14 | 32.98 ± 3.27 | 40.18 ± 2.83 | |
| C144 | 7.84 ± 0.02 | 3364 ± 47 | 0.84 | 282 ± 4 | 11.82 | 44.10 ± 2.90 | 50.00 ± 1.66 | |
| TS-11 | D0 | 6.86 ± 0.00 | 2814 ± 33 | 0 | 27 ± 0 | 0 | 7.58 ± 0.06 | 20.08 ± 0.21 |
| D7 | 7.21 ± 0.02 | 4241 ± 93 | 0.51 | 88 ± 2 | 2.26 | 21.71 ± 0.70 | 30.98 ± 1.51 | |
| D35 | 6.51 ± 0.06 | 5057 ± 69 | 0.80 | 24 ± 1 | −0.11 | 26.22 ± 1.13 | 34.01 ± 0.77 | |
| D77 | 7.99 ± 0.03 | 4887 ± 123 | 0.74 | 588 ± 12 | 20.78 | 28.78 ± 0.77 | 36.37 ± 1.11 | |
| D144 | 7.91 ± 0.12 | 4451 ± 123 | 0.58 | 466 ± 98 | 16.26 | 41.10 ± 1.73 | 49.20 ± 0.99 | |
| TS-14 | E0 | 6.75 ± 0.02 | 3206 ± 95 | 0 | 24 ± 0 | 0 | 7.83 ± 0.09 | 21.88 ± 0.64 |
| E7 | 7.09 ± 0.06 | 5370 ± 405 | 0.67 | 84 ± 16 | 2.50 | 22.57 ± 0.96 | 36.03 ± 1.99 | |
| E35 | 6.68 ± 0.04 | 6134 ± 399 | 0.91 | 41 ± 1 | 0.71 | 27.63 ± 1.49 | 38.06 ± 1.60 | |
| E77 | 8.09 ± 0.12 | 5918 ± 70 | 0.85 | 734 ± 54 | 29.58 | 31.04 ± 2.43 | 39.51 ± 1.92 | |
| E144 | 7.85 ± 0.05 | 5778 ± 0 | 0.80 | 540 ± 32 | 21.50 | 37.19 ± 2.06 | 46.91 ± 1.88 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Zhang, Y.; Sui, Q.; Zhang, J.; Wang, R.; Yu, D.; Wang, Y.; Wei, Y. Biochemical Conversion and Microbial Community in Response to Ternary pH Buffer System during Anaerobic Digestion of Swine Manure. Energies 2018, 11, 2991. https://doi.org/10.3390/en11112991
Meng X, Zhang Y, Sui Q, Zhang J, Wang R, Yu D, Wang Y, Wei Y. Biochemical Conversion and Microbial Community in Response to Ternary pH Buffer System during Anaerobic Digestion of Swine Manure. Energies. 2018; 11(11):2991. https://doi.org/10.3390/en11112991
Chicago/Turabian StyleMeng, Xiaoshan, Yuxiu Zhang, Qianwen Sui, Junya Zhang, Rui Wang, Dawei Yu, Yawei Wang, and Yuansong Wei. 2018. "Biochemical Conversion and Microbial Community in Response to Ternary pH Buffer System during Anaerobic Digestion of Swine Manure" Energies 11, no. 11: 2991. https://doi.org/10.3390/en11112991






