Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Crude Oil
2.3. Production of Biodiesel through Two-Step Process
2.3.1. Esterification Step
2.3.2. Transesterification Step
2.4. Analysis
3. Results and Discussion
3.1. Properties of Soursop Seed Oil
3.2. Conversion of FFAs into Biodiesel through Acid-Catalyzed Esterification
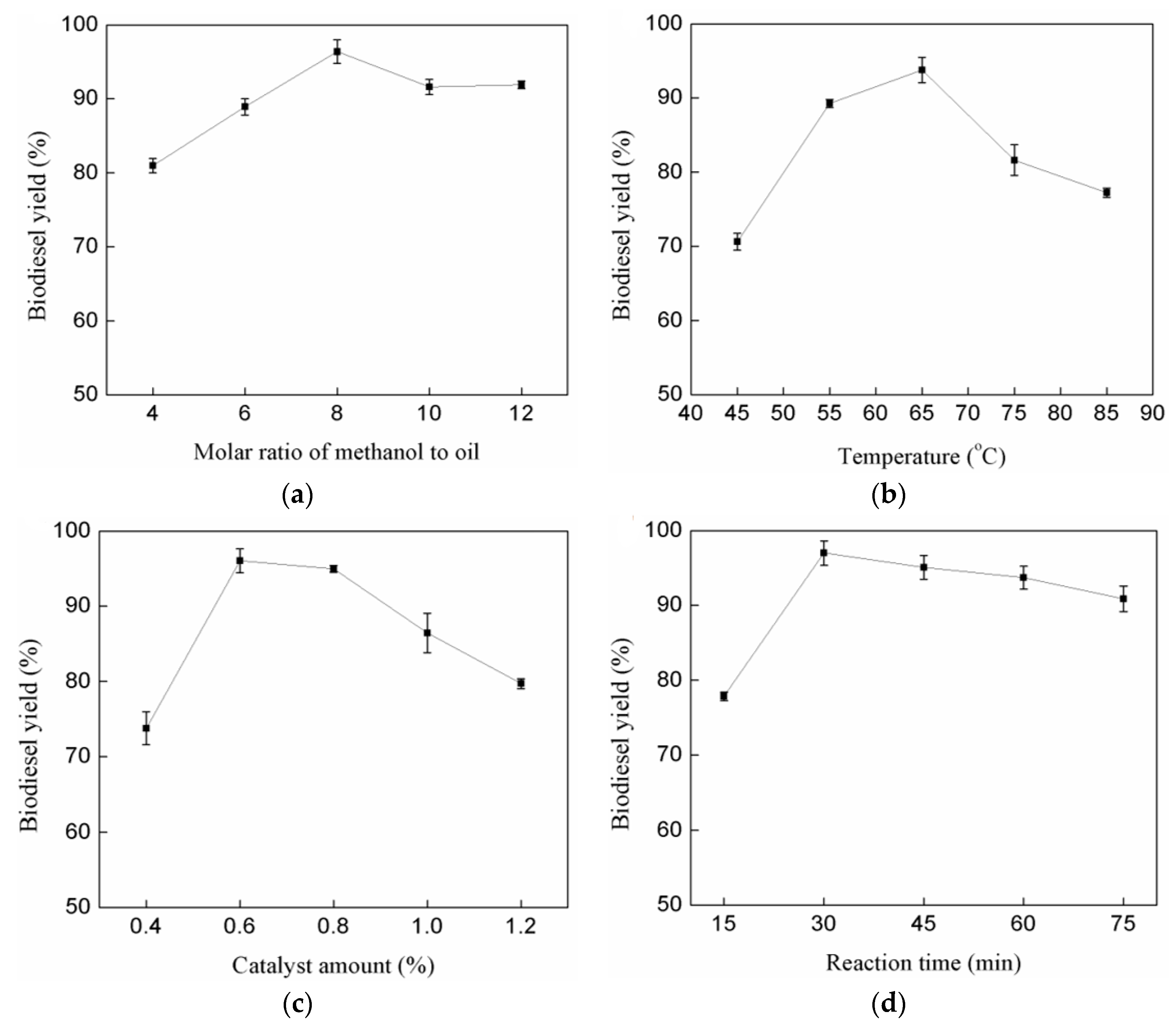
3.2.1. Effect of Methanol to Oil Molar Ratio
3.2.2. Effect of Temperature
3.2.3. Effect of Catalyst Amount
3.2.4. Effect of Reaction Time
3.3. Conversion of Triglyceride into Biodiesel through Alkali-Catalyzed Transesterification
3.3.1. Effect of Methanol to Oil Molar Ratio
3.3.2. Effect of Temperature
3.3.3. Effect of Catalyst Amount
3.3.4. Effect of Reaction Time
3.4. Fatty Acid Profiles of Soursop Biodiesel
3.5. Properties of Soursop Biodiesel
3.6. The Feasibility of Soursop Seed Oil as Biodiesel Feedstock
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mosarof, M.; Kalam, M.; Masjuki, H.; Alabdulkarem, A.; Ashraful, A.; Arslan, A.; Rashedul, H.; Monirul, I. Optimization of performance, emission, friction and wear characteristics of palm and Calophyllum inophyllum biodiesel blends. Energy Convers. Manag. 2016, 118, 119–134. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Tong, C.W.; Mahlia, T.M.I.; Silitonga, A.S. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ. Sci. Pollut. Res. 2018, 25, 15307–15325. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Abdul Malik, M.S.; Shaiful, A.I.M.; Mohd Ismail, M.S.; Mohd Jaafar, M.N.; Mohamad Sahar, A. Combustion and emission characteristics of coconut-based biodiesel in a liquid fuel burner. Energies 2017, 10, 458. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Bhuyan, M.S.U.S.; Alam, A.H.M.A.; Chu, Y.; Seo, Y.C. Biodiesel production potential from littered edible oil fraction using directly synthesized S-TiO2/MCM-41 catalyst in esterification process via non-catalytic subcritical hydrolysis. Energies 2017, 10, 1290. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Lee, H. A review on latest developments and future prospects of heterogeneous catalyst in biodiesel production from non-edible oils. Renew. Sustain. Energy Rev. 2017, 67, 1225–1236. [Google Scholar] [CrossRef]
- Martindale, W.; Trewavas, A. Fuelling the 9 billion. Nat. Biotechnol. 2008, 26, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Liang, S.H.; Doan, T.T.; Su, C.H.; Yang, P.C. Lipase-catalyzed synthesis of biodiesel from black soldier fly (Hermetica illucens): Optimization by using response surface methodology. Energy Convers. Manag. 2017, 145, 335–342. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crops Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- El Shimi, H.I.; Moustafa, S.S. Biodiesel production from microalgae grown on domestic wastewater: Feasibility and Egyptian case study. Renew. Sustain. Energy Rev. 2018, 82, 4238–4244. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rahimpour, M.R.; Ghasemi, Y.; Raeissi, S. The biodiesel of microalgae as a solution for diesel demand in Iran. Energies 2018, 11, 950. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crops Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Lin, J.J.; Chen, Y.W. Production of biodiesel by transesterification of Jatropha oil with microwave heating. J. Taiwan Inst. Chem. Eng. 2017, 75, 43–50. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Hossain, M.N.; Siddik Bhuyan, M.S.U.; Alam, A.H.M.A.; Seo, Y.C. Biodiesel from hydrolyzed waste cooking oil using a S-ZrO2/SBA-15 super acid catalyst under sub-critical conditions. Energies 2018, 11, 299. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Sanjel, N.; Shah, M.; Oh, S.C. Comparison of biodiesel obtained from virgin cooking oil and waste cooking oil using supercritical and catalytic transesterification. Energies 2017, 10, 546. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Exploring the potential of grease from yellow mealworm beetle (Tenebrio molitor) as a novel biodiesel feedstock. Appl. Energy 2013, 101, 618–621. [Google Scholar] [CrossRef]
- Surendra, K.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Barekati-Goudarzi, M.; Muley, P.D.; Clarens, A.; Nde, D.B.; Boldor, D. Continuous microwave-assisted in-situ transesterification of lipids in seeds of invasive Chinese tallow trees (Triadica sebifera L.): Kinetic and thermodynamic studies. Biomass Bioenergy 2017, 107, 353–360. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Quesada-Medina, J.; de los Ríos, A.P.; Chica, A.; Beneito-Ruiz, R.; Carratalá-Abril, J. Optimization of non-catalytic transesterification of tobacco (Nicotiana tabacum) seed oil using supercritical methanol to biodiesel production. Energy Convers. Manag. 2017, 131, 99–108. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.D.; Kusumo, F.; Mazaheri, H.; Ilham, Z. Biodiesel production by lipase-catalyzed transesterification of Ocimum basilicum L. (sweet basil) seed oil. Energy Convers. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef]
- Hasni, K.; Ilham, Z.; Dharma, S.; Varman, M. Optimization of biodiesel production from Brucea javanica seeds oil as novel non-edible feedstock using response surface methodology. Energy Convers. Manag. 2017, 149, 392–400. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Tongcumpou, C. Sequential extraction and reactive extraction processing of spent coffee grounds: An alternative approach for pretreatment of biodiesel feedstocks and biodiesel production. Ind. Crops Prod. 2018, 117, 359–365. [Google Scholar] [CrossRef]
- Sakuragi, K.; Li, P.; Otaka, M.; Makino, H. Recovery of bio-oil from industrial food waste by liquefied dimethyl ether for biodiesel production. Energies 2016, 9, 106. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, W.; Chen, X.; Wang, J. Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour. Technol. 2012, 111, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Cardoso, C.C.; Pasa, V.M. Production of cold-flow quality biodiesel from high-acidity on-edible oils—Esterification and transesterification of Macauba (Acrocomia aculeata) oil using various alcohols. BioEnergy Res. 2016, 9, 864–873. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Antony, J.V.; Vengalil, R.; Kochimoolayil, G.E.; Joseph, R. Esterification of free fatty acids in non-edible oils using partially sulfonated polystyrene for biodiesel feedstock. Ind. Crops Prod. 2017, 95, 66–74. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.Y.; Su, C.H.; Chien, C.C. Liquid lipase-catalyzed esterification of oleic acid with methanol for biodiesel production in the presence of superabsorbent polymer: Optimization by using response surface methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef]
- Çaylı, G.; Küsefoğlu, S. Increased yields in biodiesel production from used cooking oils by a two step process: Comparison with one step process by using TGA. Fuel Process. Technol. 2008, 89, 118–122. [Google Scholar] [CrossRef]
- Hayyan, A.; Alam, M.Z.; Mirghani, M.E.; Kabbashi, N.A.; Hakimi, N.I.N.M.; Siran, Y.M.; Tahiruddin, S. Reduction of high content of free fatty acid in sludge palm oil via acid catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 920–924. [Google Scholar] [CrossRef]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2016, 11, 662–691. [Google Scholar] [CrossRef]
- Schroeder, P.; do Nascimento, B.P.; Romeiro, G.A.; Figueiredo, M.K.K.; da Cunha Veloso, M.C. Chemical and physical analysis of the liquid fractions from soursop seed cake obtained using slow pyrolysis conditions. J. Anal. Appl. Pyrolysis 2017, 124, 161–174. [Google Scholar] [CrossRef]
- Fasakin, A.; Fehintola, E.; Obijole, O.; Oseni, O. Compositional analyses of the seed of sour sop, Annona muricata L., as a potential animal feed supplement. Sci. Res. Essays 2008, 3, 521–523. [Google Scholar]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.C.; Alves, R. Soursop (Annona muricata L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Mangosteen to White Sapote; Yahia, E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 363–392. [Google Scholar]
- Gajalakshmi, S.; Vijayalakshmi, S.; Devi Rajeswari, V. Phytochemical and pharmacological properties of Annona muricata: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 3–6. [Google Scholar]
- Awan, J.; Kar, A.; Udoudoh, P. Preliminary studies on the seeds of Annona muricata Linn. Plant Foods Hum. Nutr. 1980, 30, 163–168. [Google Scholar] [CrossRef]
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, nutritional value, medicinal uses, and toxicology. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Oxford, UK, 2010; pp. 621–643. [Google Scholar]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from Annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty acids analysis, antioxidant and biological activity of fixed oil of Annona muricata L. seeds. J. Chem. 2016, 6. [Google Scholar] [CrossRef]
- Phoo, Z.W.M.M.; Ilham, Z.; Goembira, F.; Razon, L.; Saka, S. Physico-chemical properties of biodiesel from various feedstocks. In Zero-Carbon Energy Kyoto 2012; Yao, T., Ed.; Springer: Berlin, Germany, 2013; pp. 113–121. [Google Scholar]
- Schroeder, P.; dos Santos Barreto, M.; Romeiro, G.A.; Figueiredo, M.K.K. Development of energetic alternatives to use of waste of Annona muricata L. Waste Biomass Valorization 2018, 9, 1459–1467. [Google Scholar] [CrossRef]
- Su, C.H.; Fu, C.C.; Gomes, J.; Chu, I.; Wu, W.T. A heterogeneous acid-catalyzed process for biodiesel production from enzyme hydrolyzed fatty acids. AIChE J. 2008, 54, 327–336. [Google Scholar] [CrossRef]
- Vicente, G.; Martınez, M.; Aracil, J. Integrated biodiesel production: A comparison of different homogeneous catalysts systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H. Recoverable and reusable hydrochloric acid used as a homogeneous catalyst for biodiesel production. Appl. Energy 2013, 104, 503–509. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Annual Book of ASTM Standards. Available online: https://www.astm.org/BOOKSTORE/BOS/index.html (accessed on 26 September 2018).
- Ramadhas, A.; Jayaraj, S.; Muraleedharan, C. Characterization and effect of using rubber seed oil as fuel in the compression ignition engines. Renew. Energy 2005, 30, 795–803. [Google Scholar] [CrossRef]
- Emil, A.; Yaakob, Z.; Kumar, M.S.; Jahim, J.M.; Salimon, J. Comparative evaluation of physicochemical properties of Jatropha seed oil from Malaysia, Indonesia and Thailand. J. Am. Oil Chem. Soc. 2010, 87, 689–695. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Biodiesel production from Croton megalocarpus oil and its process optimization. Fuel 2010, 89, 2556–2560. [Google Scholar] [CrossRef]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Yang, S.; Li, Q.; Gao, Y.; Zheng, L.; Liu, Z. Biodiesel production from swine manure via housefly larvae (Musca domestica L.). Renew. Energy 2014, 66, 222–227. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Automotive Fuels-Fatty Acid Methyl Esters (FAME) for Diesel Engines-Requirements and Test Methods. Available online: http://agrifuelsqcs-i.com/attachments/1598/en14214.pdf (accessed on 25 September 2018).
- Bala, V.S.S.; Thiruvengadaravi, K.V.; Kumar, P.S.; Premkumar, M.P.; Kumar, M.H.; Sivanesan, S. Removal of free fatty acids in Pongamia pinnata (Karanja) oil using divinylbenzene-styrene copolymer resins for biodiesel production. Biomass Bioenergy 2012, 37, 335–341. [Google Scholar] [CrossRef]

| Fat Yield (%) | Acid Value (mg KOH/g) | Saponification Value (mg KOH/g) |
|---|---|---|
| 29.6 ± 0.2 | 54.4 ± 0.4 | 244.7 ± 1.6 |
| Composition | Rapeseed Biodiesel a (%) | Soursop Biodiesel b (%) |
|---|---|---|
| Palmitic acid methyl ester (C16:0) | 3.5 | 18.14 |
| Palmitoleic acid methyl ester (C16:1) | na c | 0.81 |
| Stearic acid methyl ester (18:0) | 0.8 | 3.79 |
| Oleic acid methyl ester (C18:1) | 64.4 | 43.68 |
| Linoleic acid methyl ester (C18:2) | 22.3 | 32.45 |
| Linolenic acid methyl ester (C18:3) | 8.2 | 1.13 |
| Properties | ASTM Method | ASTM D6751 a | EN 14214 | Rapeseed Biodiesel b | This Study |
|---|---|---|---|---|---|
| Acid value (mg KOH/g) | D664 | <0.5 | <0.5 | 0.31 | <0.8 |
| Sulfur content (wt. %) | D5453 | <0.05 | <0.05 | <0.01 | 0.04 |
| Ester content (%) | D7371 | na c | >96.5 | na c | 98.6 |
| Viscosity at 40 °C (mm2/s) | D445 | 1.9–6.0 | 3.5–5.0 | 6.35 | 5.5 |
| Water content (mg/kg) | D95 | na c | <500 | 300 | 300 |
| Cetane number | D613 | >47 | >51 | 45 | 53 |
| Density (kg/m3) | D1480 | na c | 860–900 | 880 | 868 |
| Flash point (closed cup) (°C) | D93 | 100–170 | >120 | na c | 123 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-H.; Nguyen, H.C.; Pham, U.K.; Nguyen, M.L.; Juan, H.-Y. Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies 2018, 11, 2562. https://doi.org/10.3390/en11102562
Su C-H, Nguyen HC, Pham UK, Nguyen ML, Juan H-Y. Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies. 2018; 11(10):2562. https://doi.org/10.3390/en11102562
Chicago/Turabian StyleSu, Chia-Hung, Hoang Chinh Nguyen, Uyen Khanh Pham, My Linh Nguyen, and Horng-Yi Juan. 2018. "Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil" Energies 11, no. 10: 2562. https://doi.org/10.3390/en11102562
APA StyleSu, C.-H., Nguyen, H. C., Pham, U. K., Nguyen, M. L., & Juan, H.-Y. (2018). Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil. Energies, 11(10), 2562. https://doi.org/10.3390/en11102562