Optimized Conversion of Waste Cooking Oil to Biodiesel Using Calcium Methoxide as Catalyst under Homogenizer System Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Transesterification Reaction Procedure
2.5. Analytical Methods
3. Results and Discussion
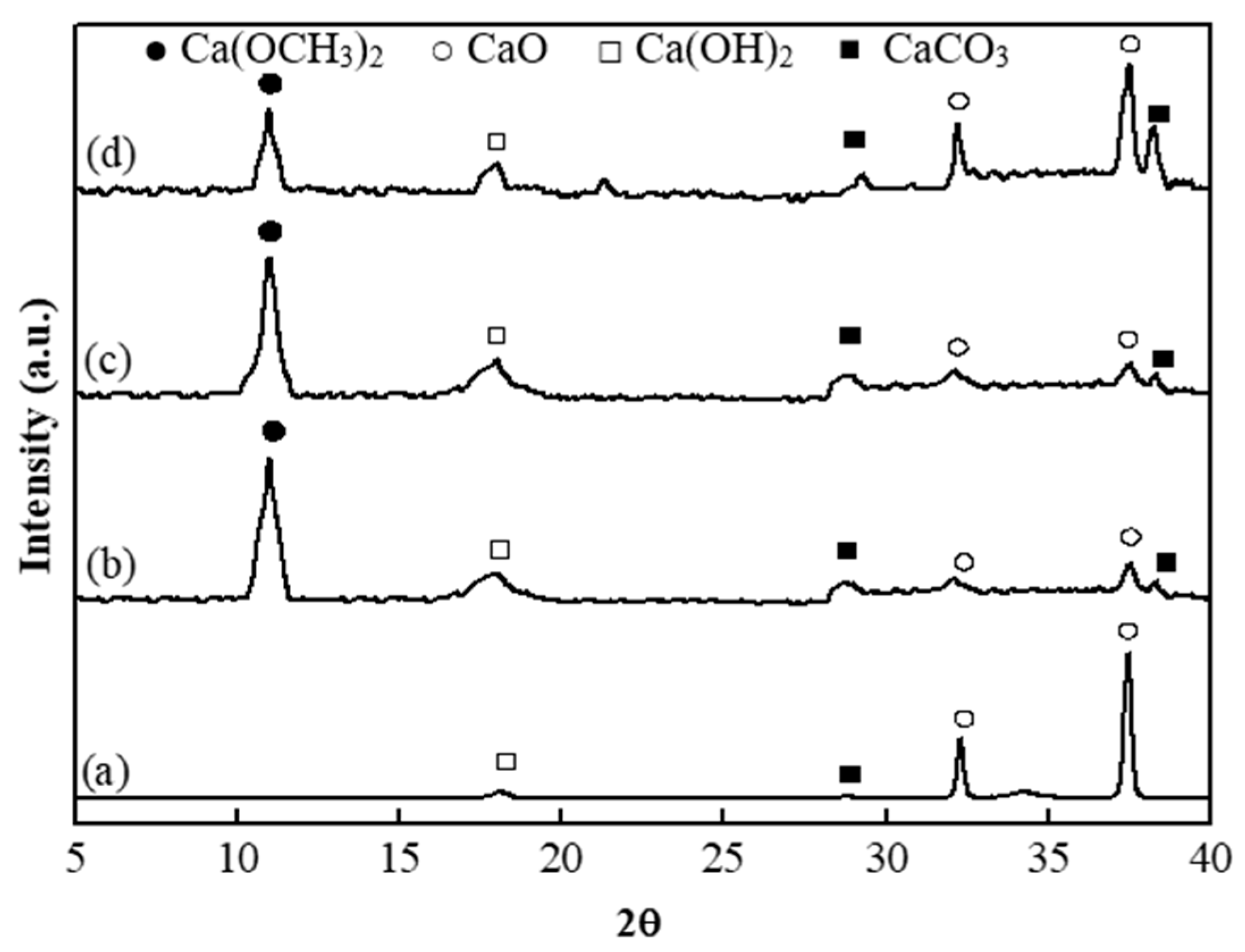
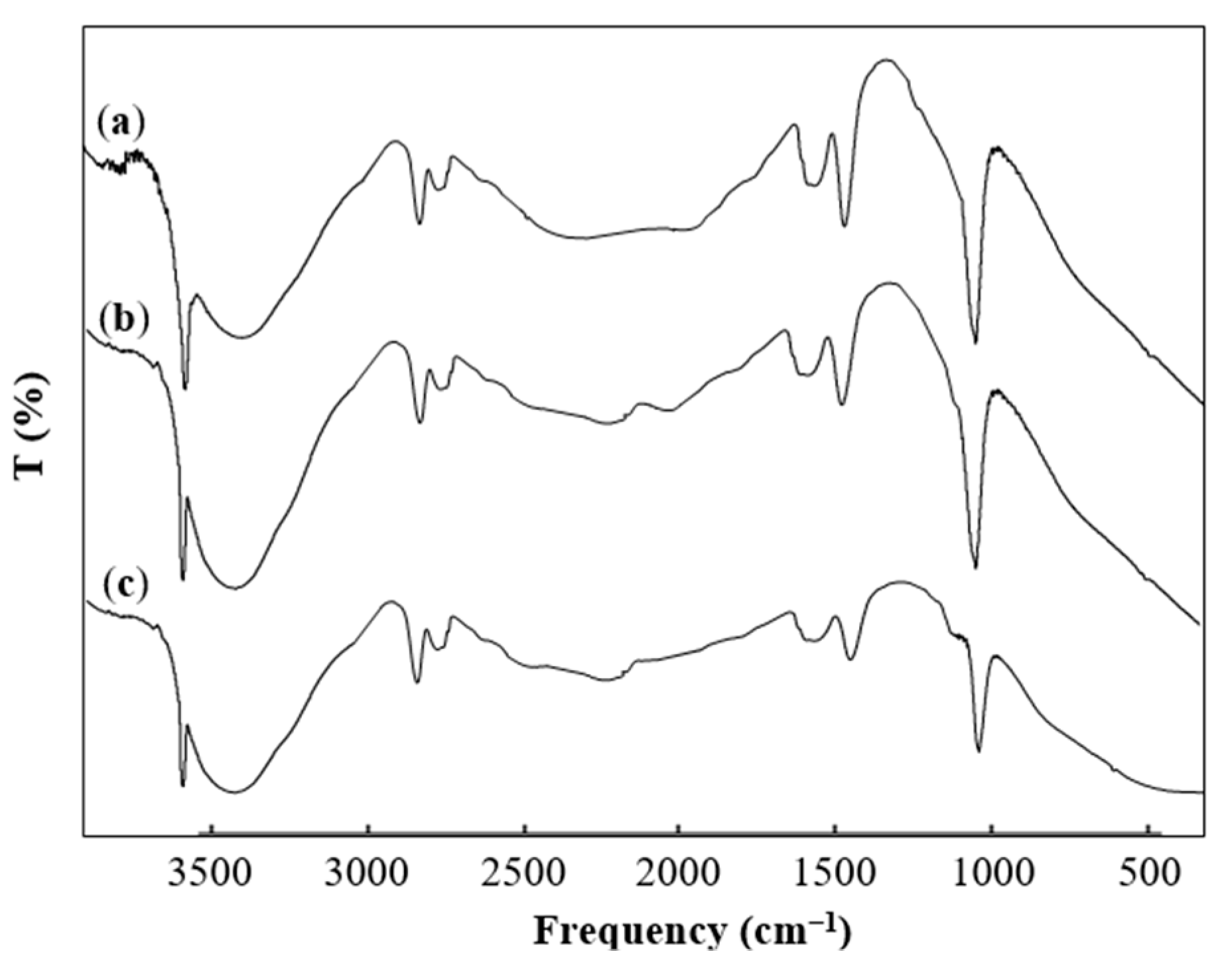
3.1. Characterization of the Catalyst
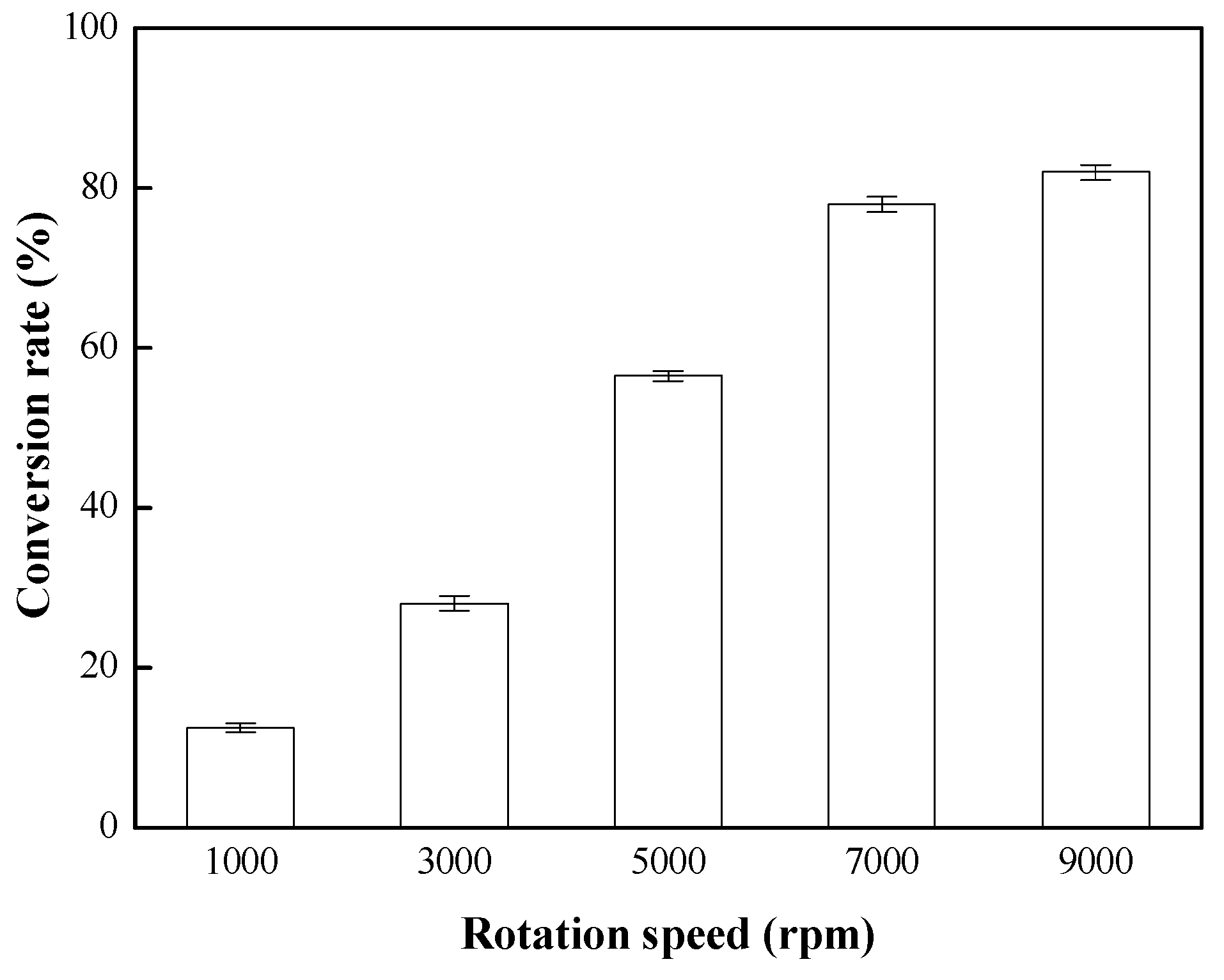
3.2. Effects of Rotation Speed on the Conversion Rate of Biodiesel
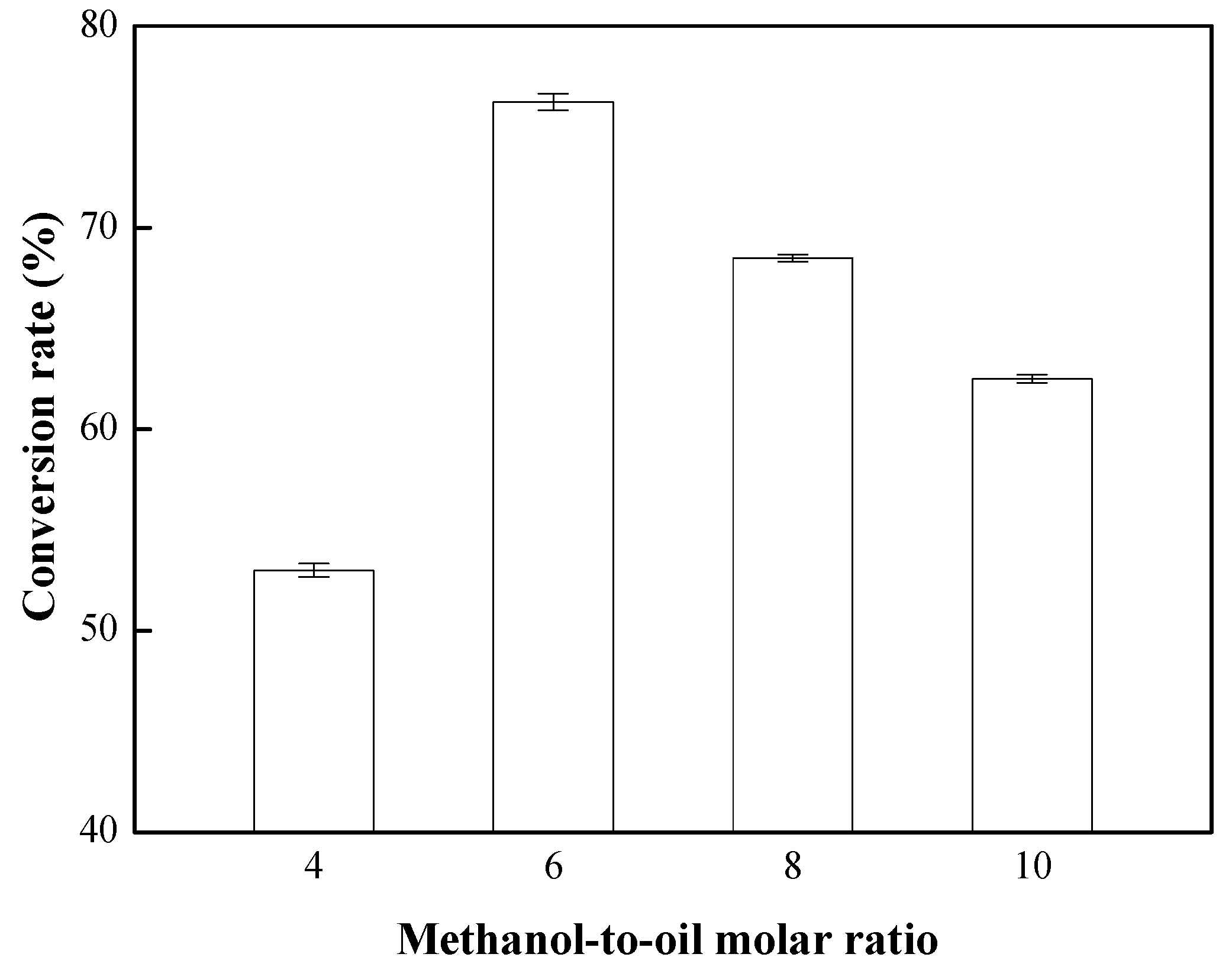
3.3. Effects of Methanol-to-Oil Molar Ratio on the Conversion Rate of Biodiesel
3.4. Effects of Amount of Catalyst on the Conversion Rate of Biodiesel
3.5. Effects of Temperature on the Conversion Rate of Biodiesel
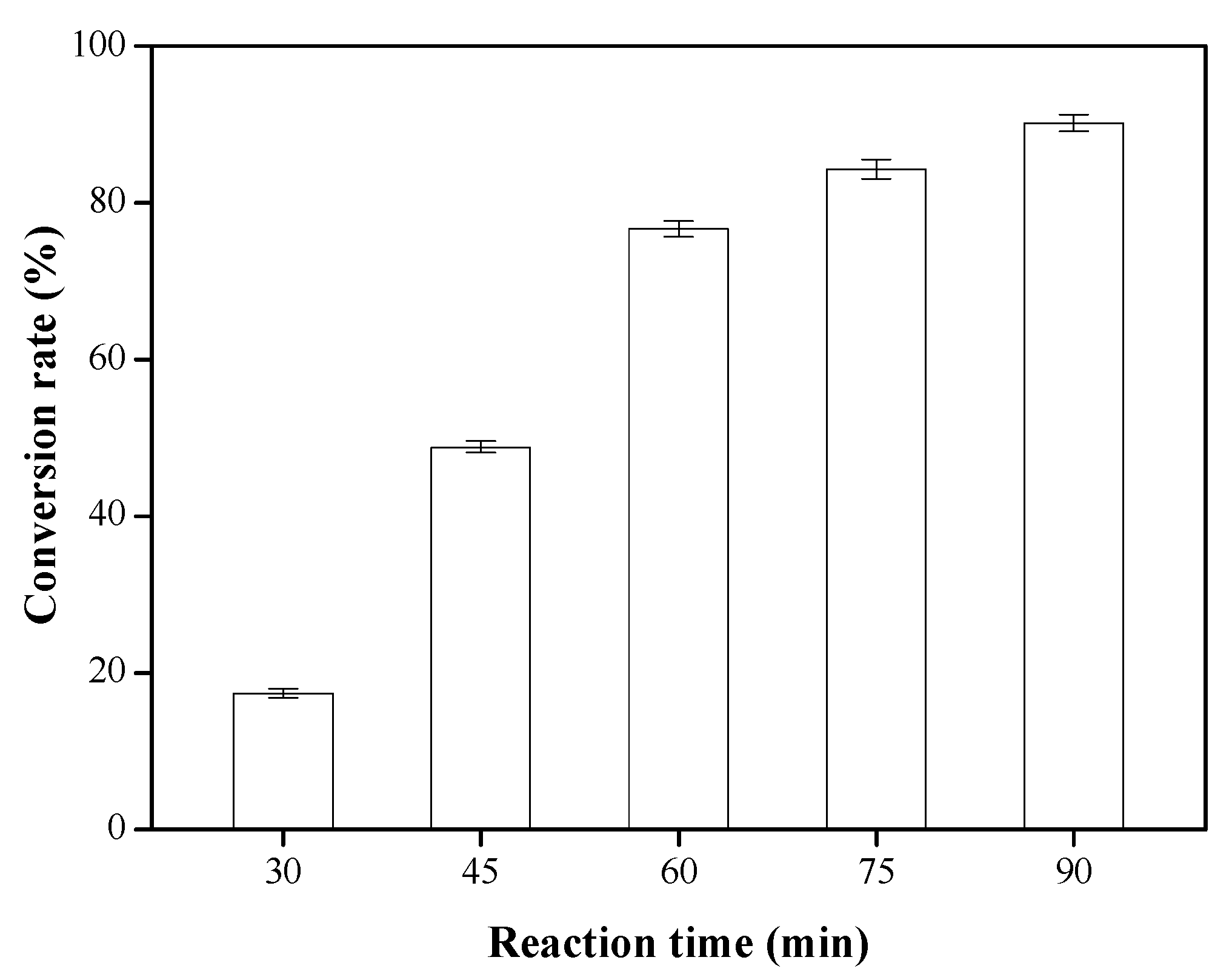
3.6. Effects of Reaction Time on the Conversion Rate of Biodiesel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Mittelbach, M.; Enzelsberger, H. Transesterification of heated rapeseed oil for extending diesel fuel. J. Am. Oil Chem. Soc. 1999, 76, 545–550. [Google Scholar] [CrossRef]
- Alcantara, R.; Amores, J.; Canoira, L.; Fidalgo, E.; Franco, M.J.; Navarro, A. Catalytic production of biodiesel from soybean oil, used frying oil and tallow. Biomass Bioenergy 2000, 18, 515–527. [Google Scholar] [CrossRef]
- Mittelbach, M.; Gangl, S. Long storage stability of biodiesel made from rapeseed and used frying oil. J. Am. Oil Chem. Soc. 2001, 78, 573–577. [Google Scholar] [CrossRef]
- Murayama, T.; Fujiwara, Y.; Noto, T. Evaluating waste vegetable oils as a diesel fuel. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2000, 214, 141–148. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Arnal, J.M.; Gomez, J.; Lopez Gimenez, F.J. Testing waste olive oil methyl ester as a fuel in a diesel engine. Energy Fuels 2001, 17, 1560–1565. [Google Scholar] [CrossRef]
- Tashtoush, G.; AlWidyan, M.I.; AlShyoukh, A.O. Combustion performance and emissions of ethyl ester of a waste vegetable oil in a water-cooled furnace. Appl. Therm. Eng. 2003, 23, 285–293. [Google Scholar] [CrossRef]
- Hsien, H.T. Green energy-biodiesel demonstration plant. Energy Mag. 2004, 12, 22–24. (In Chinese) [Google Scholar]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Ghadge, S.V.; Raheman, H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy 2005, 2, 601–605. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Jitputti, J.; Kitiyanan, B.; Rangsunvigit, P.; Bunyakiat, K.; Attanatho, L.; Jenvanitpanjakul, P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chem. Eng. J. 2006, 116, 61–66. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J. Transesterification of soybean oil with nano-MgO or not in supercritical and subcritical methanol. Fuel 2007, 8, 328–333. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, W. Soybean oil transesterification over zinc oxide modified with alkali earth metals. Fuel Process. Technol. 2007, 88, 631–638. [Google Scholar] [CrossRef]
- Xie, W.; Yang, Z. Ba-ZnO catalysts for soybean oil transesterification. Catal. Lett. 2007, 117, 159–165. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Jiménez-Urbistondo, I.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Rodríguez-Castellón, E.; Jiménez-López, A.; Azevedo, D.C.S.; Cavalcante, C.L., Jr.; et al. CaO supported on mesoporous silicas as basic catalysts for transesterification reactions. Appl. Catal. A 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, B.S.; Kim, M.J.; Park, Y.M.; Kim, D.K.; Lee, J.S. Transesterification of vegetable oil to biodiesel using heterogeneous base catalyst. Catal. Today 2004, 93, 315–320. [Google Scholar] [CrossRef]
- Cantrell, D.G.; Gillie, L.J.; Lee, A.F.; Wilson, K. Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl. Catal. A 2005, 287, 183–190. [Google Scholar] [CrossRef]
- Gao, L.; Teng, G.; Xiao, G.; Wei, R. Biodiesel from palm oil via loading KF/Ca-Al hydrotalcite catalyst. Biomass Bioenergy 2010, 34, 1283–1288. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Bernardo, J.; Felizardo, P.; Correia, M.J.N. Biodiesel production over thermal activated cerium modified Mg-Al hydrotalcites. Energy 2012, 41, 344–353. [Google Scholar] [CrossRef]
- Li, X.; Lu, G.; Guo, Y.; Wang, Y.; Zhang, Z.; Liu, X. A novel solid superbase of Eu2O3/Al2O3 and its catalytic performance for the transesterification of soybean oil to biodiesel. Catal. Commun. 2007, 8, 1969–1972. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Kawi, S. An active and stable CaO-CeO2 catalyst for transesterification of oil to biodiesel. Green Chem. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Shibasaki-Kitakawa, N.; Honda, H.; Kuribayashi, H.; Toda, T.; Fukumura, T.; Yonemoto, T. Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst. Bioresour. Technol. 2007, 98, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ding, Y.; Duan, J.; Zhang, Q.; Wang, Z.; Lou, H. Transesterification of sunflower oil to biodiesel on ZrO2 supported La2O3 catalyst. Bioresour. Technol. 2010, 101, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Wan Omar, W.N.N.; Amin, N.A.S. Biodiesel production from waste cooking oil over alkaline modified zirconia catalyst. Fuel Process. Technol. 2011, 92, 2397–2405. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Huppertz, T. Homogenization of Milk-Other Types of Homogenizer (High-Speed Mixing, Ultrasonics Microfluidizers, Membrane Emulsification). In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Elsevier: Oxford, UK; Academic Press: Oxford, UK, 2011; pp. 761–764. [Google Scholar]
- Saheki, A.; Seki, J.; Nakanishi, T.; Tamai, I. Effect of back pressure on emulsification of lipid nanodispersions in a high-pressure homogenizer. Int. J. Pharm. 2012, 422, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, P.H.; Hou, S.S. Improving biodiesel conversions from blends of high-and low-acid-value waste cooking oils using sodium methoxide as a catalyst based on a high speed homogenizer. Energies 2018, 11, 2298. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H.; Chen, L.C. Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel 2010, 89, 3618–3622. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Kuan, I.; Kao, W.C.; Chen, C.L.; Yu, C.Y. Microbial biodiesel production by direct transesterification of Rhodotorula glutinis biomass. Energies 2018, 11, 1036. [Google Scholar] [CrossRef]
- Chen, K.S.; Lin, Y.C.; Hsu, K.H.; Wang, H.K. Improving biodiesel yields from waste cooking oil by using sodium methoxide and a microwave heating system. Fuel 2012, 38, 151–156. [Google Scholar] [CrossRef]
- Al-Zaini, E.O.; Olsen, J.; Nguyen, T.H.; Adesina, A. Transesterification of waste cooking oil in presence of crushed seashell as a support for solid heterogeneous catalyst. SAE Int. J. Fuels Lubr. 2011, 4, 139–157. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Yamanaka, S.; Hidaka, J. Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing ethanol. Appl. Catal. A 2008, 334, 357–365. [Google Scholar] [CrossRef]
- Gryglewicz, S. Alkaline-earth metal compounds as alcoholysis catalysts for ester oils synthesis. Appl. Catal. A 2000, 192, 23–28. [Google Scholar] [CrossRef]
- Maneerung, T.; Kawi, S.; Dai, Y.; Wang, C.H. Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manag. 2016, 123, 487–497. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Yusaf, T.; Taufiq-Yap, Y.H. Transesterification of Nannochloropsis oculata microalga's oil to biodiesel using calcium methoxide catalyst. Energy 2014, 78, 63–71. [Google Scholar] [CrossRef]
- Li, F.J.; Li, H.Q.; Wang, L.G.; Cao, Y. Waste carbide slag as a solid base catalyst for effective synthesis of biodiesel via transesterification of soybean oil with methanol. Fuel Process. Technol. 2015, 131, 421–429. [Google Scholar] [CrossRef]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetic studies of production of biodiesel from waste frying oil using heterogeneous catalysts derived from snail shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, M.-C.; Hou, S.-S.; Kuo, J.-Y.; Hsieh, P.-H. Optimized Conversion of Waste Cooking Oil to Biodiesel Using Calcium Methoxide as Catalyst under Homogenizer System Conditions. Energies 2018, 11, 2622. https://doi.org/10.3390/en11102622
Hsiao M-C, Hou S-S, Kuo J-Y, Hsieh P-H. Optimized Conversion of Waste Cooking Oil to Biodiesel Using Calcium Methoxide as Catalyst under Homogenizer System Conditions. Energies. 2018; 11(10):2622. https://doi.org/10.3390/en11102622
Chicago/Turabian StyleHsiao, Ming-Chien, Shuhn-Shyurng Hou, Jui-Yang Kuo, and Pei-Hsuan Hsieh. 2018. "Optimized Conversion of Waste Cooking Oil to Biodiesel Using Calcium Methoxide as Catalyst under Homogenizer System Conditions" Energies 11, no. 10: 2622. https://doi.org/10.3390/en11102622




