Analysis of Combined Cycle Power Plants with Chemical Looping Reforming of Natural Gas and Pre-Combustion CO2 Capture
Abstract
:1. Introduction
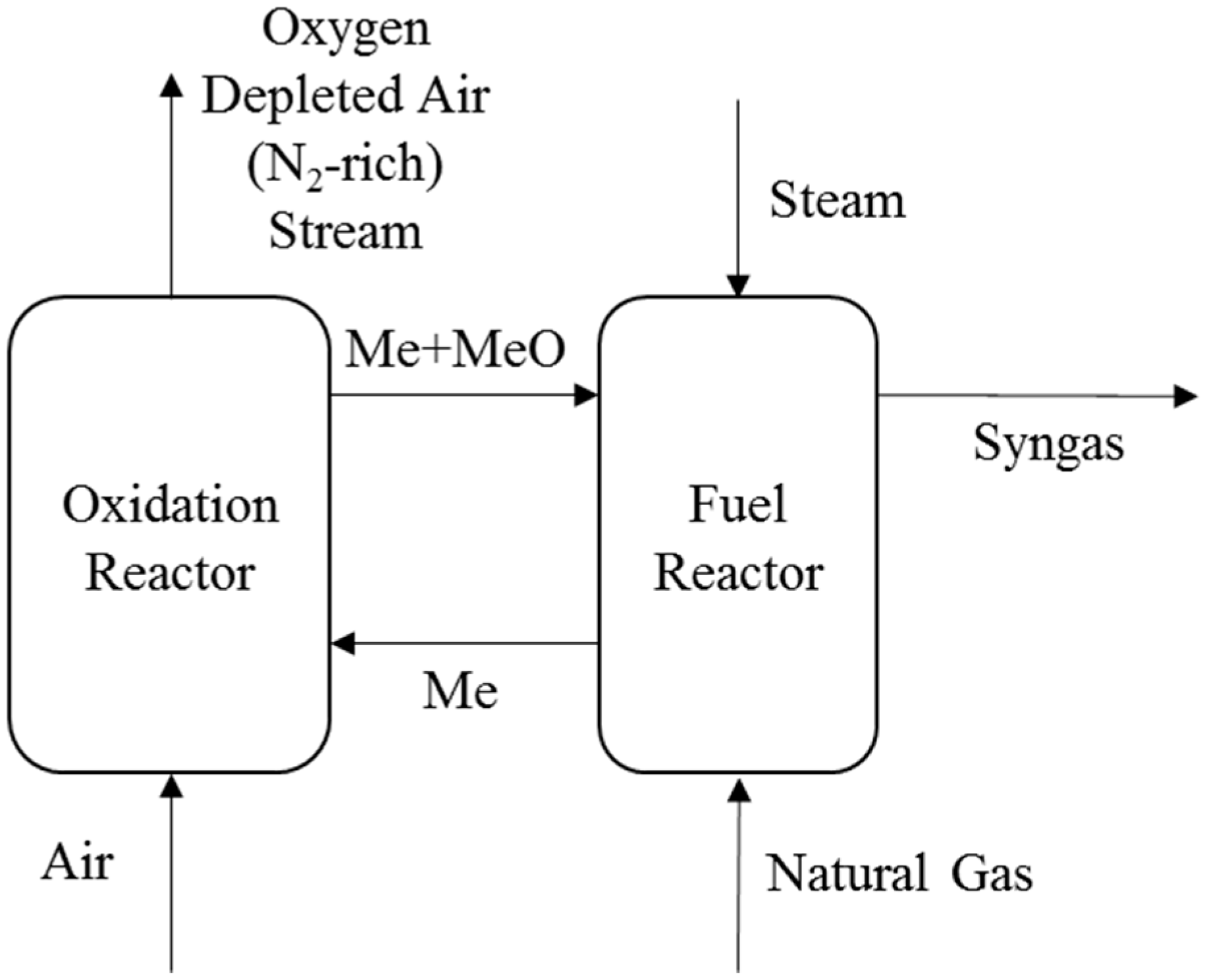
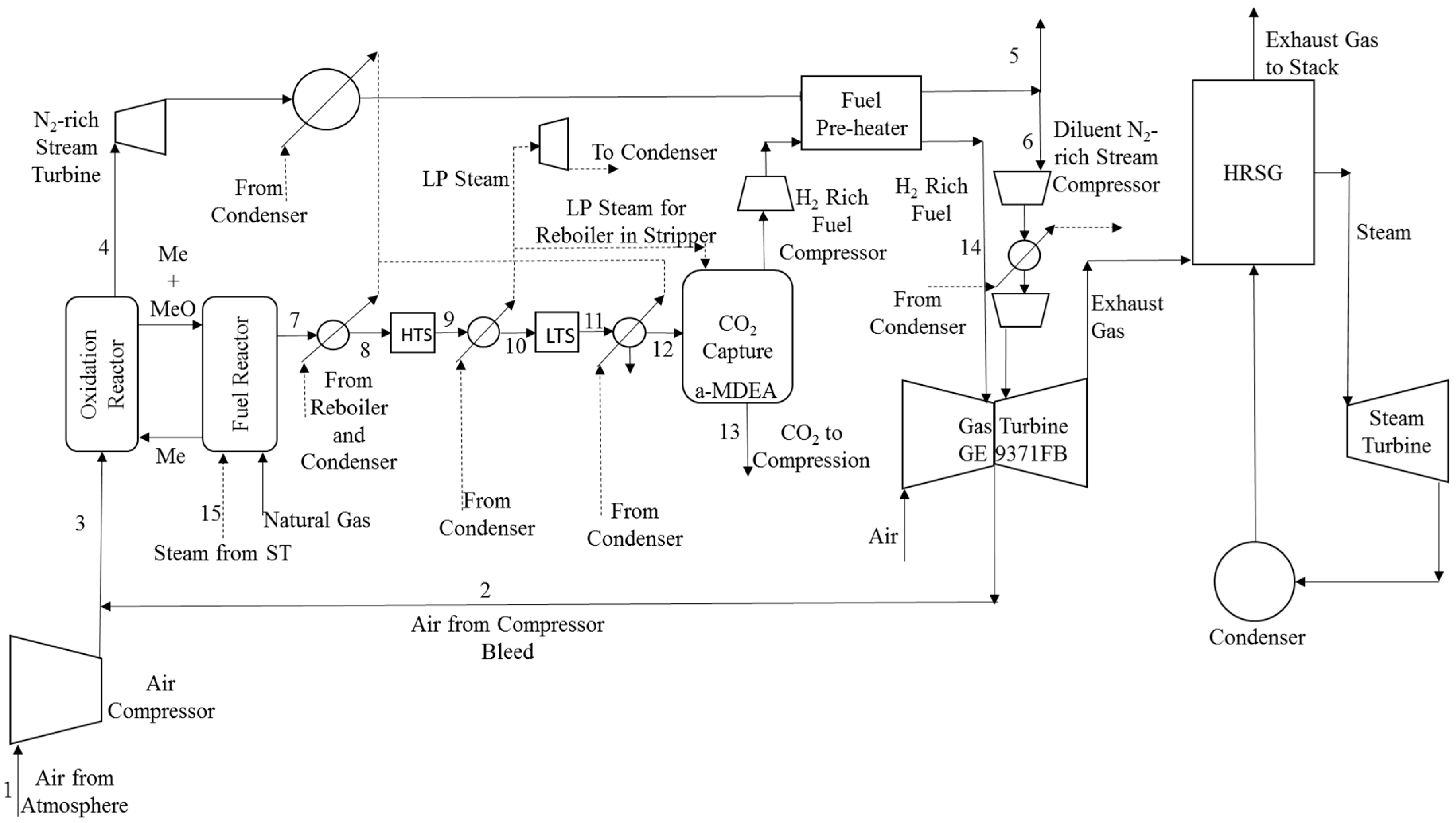
2. Process Description
3. Methodology
3.1. Different Design Pressures for the Chemical Looping Reforming
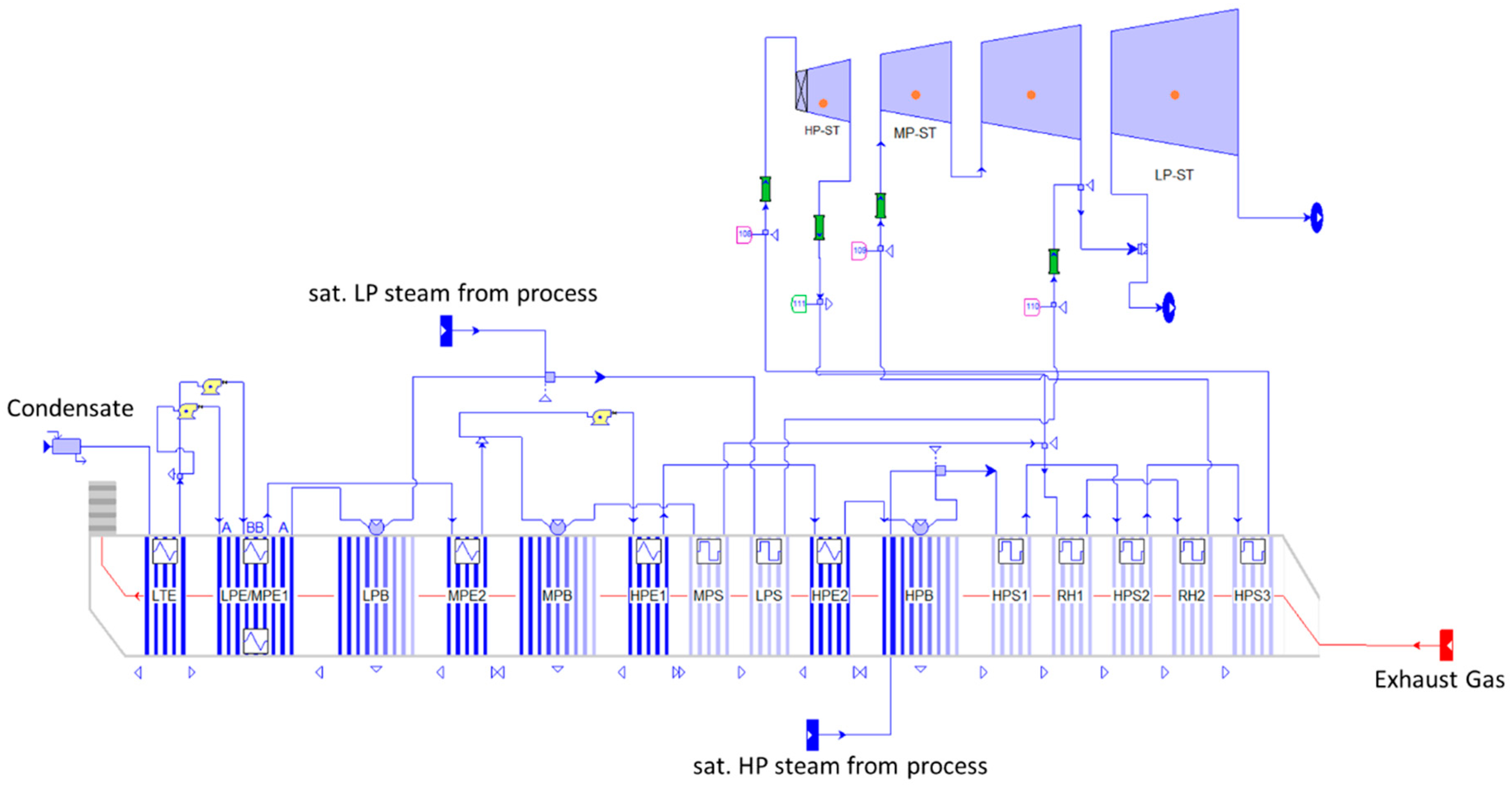
3.2. Options for Heat Integration
4. Results and Discussion
4.1. Sensitivity Study for Pressure in Chemical Looping Reforming
4.2. Heat Integration Options
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Energy Technology Perspectives (ETP). Energy Technology Perspectives 2012; International Energy Agency: Paris, France, 2012. [Google Scholar]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Kenarsari, S.D.; Yang, D.; Jiang, G.; Zhang, S.; Wang, J.; Russell, A.G.; Wei, Q.; Fan, M. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Richter, H.J.; Knoche, K.F. Reversibility of Combustion Processes; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1983; pp. 71–85. [Google Scholar]
- Ishida, M.; Jin, H. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Consonni, S.; Lozza, G.; Pelliccia, G.; Rossini, S.; Saviano, F. Chemical-looping combustion for combined cycles with CO2 capture. J. Eng. Gas Turbines Power 2006, 128, 525–534. [Google Scholar] [CrossRef]
- Naqvi, R.; Bolland, O. Multi-stage chemical looping combustion (CLC) for combined cycles with CO2 capture. Int. J. Greenh. Gas Control 2007, 1, 19–30. [Google Scholar] [CrossRef]
- Iloeje, C.; Zhao, Z.; Ghoniem, A.F. Analysis of thermally coupled chemical looping combustion-based power plants with carbon capture. Int. J. Greenh. Gas Control 2015, 35, 56–70. [Google Scholar] [CrossRef]
- Nazir, S.M.; Bolland, O.; Amini, S. Full plant scale analysis of natural gas fired power plants with pre-combustion CO2 capture and Chemical Looping Reforming (CLR). Energy Procedia 2017, 114, 2146–2155. [Google Scholar] [CrossRef]
- Nag, P.K. Power Plant Engineering, 2nd ed.; Tata McGraw-Hill Publishing Company Limited: New York, NY, USA, 2001. [Google Scholar]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef] [Green Version]
- Yahom, A.; Powell, J.; Pavarajarn, V.; Onbhuddha, P.; Charojrochkul, S.; Assabumrungrat, S. Simulation and thermodynamic analysis of chemical looping reforming and CO2 enhanced chemical looping reforming. Chem. Eng. Res. Des. 2014, 92, 2575–2583. [Google Scholar] [CrossRef]
- Bischi, A.; Langørgen, Ø.; Morin, J.X.; Bakken, J.; Ghorbaniyan, M.; Bysveen, M.; Bolland, O. Hydrodynamic viability of chemical looping processes by means of cold flow model investigation. Appl. Energy 2012, 97, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Pröll, T.; Kolbitsch, P.; Bolhàr-Nordenkampf, J.; Hofbauer, H. Chemical looping pilot plant results using a nickel-based oxygen carrier. Oil Gas Sci. Technol. 2011, 66, 173–180. [Google Scholar] [CrossRef]
- Pröll, T.; Bolhàr-Nordenkampf, J.; Kolbitsch, P.; Hofbauer, H. Syngas and a separate nitrogen/argon stream via chemical looping reforming—A 140 kW pilot plant study. Fuel 2010, 89, 1249–1256. [Google Scholar] [CrossRef]
- Ortiz, M.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Gayán, P.; Adánez, J. Hydrogen production by auto-thermal chemical-looping reforming in a pressurized fluidized bed reactor using Ni-based oxygen carriers. Int. J. Hydrog. Energy 2010, 35, 151–160. [Google Scholar] [CrossRef] [Green Version]
- De Diego, L.F.; Ortiz, M.; García-Labiano, F.; Adánez, J.; Abad, A.; Gayán, P. Hydrogen production by chemical-looping reforming in a circulating fluidized bed reactor using Ni-based oxygen carriers. J. Power Sources 2009, 192, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Rydén, M.; Lyngfelt, A.; Mattisson, T. Synthesis gas generation by chemical-looping reforming in a continuously operating laboratory reactor. Fuel 2006, 85, 1631–1641. [Google Scholar] [CrossRef]
- Martínez, I.; Murillo, R.; Grasa, G.; Fernández, J.R.; Abanades, J.C. Integrated combined cycle from natural gas with CO2 capture using a Ca-Cu chemical loop. AIChE J. 2013, 59, 2780–2794. [Google Scholar] [CrossRef]
- Romano, M.C.; Chiesa, P.; Lozza, G. Pre-combustion CO2 capture from natural gas power plants, with ATR and MDEA processes. Int. J. Greenh. Gas Control 2010, 4, 785–797. [Google Scholar] [CrossRef]
- Nord, L.O.; Anantharaman, R.; Bolland, O. Design and off-design analyses of a pre-combustion CO2 capture process in a natural gas combined cycle power plant. Int. J. Greenh. Gas Control 2009, 3, 385–392. [Google Scholar] [CrossRef]
- Ertesvåg, I.S.; Kvamsdal, H.M.; Bolland, O. Exergy analysis of a gas-turbine combined-cycle power plant with precombustion CO2 capture. Energy 2005, 30, 5–39. [Google Scholar] [CrossRef]
- Corradetti, A.; Desideri, U. Analysis of gas-steam combined cycles with natural gas reforming and CO2 capture. J. Eng. Gas Turbines Power 2005, 127, 545–552. [Google Scholar] [CrossRef]
- Lozza, G.; Chiesa, P. Natural gas decarbonization to reduce CO2 emission from combined cycles—Part I: Partial oxidation. J. Eng. Gas Turbines Power 2000, 124, 82–88. [Google Scholar] [CrossRef]
- Zohrabian, A.; Mansouri Majoumerd, M.; Soltanieh, M.; Sattari, S. Techno-economic evaluation of an integrated hydrogen and power co-generation system with CO2 capture. Int. J. Greenh. Gas Control 2016, 44, 94–103. [Google Scholar] [CrossRef]
- Lozza, G.; Chiesa, P. Natural gas decarbonization to reduce CO2 emission from combined cycles—Part II: Steam-Methane reforming. J. Eng. Gas Turbines Power 2000, 124, 89–95. [Google Scholar] [CrossRef]
- Spallina, V.; Gallucci, F.; Romano, M.C.; Van Sint Annaland, M. Pre-combustion packed bed chemical looping (PCCL) technology for efficient H2-rich gas production processes. Chem. Eng. J. 2016, 294, 478–494. [Google Scholar] [CrossRef]
- Kathe, M.V.; Empfield, A.; Na, J.; Blair, E.; Fan, L.-S. Hydrogen production from natural gas using an iron-based chemical looping technology: Thermodynamic simulations and process system analysis. Appl. Energy 2016, 165, 183–201. [Google Scholar] [CrossRef]
- Diglio, G.; Bareschino, P.; Mancusi, E.; Pepe, F. Simulation of hydrogen production through chemical looping reforming process in a packed-bed reactor. Chem. Eng. Res. Des. 2016, 105, 137–151. [Google Scholar] [CrossRef]
- Antzara, A.; Heracleous, E.; Bukur, D.B.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via chemical looping steam methane reforming coupled with in situ CO2 capture. Int. J. Greenh. Gas Control 2015, 32, 115–128. [Google Scholar] [CrossRef]
- Cormos, C.C.; Petrescu, L.; Cormos, A.M. Assessment of hydrogen production systems based on natural gas conversion with carbon capture and storage. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 1081–1086. [Google Scholar]
- Diglio, G.; Hanak, D.P.; Bareschino, P.; Mancusi, E.; Pepe, F.; Montagnaro, F.; Manovic, V. Techno-economic analysis of sorption-enhanced steam methane reforming in a fixed bed reactor network integrated with fuel cell. J. Power Sources 2017, 364, 41–51. [Google Scholar] [CrossRef]
- Diglio, G.; Bareschino, P.; Mancusi, E.; Pepe, F. Novel quasi-autothermal hydrogen production process in a fixed-bed using a chemical looping approach: A numerical study. Int. J. Hydrog. Energy 2017, 42, 15010–15023. [Google Scholar] [CrossRef]
- Newsome, D.S. The water-gas shift reaction. Catal. Rev. 1980, 21, 275–318. [Google Scholar] [CrossRef]
- EBTF (European Tenpin Bowling Federation). European Best Practice Guidelines for Assessment of CO2 Capture Technologies; CESAR—Project 7th FrameWork Programme. Collaborative Project—GA No. 213569; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- AspenPlus. Aspen Plus V8.6 User Guide; Aspen Technology Inc.: Bedford, MA, USA, 2017. [Google Scholar]
- AspenHYSYS. Aspen HYSYS V8.6 User Guide; Aspen Technology Inc.: Bedford, MA, USA, 2017. [Google Scholar]
- Thermoflow. Thermoflow Suite V26 User Guide; Thermoflow Inc.: Southborough, MA, USA, 2017. [Google Scholar]
- Cho, W.C.; Kim, C.G.; Jeong, S.U.; Park, C.S.; Kang, K.S.; Lee, D.Y.; Kim, S.D. Activation and reactivity of iron oxides as oxygen carriers for hydrogen production by chemical looping. Ind. Eng. Chem. Res. 2015, 54, 3091–3100. [Google Scholar] [CrossRef]
- Appl, M. Ammonia: Principles and Industrial Practice; WILEY-VCH Verlag GmbH: Weinheim, Germany, 1999. [Google Scholar]
- Young, D.J.; Zhang, J.; Geers, C.; Schütze, M. Recent advances in understanding metal dusting: A review. Mater. Corros. 2011, 62, 7–28. [Google Scholar] [CrossRef]
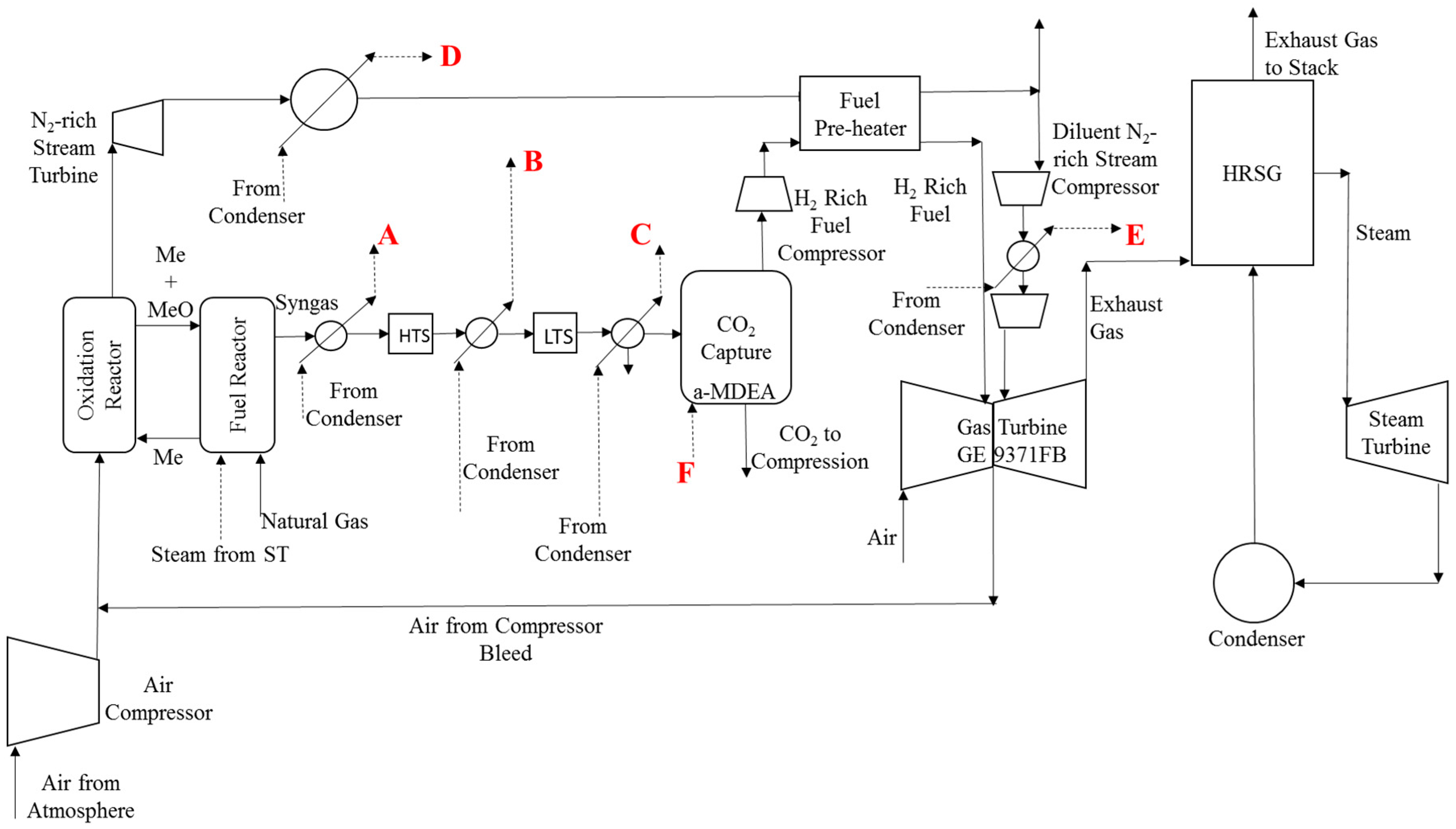
| Steam Type | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Base Case | sat. LP | sat. LP | sat. LP | sat. LP | sat. LP | sat. LP |
| Case 1 | sup. HP | sat. LP | sat. LP | sup. MP | sat. LP | sat. LP |
| Case 2 | sat. HP | sat. LP | sat. LP | sup. MP | sat. LP | sat. LP |
| Case 3 | sat. HP | sat. LP | sat. LP | sat. HP | sat. LP | sat. LP |
| Case 4 | sat. HP | sat. HP | sat. LP | sat. HP | sat. HP | sup. LP |
| Stream | P (bar) | T (°C) | Flow TPH | H2O mol % | CO2 mol % | CH4 mol % | CO mol % | H2 mol % | N2 mol % | O2 mol % | Ar mol % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.01 | 15 | 505 | 1.01 | 0.03 | - | - | - | 77.3 | 20.74 | 0.92 |
| 2 | 18 | 416.7 | 555 | 1.01 | 0.03 | - | - | - | 77.3 | 20.74 | 0.92 |
| 3 | 18 | 416.7 | 1060 | 1.01 | 0.03 | - | - | - | 77.3 | 20.74 | 0.92 |
| 4 | 17.1 | 1199.3 | 816 | 1.22 | 0.03 | - | - | - | 97.52 | - | 1.16 |
| 5 | 1.02 | 134.8 | 261 | 1.22 | 0.03 | - | - | - | 97.52 | - | 1.16 |
| 6 | 1.02 | 134.8 | 555 | 1.22 | 0.03 | - | - | - | 97.52 | - | 1.16 |
| 7 | 16.25 | 984.5 | 575 | 31.09 | 8.31 | 0.17 | 17.87 | 42.34 | 0.22 | - | - |
| 8 | 15.60 | 400 | 575 | 31.09 | 8.31 | 0.17 | 17.87 | 42.34 | 0.22 | - | - |
| 9 | 15.13 | 503.7 | 575 | 21.54 | 17.86 | 0.17 | 8.33 | 51.89 | 0.22 | - | - |
| 10 | 14.83 | 200 | 575 | 21.54 | 17.86 | 0.17 | 8.33 | 51.89 | 0.22 | - | - |
| 11 | 14.38 | 278.4 | 575 | 14.85 | 24.55 | 0.17 | 1.64 | 58.58 | 0.22 | - | - |
| 12 | 13.82 | 50 | 479 | 0.98 | 28.54 | 0.19 | 1.90 | 68.13 | 0.25 | - | - |
| 13 | 110 | 25 | 391 | 0.27 | 99.40 | - | - | 0.32 | - | - | - |
| 14 | 13.34 | 140 | 86 | 0.60 | 1.80 | 0.27 | 2.64 | 94.34 | 0.35 | - | - |
| 15 | 18 | 283 | 166 | 100 | - | - | - | - | - | - | - |
| Pressure | Case (bar) | P5 | P10 | P15 | Base Case (P18) | P25 | P30 |
|---|---|---|---|---|---|---|---|
| Gas Turbine | %—LHV input | 30.3 | 29.4 | 28.9 | 28.5 | 28.6 | 28.7 |
| Steam Turbine | %—LHV input | 14.5 | 14.2 | 14.0 | 13.9 | 13.7 | 13.8 |
| N2-rich Stream Turbine | %—LHV input | 5.3 | 7.2 | 8.1 | 8.5 | 9.2 | 9.5 |
| Additional LP steam Turbine | %—LHV input | 2.7 | 2.8 | 3.0 | 3.1 | 3.3 | 3.4 |
| Diluent N2-rich Stream Compressor | %—LHV input | −4.8 | −4.7 | −4.7 | −4.6 | −4.5 | −4.4 |
| H2 rich fuel Compressor | %—LHV input | −3.1 | −1.9 | −1.3 | −1.0 | −0.6 | −0.4 |
| Air Compressor | %—LHV input | −1.3 | −2.1 | −2.6 | −2.9 | −3.9 | −4.5 |
| Pump for Regenerated Amine | %—LHV input | −0.1 | −0.1 | −0.1 | −0.1 | −0.2 | −0.2 |
| CO2 Compression | %—LHV input | −1.9 | −1.9 | −1.8 | −1.8 | −1.8 | −1.8 |
| Auxiliaries | %—LHV input | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 |
| LHV of NG—Input | GW | 2.11 | 2.14 | 2.14 | 2.14 | 2.14 | 2.13 |
| Net Electrical Efficiency | % | 40.6 | 41.8 | 42.3 | 42.5 | 42.7 | 42.9 |
| CO2 Avoidance | % | 85.1 | 83.8 | 83.1 | 83.7 | 83.2 | 82.8 |
| CO2 Capture | % | 89.4 | 88.6 | 88.2 | 88.6 | 88.2 | 87.8 |
| Energy to compress captured CO2 | kWh/kg CO2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Cases | Units | Base Case | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| Gas Turbine | MW | +611 | +611 | +611 | +611 | +611 |
| Steam Turbine | MW | +298 | +452 | +403 | +403 | +415 |
| N2-rich Stream Turbine | MW | +182 | +182 | +182 | +182 | +182 |
| Additional LP steam Turbine | MW | +67 | - | - | - | - |
| Diluent N2-rich Stream Compressor | MW | −99 | −99 | −99 | −99 | −99 |
| H2 rich fuel Compressor | MW | −21 | −21 | −21 | −21 | −21 |
| Air Compressor | MW | −62 | −62 | −62 | −62 | −62 |
| Pump for Regenerated Amine | MW | −3 | −3 | −3 | −3 | −3 |
| CO2 Compressors and Pump | MW | −40 | −39 | −39 | −40 | −39 |
| Auxiliaries | MW | −25 | −27 | −23 | −22 | −25 |
| Net Electrical Output | MW | 909 | 994 | 949 | 950 | 959 |
| Mass of NG Input | TPH | 166 | 166 | 166 | 166 | 166 |
| LHV of NG—Input | MW | 2139 | 2140 | 2140 | 2139 | 2140 |
| Net Electrical Efficiency | % | 42.5 | 46.5 | 44.4 | 44.4 | 44.8 |
| CO2 Avoidance | % | 83.7 | 83.2 | 83.1 | 83.3 | 83.2 |
| CO2 Capture | % | 88.5 | 88.2 | 88.2 | 88.3 | 88.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, S.M.; Bolland, O.; Amini, S. Analysis of Combined Cycle Power Plants with Chemical Looping Reforming of Natural Gas and Pre-Combustion CO2 Capture. Energies 2018, 11, 147. https://doi.org/10.3390/en11010147
Nazir SM, Bolland O, Amini S. Analysis of Combined Cycle Power Plants with Chemical Looping Reforming of Natural Gas and Pre-Combustion CO2 Capture. Energies. 2018; 11(1):147. https://doi.org/10.3390/en11010147
Chicago/Turabian StyleNazir, Shareq Mohd, Olav Bolland, and Shahriar Amini. 2018. "Analysis of Combined Cycle Power Plants with Chemical Looping Reforming of Natural Gas and Pre-Combustion CO2 Capture" Energies 11, no. 1: 147. https://doi.org/10.3390/en11010147





