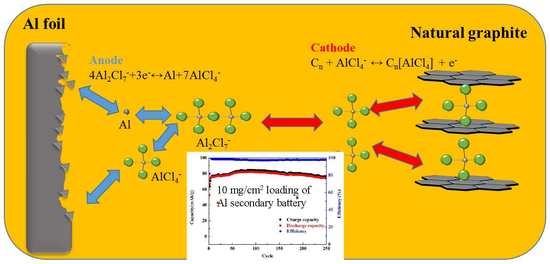
Influence of High Loading on the Performance of Natural Graphite-Based Al Secondary Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Natural Graphite Electrodes
2.2. Preparation of AlCl3/BMIC Electrolytes
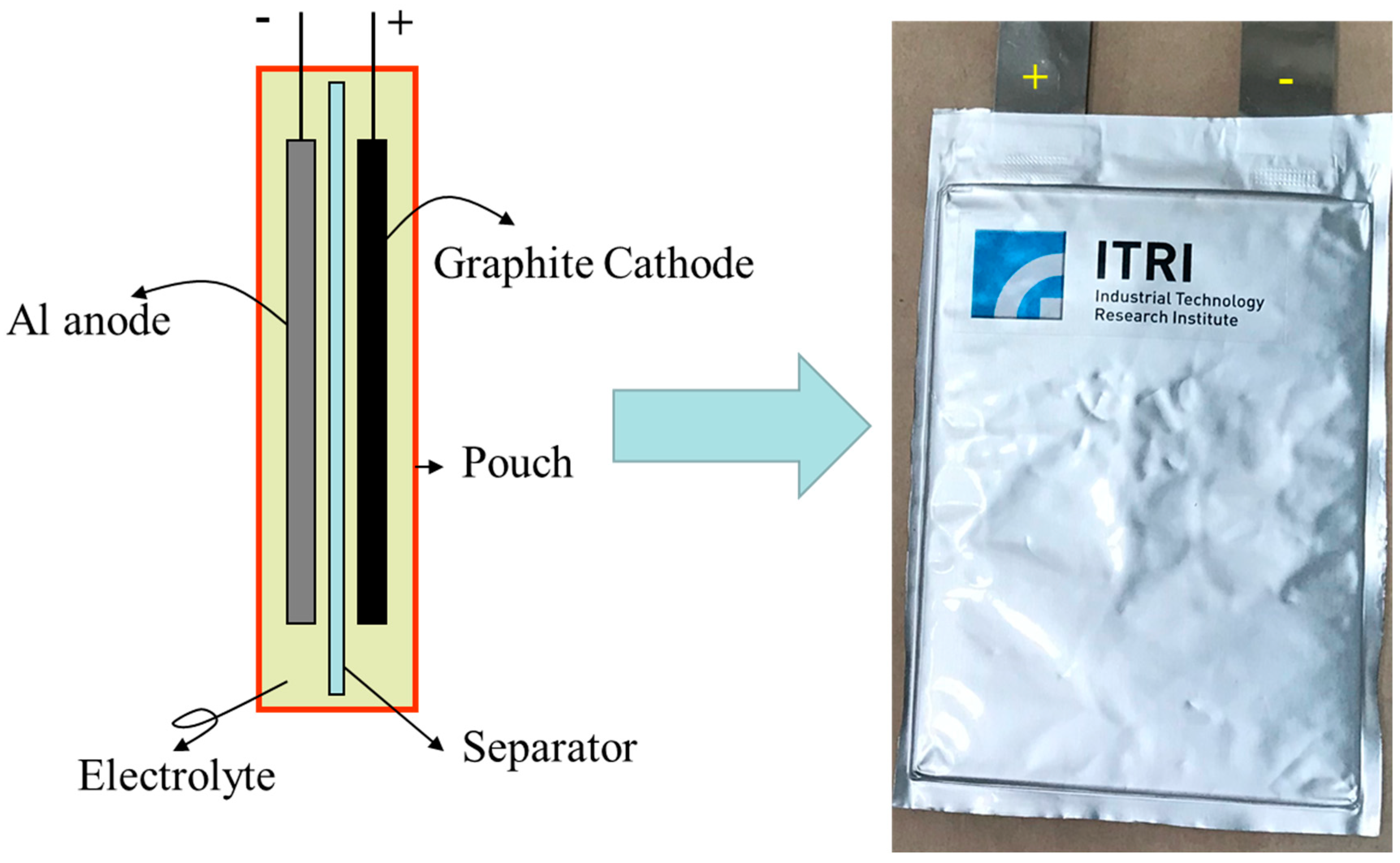
2.3. Fabrication of Pouch Cells
2.4. Characterizations
2.5. Electrochemical Performance Measurements
3. Results and Discussion
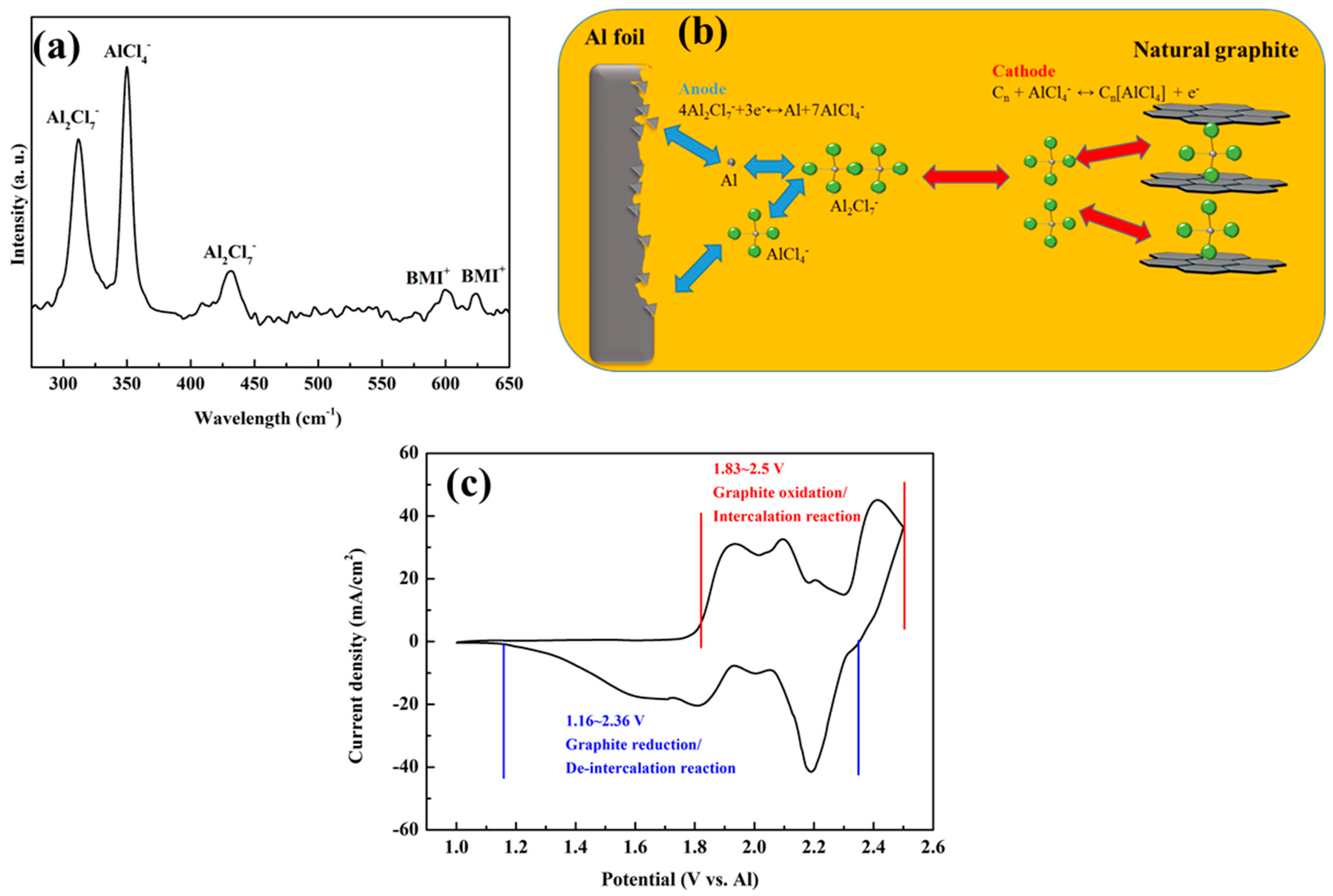
3.1. Properties of Electrolyte and Proposed Charging–Discharging Reactions
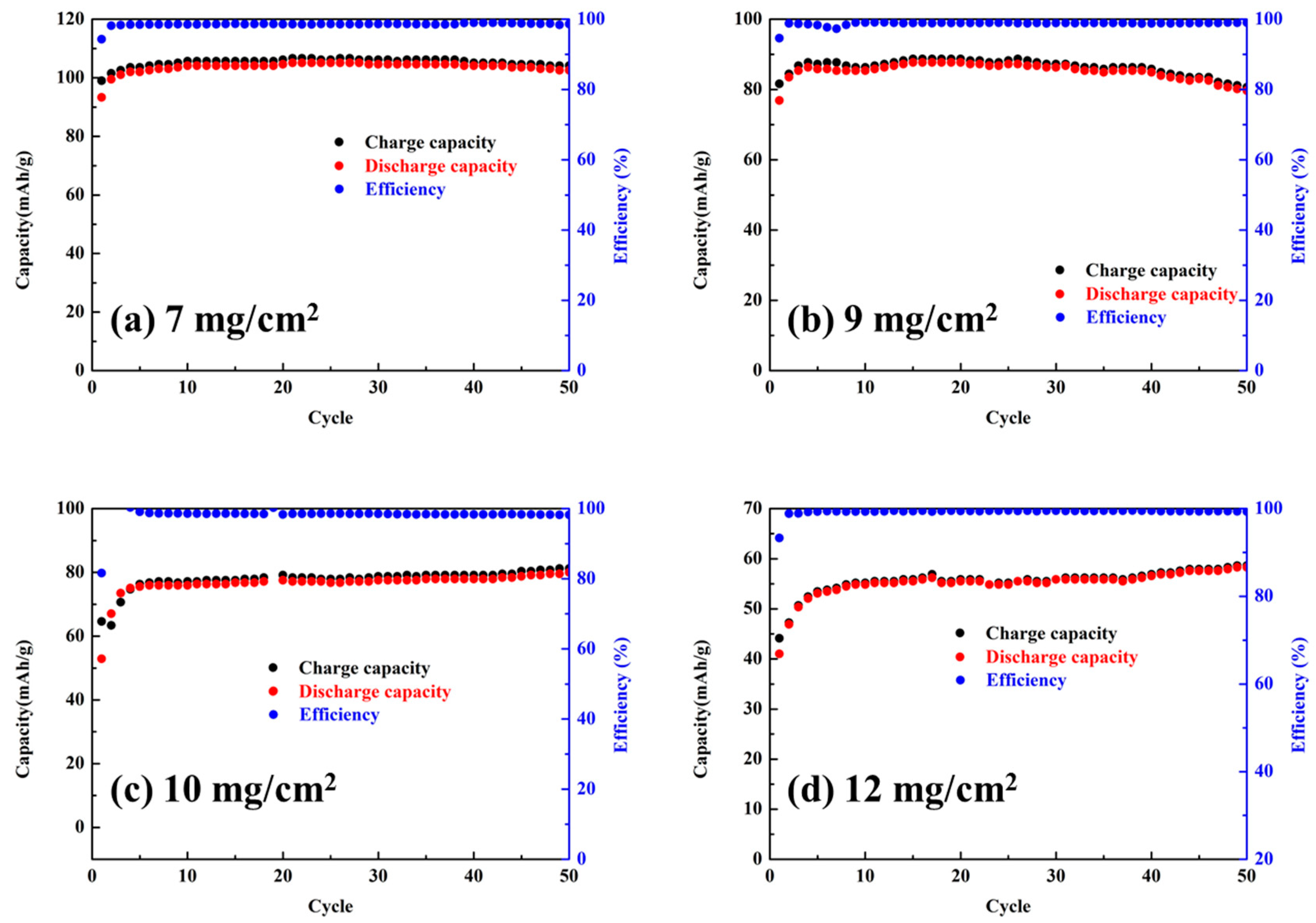
3.2. Effects of Graphite Loading on Electrochemical Performance
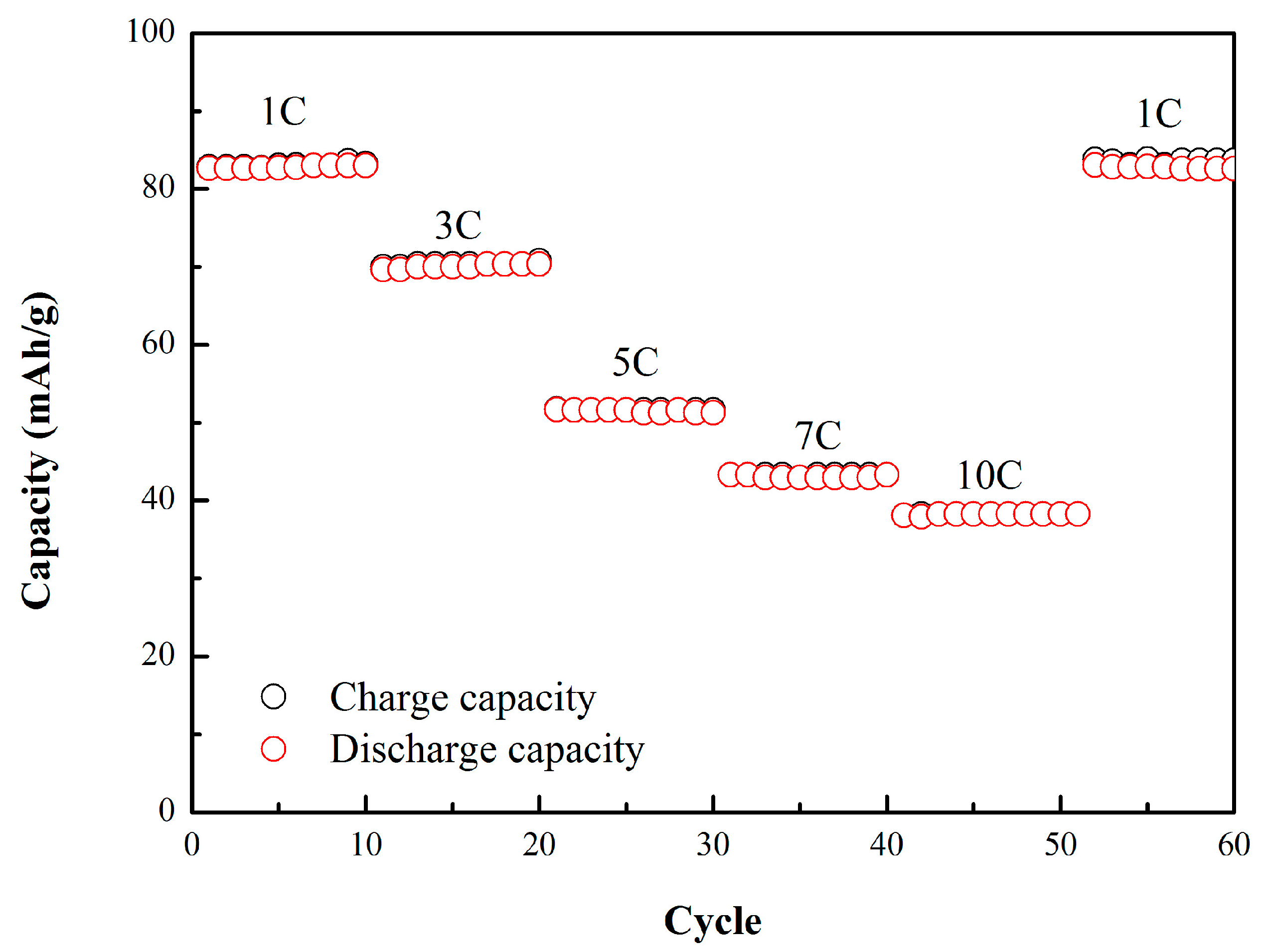
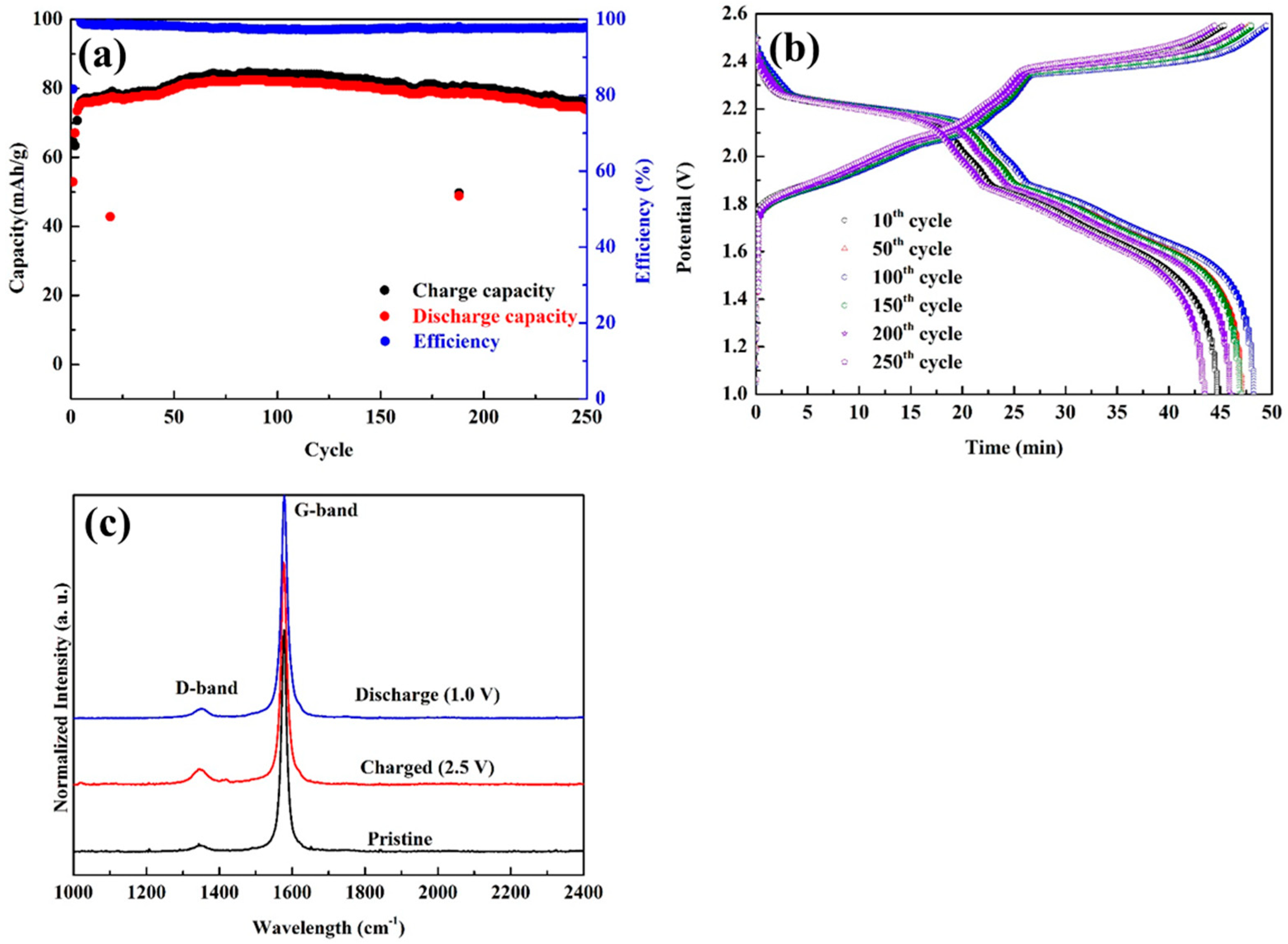
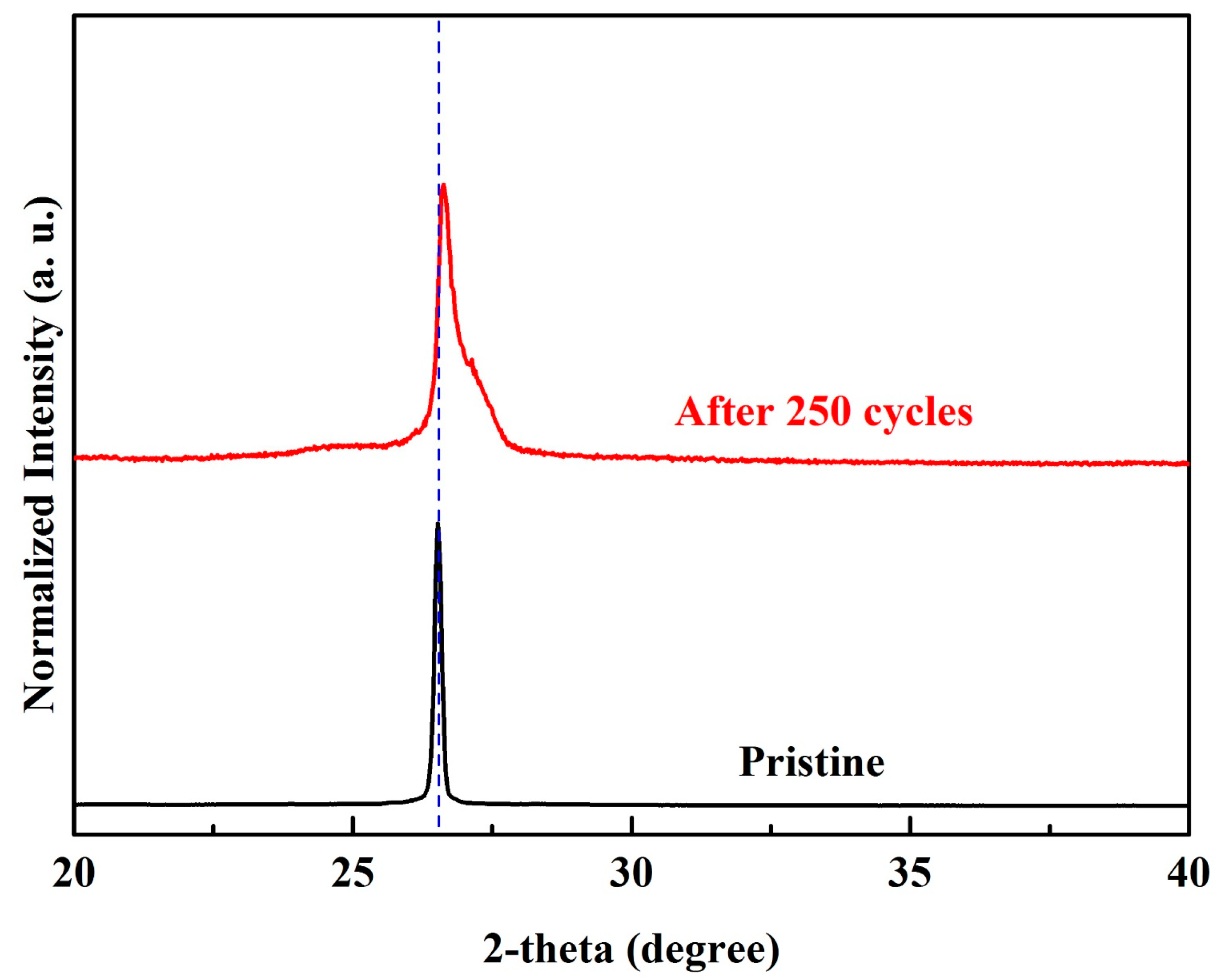
3.3. Characterizations and Electrochemical Performance of GABs with Graphite Loading of 10 mg/cm2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Al | Aluminum |
| AlCl3 | Aluminum chloride |
| AlCl4− | Chloroaluminate |
| Al2Cl7− | Dialuminum heptachloride |
| mAh/g | Capacity |
| mA/g | Charge–discharge rate |
| mg/cm2 | Graphite loading |
| Wh/L | Energy density |
| ω | Frequency |
| σ | Warburg coefficient |
| D | Diffusion coefficient |
| n | Number of electrons involved |
| A | Surface area of the electrode |
| C | Bulk concentration of diffusing species |
| cm−1 | Wavenumber |
| Cn | Molar ratio of carbon atoms |
| V | Potential vs. Al |
| ID | Intensity of D-band |
| IG | Intensity of G-band |
| mS/cm | Ionic conductivity |
| Abbreviations | |
| EMIC | 1-ethyl-3-methylimidazolium chloride |
| BMIC | 1-butyl-3-methylimidazolium chloride |
| GABs | Graphite-based Al secondary batteries |
| PVDF | Polyvinylidene difluoride |
| NMP | Methylpyrrolidone |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| CV | Cyclic voltammetry |
| EIS | Electrochemistry impedance spectra |
| 1C–10C | 100 to 1000 mA/g |
| Superscripts | |
| 2 | Square |
| −1 | To the negative one |
References
- Walter, M.; Kravchyk, K.V.; Böfer, C.; Widmer, R.; Kovalenko, M.V. Polypyrenes as High-Performance Cathode Materials for Aluminum Batteries. Adv. Mater. 2018, 30, 1705644. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wang, C.; Tu, J.; Tian, D.; Jiao, S. A rechargeable Al-ion battery: Al/molten AlCl3–urea/graphite. Chem. Commun. 2017, 53, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Angell, M.; Pan, C.-J.; Rong, Y.; Yuan, C.; Lin, M.-C.; Hwang, B.-J.; Dai, H. High Coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl. Acad. Sci. USA 2017, 114, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, F.; Du, C.; Kang, J.; Turkson, R.F. Evaluating the Degradation Mechanism and State of Health of LiFePO4 Lithium-Ion Batteries in Real-World Plug-in Hybrid Electric Vehicles Application for Different Ageing Paths. Energies 2017, 10, 110. [Google Scholar] [CrossRef]
- Cui, X.; Jing, Z.; Luo, M.; Guo, Y.; Qiao, H. A New Method for State of Charge Estimation of Lithium-Ion Batteries Using Square Root Cubature Kalman Filter. Energies 2018, 11, 209. [Google Scholar] [CrossRef]
- Lu, Y.C.; Dimov, N.; Okada, S.; Bui, T.H. SnSb Alloy Blended with Hard Carbon as Anode for Na-Ion Batteries. Energies 2018, 11, 1614. [Google Scholar] [CrossRef]
- Sun, H.; Wang, W.; Yu, Z.; Yuan, Y.; Wang, S.; Jiao, S. A new aluminium-ion battery with high voltage, high safety and low cost. Chem. Commun. 2015, 51, 11892–11895. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S. Impact of Vehicle Charge and Discharge Cycles on the Thermal Characteristics of Lithium-Ion Batteries. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2014. [Google Scholar]
- Panchal, S.; Rashid, M.; Long, F.; Mathew, M.; Fraser, R.; Fowler, M. Degradation Testing and Modeling of 200 Ah LiFePO4 Battery; SAE Technical Paper: Waterloo, ON, Canada, 3 April 2018. [Google Scholar]
- Beaudin, M.; Zareipour, H.; Schellenberglabe, A.; Rosehart, W. Energy storage for mitigating the variability of renewable electricity sources: An updated review. Energy Sustain. Dev. 2010, 14, 302–304. [Google Scholar] [CrossRef]
- Zafar, Z.A.; Imtiaz, S.; Li, R.; Zhang, J.; Razaq, R.; Xin, Y.; Li, Q.; Zhang, Z.; Huang, Y. A super-long life rechargeable aluminum battery. Solid State Ion. 2018, 320, 70–75. [Google Scholar] [CrossRef]
- Li, Z.; Niu, B.; Liu, J.; Li, J.; Kang, F. Rechargeable Aluminum-Ion Battery Based on MoS2 Microsphere Cathode. ACS Appl. Mater. Interfaces 2018, 10, 9451–9459. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Wei, C.-Y.; Lin, M.-C.; Pan, C.-J.; Chou, H.-L.; Chen, H.-A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced rechargeable aluminium ion battery with a high-quality natural graphite cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kravchyk, K.V.; Wang, S.; Piveteau, L.; Kovalenko, M.V. Efficient Aluminum Chloride−Natural Graphite Battery. Chem. Mater. 2017, 29, 4484–4492. [Google Scholar] [CrossRef]
- Chan, C.C.; Wong, Y.S. Electric vehicles charge forward. IEEE Power Energy Mag. 2004, 2, 24–33. [Google Scholar] [CrossRef]
- Koniak, M.; Czerepicki, A. Selection of the battery pack parameters for an electric vehicle based on performance requirements. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 21–23 April 2017. [Google Scholar]
- Battery Comparison of Energy Density. Available online: https://www.epectec.com/batteries/cell-comparison.html (accessed on 1 October 2018).
- Reed, L.D.; Menke, E. The Roles of V2O5 and Stainless Steel in Rechargeable Al–Ion Batteries. J. Electrochem. Soc. 2013, 160, A915–A917. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, M.; Lin, M.-C.; Yuan, C.; Angell, M.; Huang, L.; Wang, D.-Y.; Zhang, X.; Yang, J.; Hwang, B.-J.; et al. 3D Graphitic Foams Derived from Chloroaluminate Anion Intercalation for Ultrafast Aluminum-Ion Battery. Adv. Mater. 2016, 28, 9218–9222. [Google Scholar] [CrossRef] [PubMed]
- Elia, G.A.; Hasa, I.; Greco, G.; Diemant, T.; Marquardt, K.; Hoeppner, K.; Behm, R.J.; Hoell, A.; Passerini, S.; Hahn, R. Insights into the reversibility of aluminum graphite batteries. J. Mater. Chem. A 2017, 5, 9682–9690. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Kravchyk, V.K.; Krumeich, F.; Kovalenko, V.M. Kish Graphite Flakes as a Cathode Material for an Aluminum Chloride–Graphite Battery. ACS Appl. Mater. Interfaces 2017, 9, 28478–28485. [Google Scholar] [CrossRef] [PubMed]
- Uemura, Y.; Chen, C.-Y.; Hashimoto, Y.; Tsuda, T.; Matsumoto, H.; Kuwabata, S. Graphene Nanoplatelet Composite Cathode for a Chloroaluminate Ionic Liquid-Based Aluminum Secondary Battery. ACS Appl. Energy Mater. 2018, 1, 2269–2274. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Wang, S.; Huang, T.; Xi, J.; Cai, S.; Guo, F.; Xu, Z.; Gao, W.; Gao, C. Ultrafast all-climate aluminum-graphene battery with quarter-million cycle life. Sci. Adv. 2017, 3, eaao7233. [Google Scholar] [CrossRef] [PubMed]
- Vedalakshmi, R.; Saraswathy, V.; Song, H.-W.; Palaniswamy, N. Determination of diffusion coefficient of chloride in concrete using Warburg diffusion coefficient. Corros. Sci. 2009, 51, 1299–1307. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Bae, J.-S. Diffusion impedance study of electrochemical lithium intercalation into porous vanadium oxide electrode. Electrochim. Acta 1996, 41, 919–925. [Google Scholar] [CrossRef]
- Huang, J. Diffusion impedance of electroactive materials, electrolytic solutions and porous electrodes: Warburg impedance and beyond. Electrochim. Acta 2018, 218, 170–188. [Google Scholar] [CrossRef]
- Li, J.; Tu, J.; Jiao, H.; Wang, C.; Jiao, S. Ternary AlCl3-Urea-[EMIm]Cl Ionic Liquid Electrolyte for Rechargeable Aluminum-Ion Batteries. J. Electrochem. Soc. 2017, 164, A3093–A3100. [Google Scholar] [CrossRef]
- Rubim, J.C.; Trindade, F.A.; Gelesky, M.A.; Aroca, R.F.; Dupont, J. Surface-Enhanced Vibrational Spectroscopy of Tetrafluoroborate 1-n-Butyl-3-methylimidazolium (BMIBF4) Ionic Liquid on Silver Surfaces. J. Phys. Chem. C 2008, 112, 19670–19675. [Google Scholar] [CrossRef]
- Xu, H.; Bai, T.; Chen, H.; Guo, F.; Xi, J.; Huang, T.; Cai, S.; Chu, X.; Ling, J.; Gao, W.; et al. Low-Cost AlCl3/Et3NHCl Electrolyte for High-Performance Aluminum-ion Battery. Energy Storage Mater. 2018, in press. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Hansen, T.A.; Ammermann, S.; McBride, J.D.; Beebe, T.P. Nanometer-Size Monolayer and Multilayer Molecule Corrals on HOPG: A Depth-Resolved Mechanistic Study by STM. J. Phys. Chem. B 2001, 105, 7632–7638. [Google Scholar] [CrossRef]
- Wei, J.; Chen, W.; Chen, D.; Yang, K. An amorphous carbon-graphite composite cathode for long cycle life rechargeable aluminum ion batteries. J. Mater. Sci. Technol. 2018, 34, 983–989. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]


| Properties | Al Batteries | Pb–Acid Batteries | Li-Ion Batteries | ||
|---|---|---|---|---|---|
| NCM | LiFePO4 | LTO | |||
| Energy density (Wh/L) | 45 to 91 | 50 to 90 | 150 to 300 | 90 to 247 | 200 |
| [12,16] | [16,17] | [18,19] | [18,19] | [18] | |
| Life cycle (times) | 250 to 250,000 | 400 to 600 | 500 to 1000 | 1000 to 3600 | 15,000 |
| [2,12,25] | [17] | [18,19] | [18,19] | [18] | |
| Efficiency (%) | 90 to 99.5% | 90% | 90%–95% | ||
| [2,4,12,25] | [16] | [16] | |||
| Discharging C-rate | 10 to 4000 | 0.2 to 5 | 2 to 3 | 3 | 5 to 10 |
| [1,2,4,12,15,25] | [19] | [18] | [18] | [18] | |
| Safety [1,2,4,8,12,13,14,15,16] | High | High | Low | Medium | High |
| Operating temperature (°C) | 20 to 150 | −20 to 50 | −20 to 45 | −30 to 45 | −30 to 55 |
| [2] | [19] | [18,19] | [18,19] | [18,19] | |
| Battery cost (USD/kWh) | – | 150 | >200 | ||
| [17] | [17] | ||||
| Loading (mg/cm2) | Thickness (µm) | Discharge Capacity (mAh/g) | Volume Energy Density (Wh/L) |
|---|---|---|---|
| 7 | 89 | 105 | 5.8 |
| 9 | 120 | 88 | 6.2 |
| 10 | 130 | 82 | 6.5 |
| 12 | 142 | 60 | 5.3 |
| Charge–Discharge Current Density (mA/g) | Discharge Capacity (mAh/g) |
|---|---|
| 100 | 82 |
| 300 | 71 |
| 500 | 51 |
| 700 | 45 |
| 1000 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.-C.; Yang, C.-H.; Chiang, C.-C.; Chiu, S.-C.; Chen, Y.-F.; Lin, C.-Y.; Wang, L.-Y.; Li, Y.-L.; Yang, C.-C.; Chang, W.-S. Influence of High Loading on the Performance of Natural Graphite-Based Al Secondary Batteries. Energies 2018, 11, 2760. https://doi.org/10.3390/en11102760
Huang M-C, Yang C-H, Chiang C-C, Chiu S-C, Chen Y-F, Lin C-Y, Wang L-Y, Li Y-L, Yang C-C, Chang W-S. Influence of High Loading on the Performance of Natural Graphite-Based Al Secondary Batteries. Energies. 2018; 11(10):2760. https://doi.org/10.3390/en11102760
Chicago/Turabian StyleHuang, Mao-Chia, Cheng-Hsien Yang, Chien-Chih Chiang, Sheng-Cheng Chiu, Yun-Feng Chen, Cong-You Lin, Lu-Yu Wang, Yen-Liang Li, Chang-Chung Yang, and Wen-Sheng Chang. 2018. "Influence of High Loading on the Performance of Natural Graphite-Based Al Secondary Batteries" Energies 11, no. 10: 2760. https://doi.org/10.3390/en11102760