Study of Dielectric Breakdown Performance of Transformer Oil Based Magnetic Nanofluids
Abstract
:1. Introduction
1.1. Breakdown Phenomenon in Insulating Fluids
1.2. Ferrofluids in Transformers
2. Materials and Methods
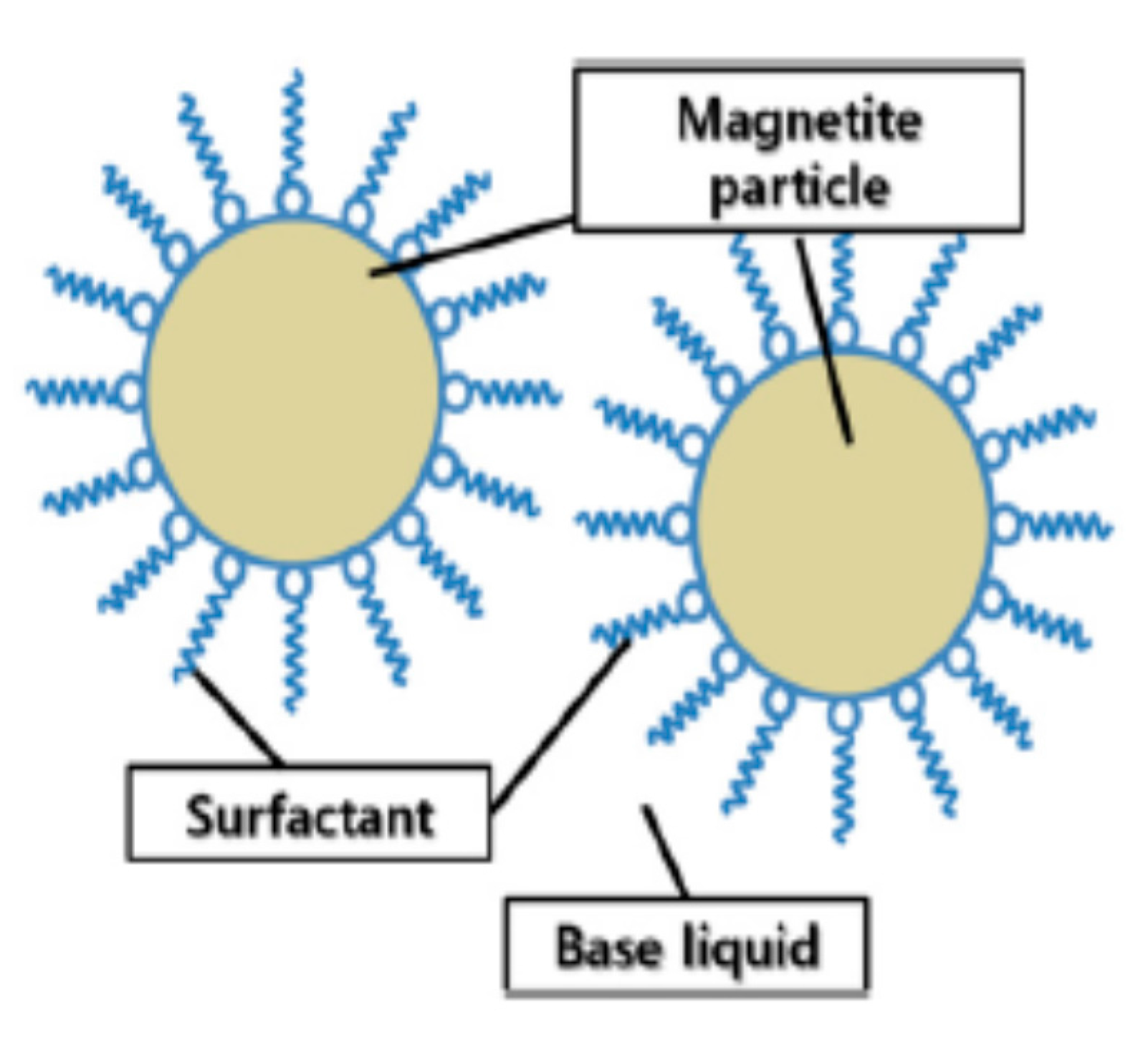
2.1. Formation of Transformer Oil-Based Ferrofluids
(a) Material Selection for Experiment
(b) Nanoparticles Synthesis
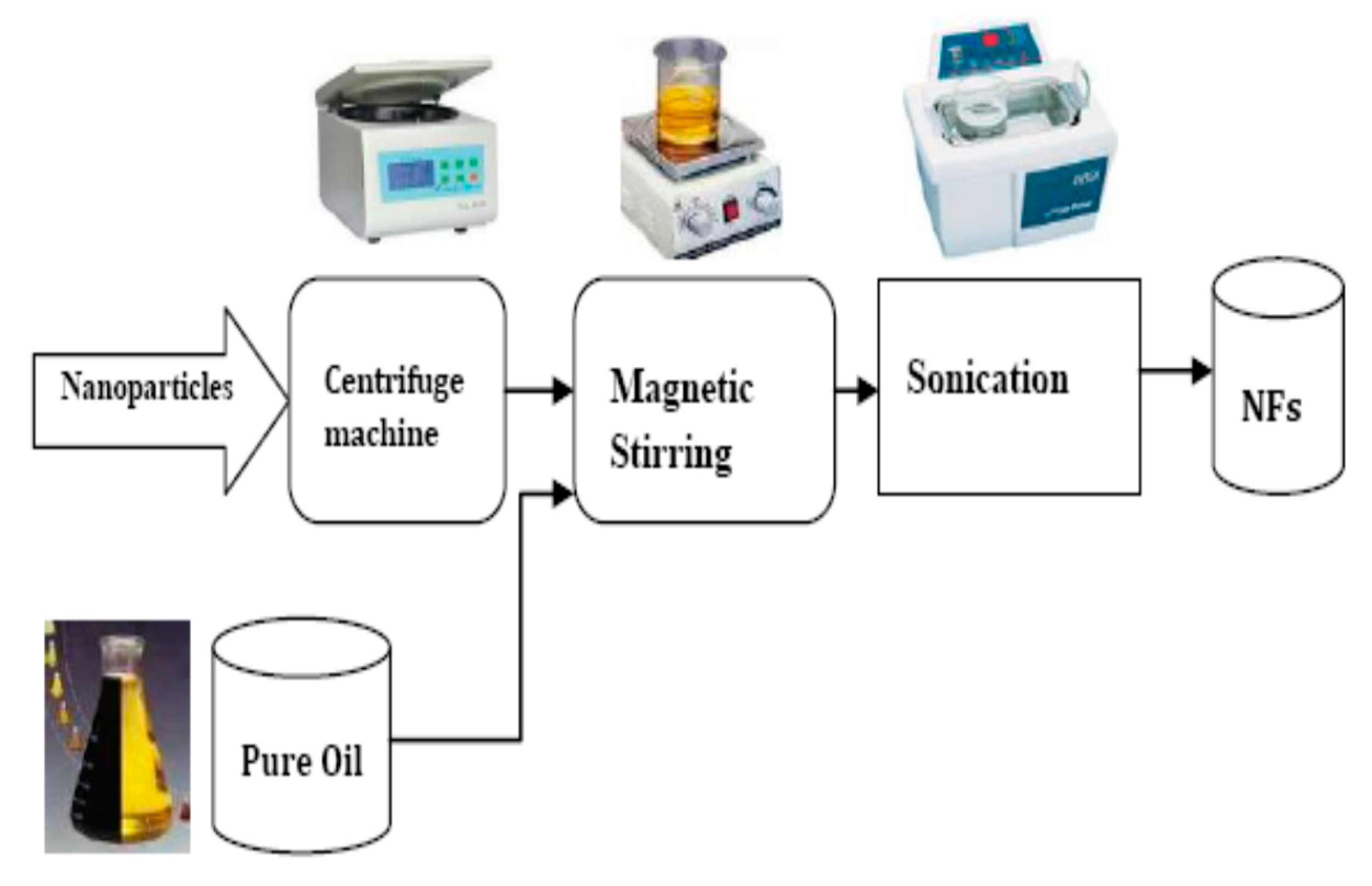
(c) Development of Nanofluids
- (1)
- The prepared nanoparticles (Fe3O4), after surface modification, are added into MO to the required concentrations, and then ultrasonic processes were applied [55].
- (2)
- The developed nanofluids are acquired and put into vacuum drying for approximately 48 h to exclude the influence of gas bubbles and moisture generated while forming NFs.
- (3)
- The moisture content of produced NFs is measured by Rishang coulmetric methods moisture meter JF-5. The flowchart for preparation of nanofluids is shown in Figure 2.
- (4)
2.2. Electrical Properties of Insulation Fluids
3. Results and Discussion
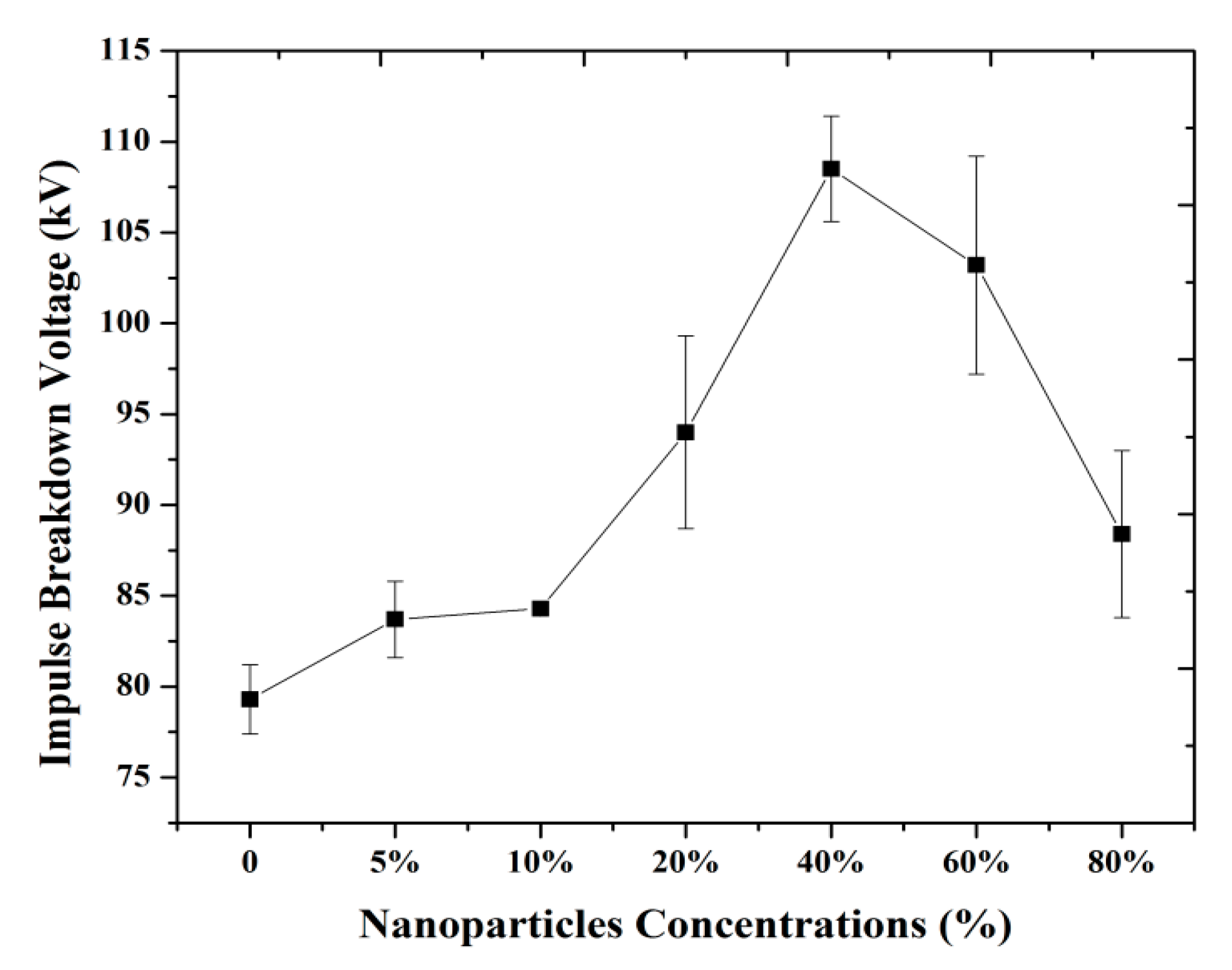
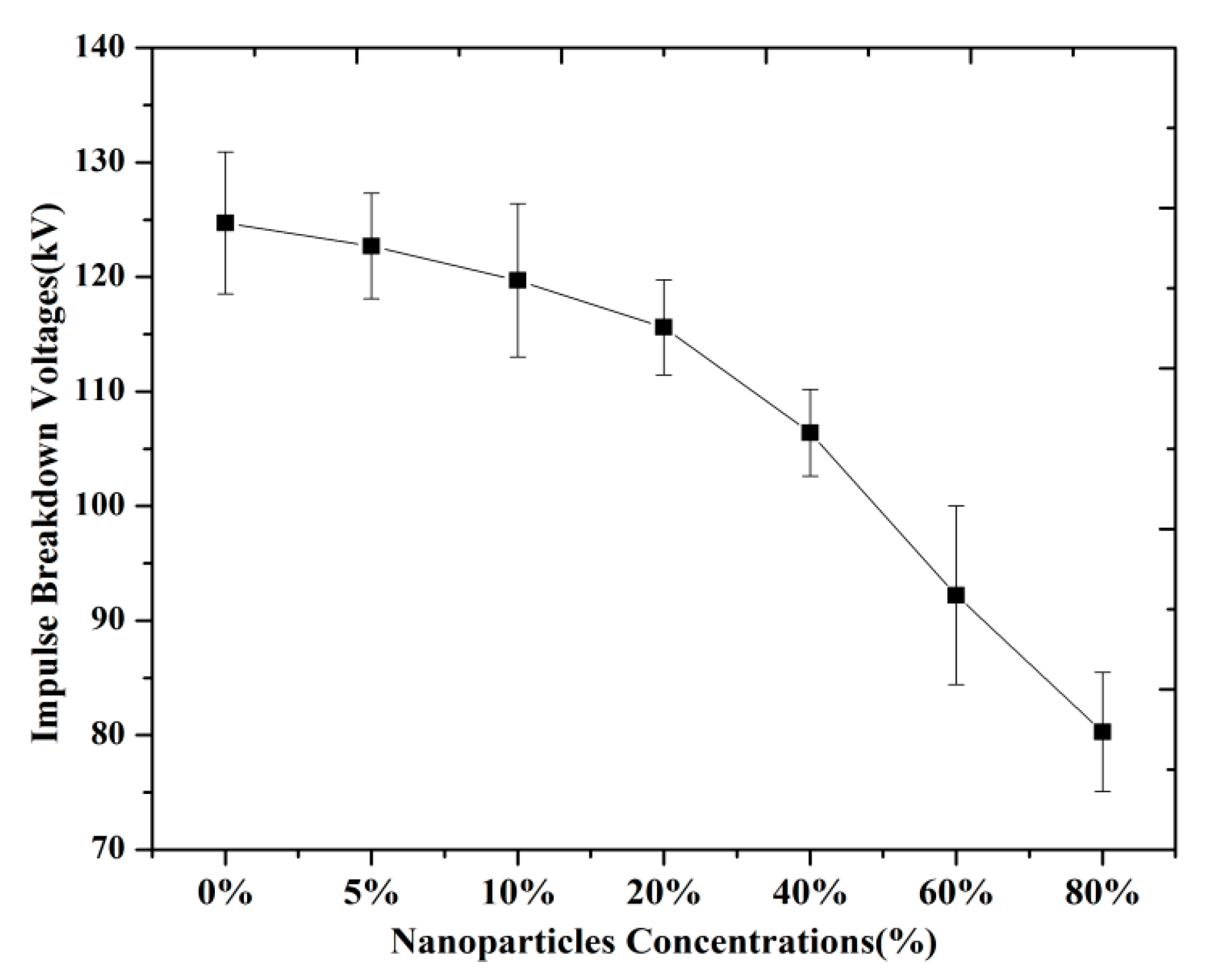
3.1. Breakdown Strength with Different Concentrations of Nanoparticles
(a) Positive LI BD Strength of Magnetic Nanofluids
(b) Negative LI BDV Strength of Magnetic Nanofluids
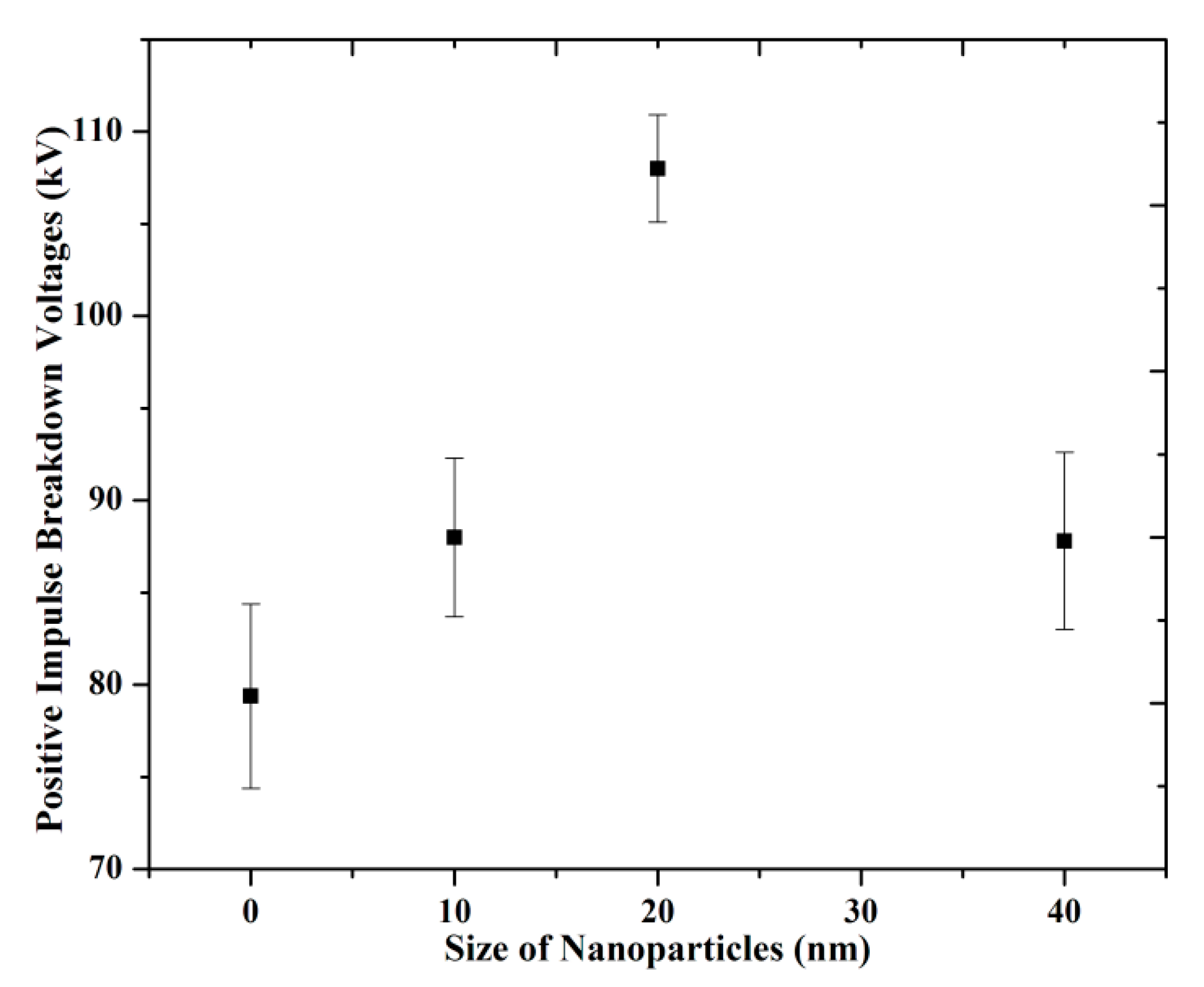
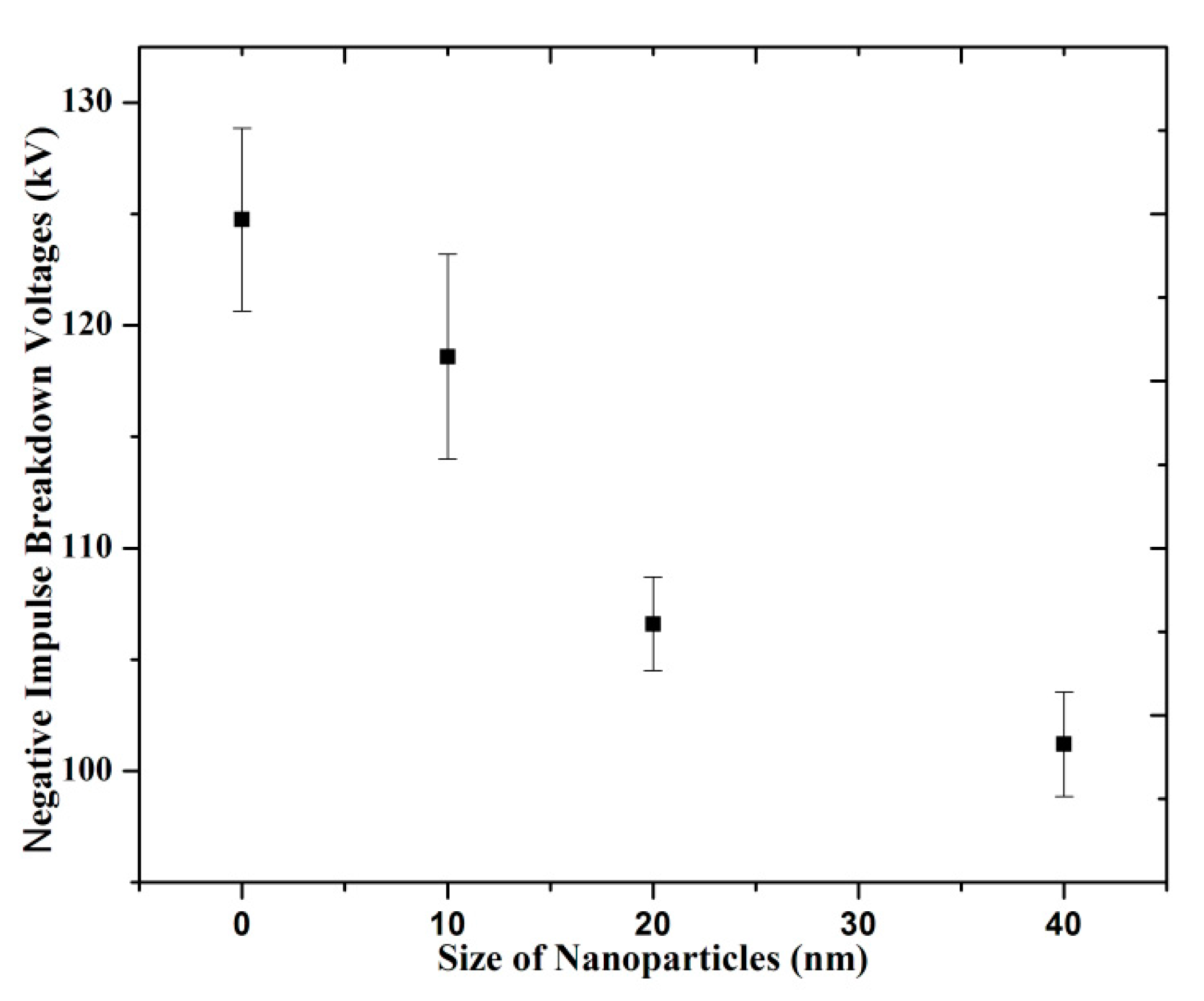
3.2. Breakdown Strength with Different Size of Nanoparticles
(a) Positive LI BDV Strength
(b) Negative LI Breakdown Strength
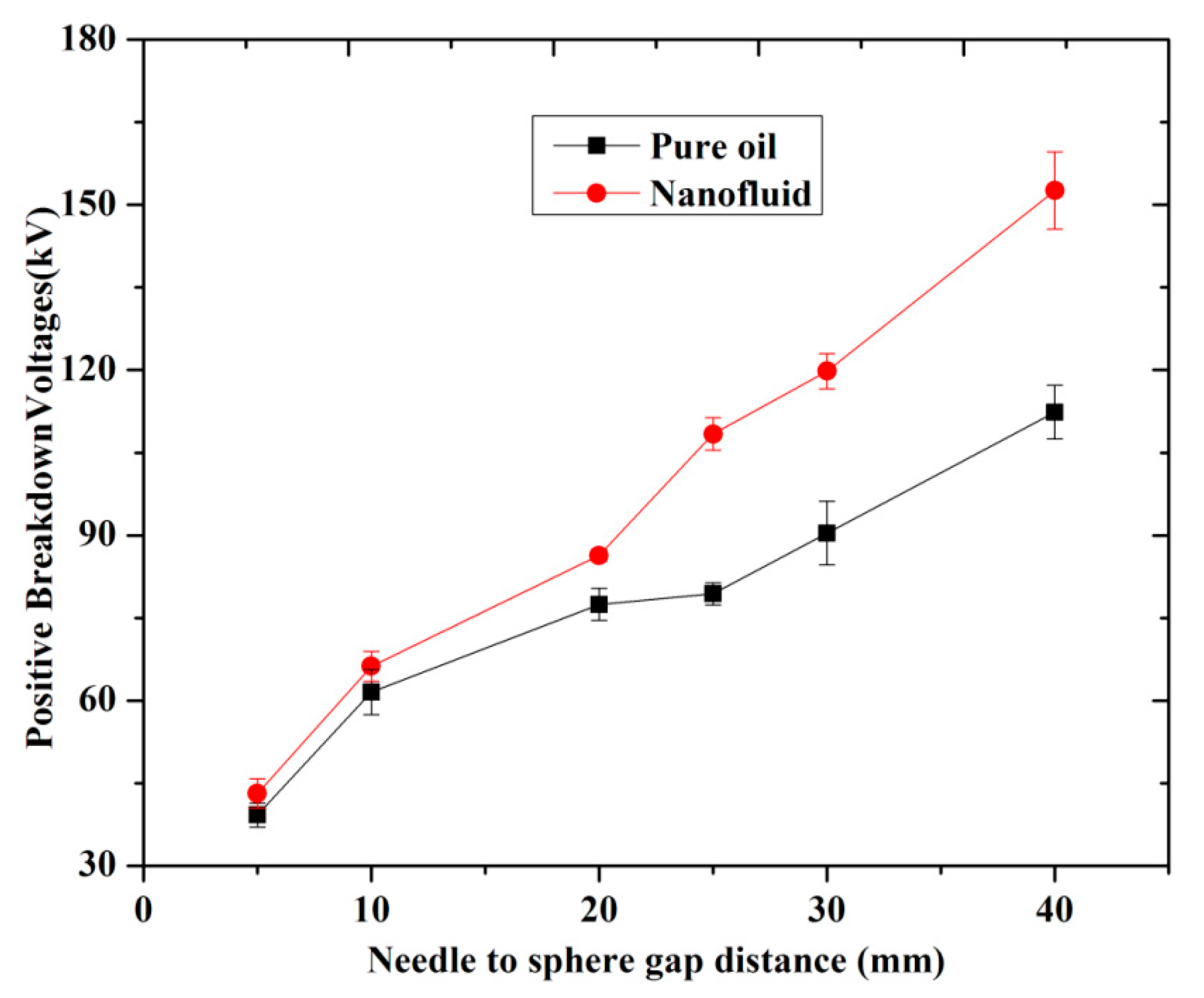
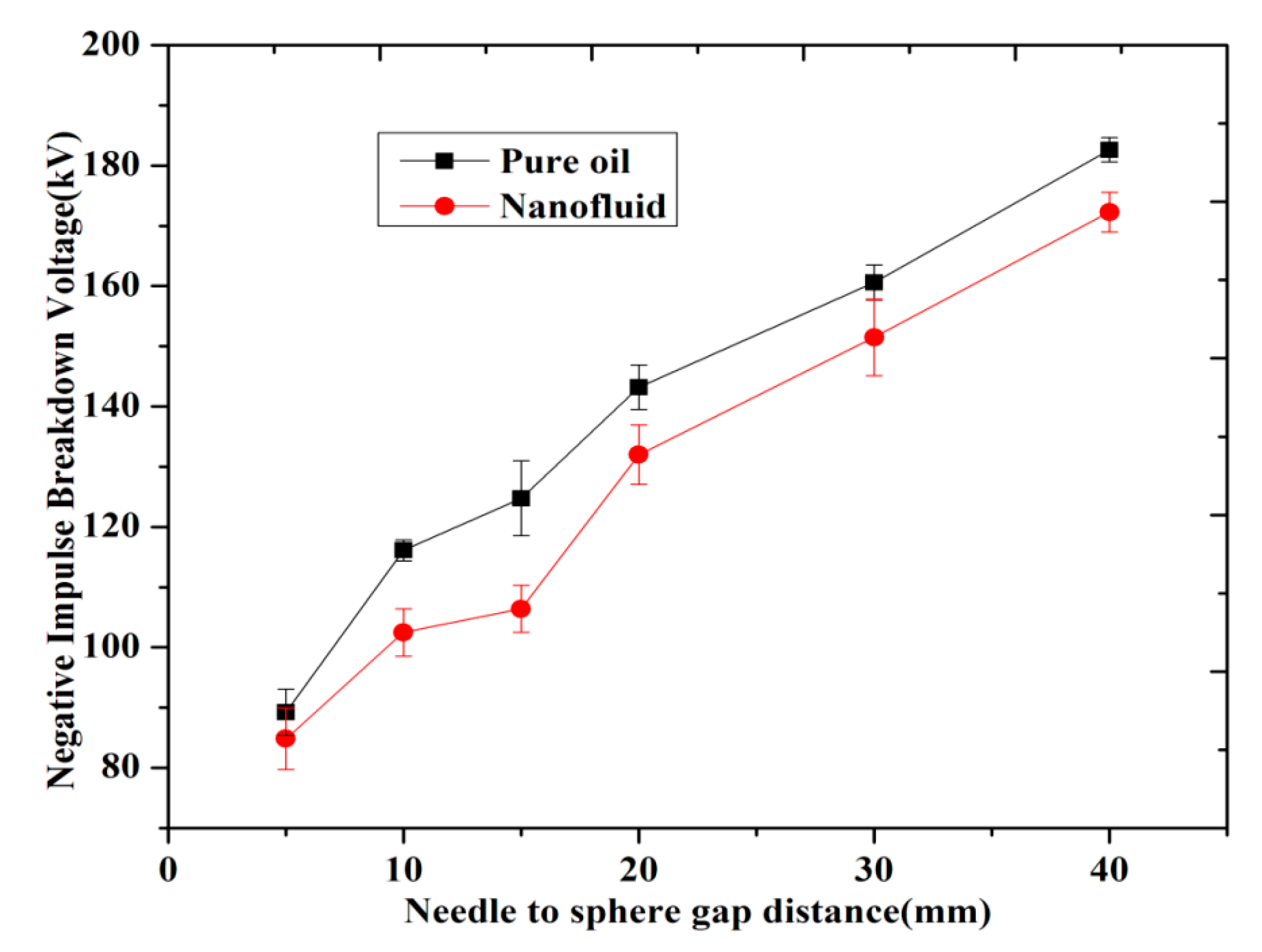
3.3. Breakdown Strength with Different Gap Distance
(a) Positive LI BDV Strength
(b) Negative LI BDV Strength
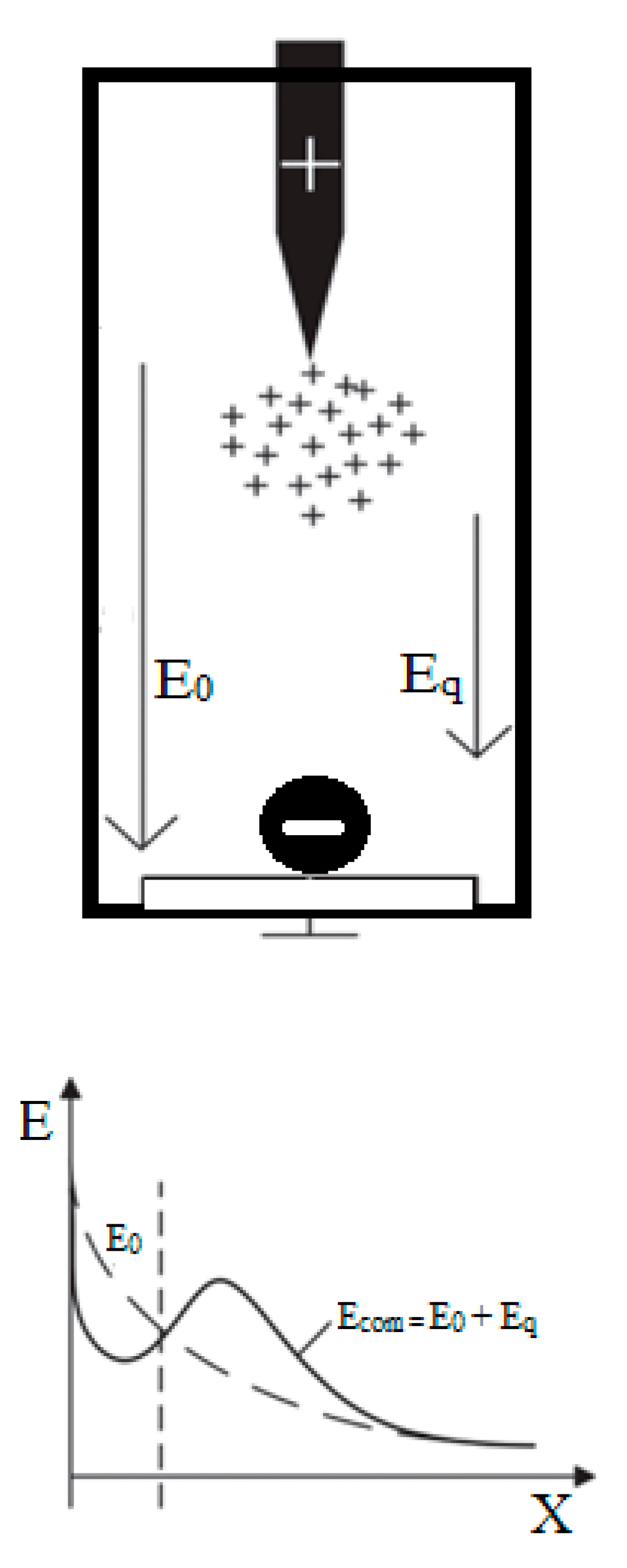
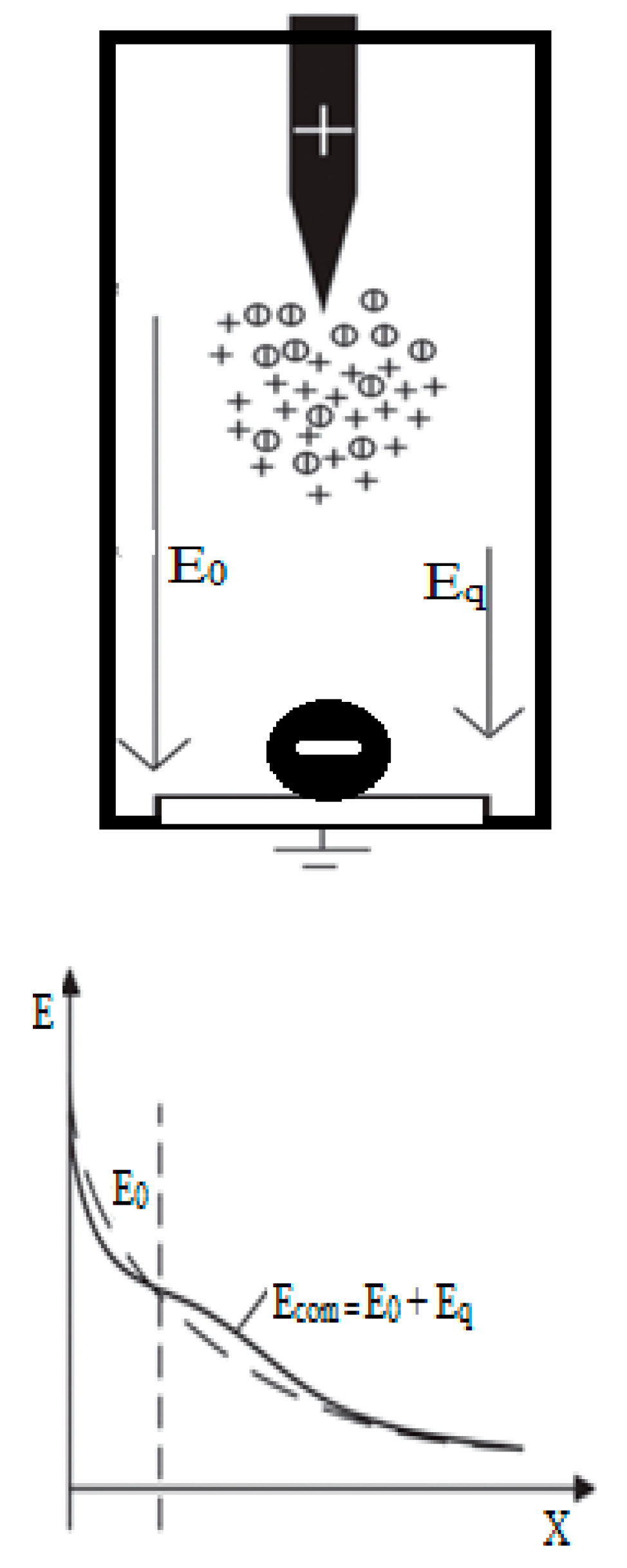
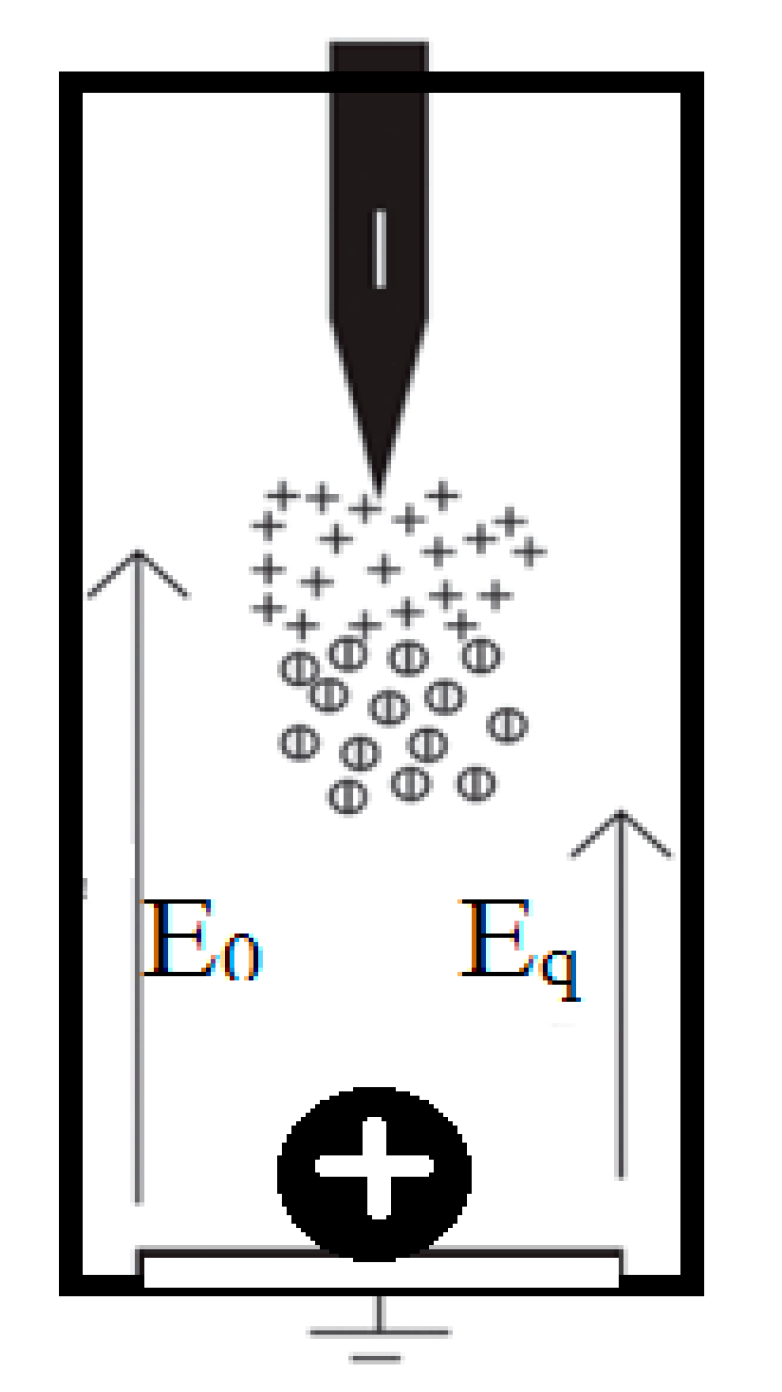
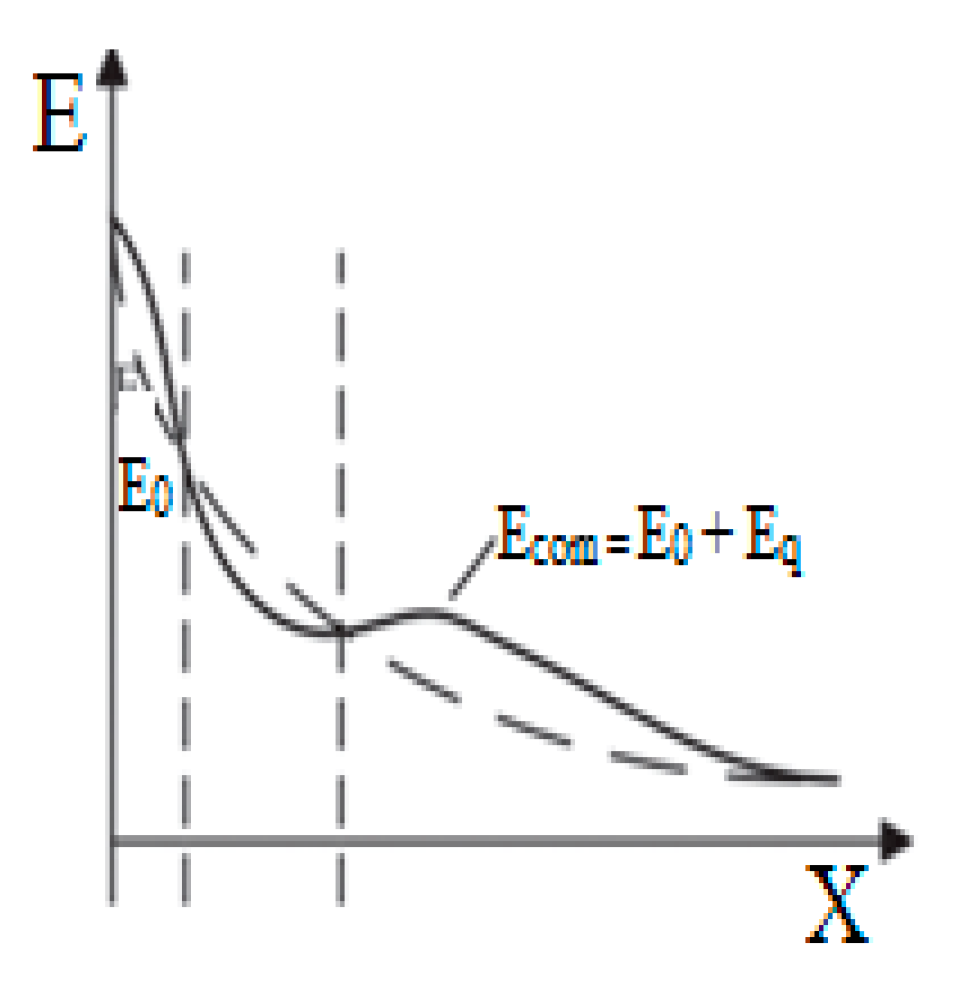
3.4. Breakdown Mechanism
(a) Mechanism of Action of Magnetic Nanoparticles
(b) Analysis of Mechanism Improved Breakdown Properties
4. Research Challenges, Technical Difficulties, and Research Gaps
4.1. Research Challenges
(a) Synthesis of Ferrofluids
(b) Stability of Ferrofluids
(c) Application of Surfactants and Surface Modification
(d) Dielectric Improvement Mechanism
(e) Other Related Issues and Challenges
4.2. Technical Problems and Difficulties
- (1)
- It is complicated to apply magnetic fluids in the existent transformers because of disparity of the electrical specifications, so it is necessary to conduct more research work regarding the application of magnetic fluids in these existing transformers.
- (2)
- More investigative studies are needed to explore more efficient synthesis processes, to decrease the production cost of FFs and to recognize the potential industrial applications of ferrofluids.
- (3)
- A significant research is necessary to curtail the severe human body and environmental impacts of transformer oil-based ferrofluids.
- (4)
- The coating chemistry of nanoparticles have a significant influence on the dielectric features of a ferrofluid, therefore more research work is required to discover a better coating chemistry for magnetic nanoparticles.
4.3. Other Problems and Research Gap
5. Advantages and Disadvantages of Transformer Oil-Based Ferrofluids
5.1. Advantages of Transformer Oil-Based Ferrofluids
- (i)
- The transformer oil-based ferrofluids have better AC and impulse breakdown performance as compared to mineral oils, so it is favorable to be used in high voltage alternating current (HVAC) and high voltage direct current (HVDC) applications.
- (ii)
- The AC BD strength of transformer oil-based ferrofluids is less influenced by moisture as compared to mineral oils, so it is helpful in improving insulation life of transformers.
- (iii)
- The transformer oil-based ferrofluids have better partial discharge characteristics, as compared to mineral oil.
- (iv)
- The transformer oil-based ferrofluids have a better anti-aging characteristic, as compared to MO, so it can improve the operational reliability and lifetime of high voltage transformers.
- (v)
- The transformer oil-based ferrofluids have a higher thermal conductivity than the transformer oil and they are more helpful in cooling the transformers.
5.2. Disadvantages of Transformer Oil-Based Ferrofluids
(a) Agglomeration of Particles
(b) Human Health Problems
(c) Higher Cost of Ferrofluids
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Peterchuck, D.; Pahwa, A. Sensitivity of transformer’s hottest-spot and equivalent aging to selected parameters. IEEE Trans. Power Deliv. 2002, 17, 996–1001. [Google Scholar] [CrossRef]
- Bartley, W.H. Investigating transformer failure. In Proceedings of the Weidmann-ACTI 5th Annual Technical Conference on New Diagnostic Concepts for Better Asset Management, Albuquerque, NM, USA, 13–15 November 2006. [Google Scholar]
- EPRI Portfolio 2007—Transmission Reliability and Performance: 37.002, Transformer Life Extension. Available online: http://www.epri.com/portfolio/ (accessed on 17 May 2016).
- Chiesa, M.; Das, S.K. Experimental investigation of the dielectric and cooling performance of colloidal suspensions in insulating media. Coll. Surf. A Physicochem. Eng. Asp. 2009, 335, 88–97. [Google Scholar] [CrossRef]
- Rouse, T.O. Mineral insulating oil in transformers. IEEE Electr. Insul. Mag. 1998, 14, 6–16. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Aging of oil-impregnated paper in power transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Li, C.R. A Review on Properties, Opportunities, and Challenges of Transformer Oil-Based Nanofluids. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Segal, M.V.; Raj, K. An investigation of Power Transformer Cooling with magnetic Fluid. Indian J. Eng. Mater. Sci. 1998, 5, 416–422. [Google Scholar]
- Kopcansky, P.; Tomco, L.; Marton, K.; Koneracka, M.; Timko, M.; Potocova, I. The DC dielectric breakdown strength of magnetic fluids based on transformer oil. J. Magn. Magn. Mater. 2005, 289, 415–418. [Google Scholar] [CrossRef]
- Erdman, H.G. Electrical Insulating Oils STP 998; ASTM Publ. Co.: Philadelphia, PA, USA, 1996. [Google Scholar]
- Choi, S. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress & Exposition, San Francisco, CA, USA, 12–17 November 1995; Volume 231, p. 99. [Google Scholar]
- Rosensweig, R.E. Ferrohydrodynamics; Cambridge Univeersity Press: Cambridge, UK, 1985; p. 7. [Google Scholar]
- Das, S.K.; Choi, S.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; John Wiley & Sons, Inc.: Madras, Indian, 2008; p. 5. [Google Scholar]
- Khurshid, H.; Nemati, J.A.Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiaran, J.M.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Parchur, A.K.; Ansari, A.A.; Singh, B.P.; Hasan, T.N.; Syed, N.A.; Rai, S.B.; Ningthoujam, R.S. Ultrafast vibrational dynamics and spectroscopy of a siloxane self-assembled monolayer. J. Chem. Phys. 2011, 134, 084701. [Google Scholar]
- Wu, Z.S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Mullen, K. Nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl. Mater. Interface 2010, 2, 3201. [Google Scholar] [CrossRef] [PubMed]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Heat transfer enhancement by magnetic nanofluids—A review. Renew. Sustain. Energy Rev. 2013, 21, 548–561. [Google Scholar] [CrossRef]
- Odenbach, S. Magnetoviscous Effects in Ferrofluids; Springer: Berlin, Germany, 2002. [Google Scholar]
- Ayoub, N.Y.; Bradbury, A.; Chantrell, R.W.; Popplewell, J. A ‘pair orientation’ model of the magnetodielectric anisotropy in ferrofluids. J. Magn. Magn. Mater. 1987, 65, 185. [Google Scholar] [CrossRef]
- Kopcansky, P.; Cernak, J.; Macko, P.; Spisak, D.; Marton, K. Dielectric behaviour of mineral-oil-based magnetic fluids-the cluster model. J. Phys. Appl. Phys. 1989, 22, 1410–1412. [Google Scholar] [CrossRef]
- Yusuf, N.A.; Shobaki, J.; Abu-Safia, H.; Abu-Aljarayesh, I. Magneto-dielectric anisotropy in magnetic fluids determined from magneto-optical measurements. J. Magn. Magn. Mater. 1995, 149, 373. [Google Scholar] [CrossRef]
- Segal, V.; Rabinovich, A.; Nattrass, D.; Raj, K.; Nunes, A. Experimental study of magnetic colloidal fluids behavior in power transformers. J. Magn. Magn. Mater. 2000, 513, 215–216. [Google Scholar] [CrossRef]
- Segal, V.; Nattrass, D.; Raj, K.; Leonard, D. Accelerated thermal aging of petroleum-based ferrofluids. J. Magn. Magn. Mater. 1999, 70, 201. [Google Scholar] [CrossRef]
- Segal, V.; Hjortsberg, A.; Rabinovich, A.; Nattrass, D.; Raj, K. AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles. In Proceedings of the Conference Record of the 1998 IEEE International Symposium on Electrical Insulation, Arlington, VA, USA, 7–10 June 1998; pp. 619–622. [Google Scholar]
- Philip, J.; Shima, P.D.; Raj, B. Enhancement of thermal conductivity in magnetite based nanofluid due to chainlike structures. Appl. Phys. Lett. 2007, 91, 203108. [Google Scholar] [CrossRef]
- Cha, H.G.; Kim, C.W.; Kang, S.W.; Kim, B.K.; Kang, Y.S. Preparation and characterization of the magnetic fluid of trimethoxyhexadecylsilane-coated Fe3O4 nanoparticles. J. Phys. Chem. 2010, 114, 9802–9807. [Google Scholar]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magen. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Sima, W.X.; Cao, X.F.; Yang, Q. Preparation of three transformer oil-based nanofluids and comparison of their impulse breakdown characteristics. Nanosci. Nanotechnol. Lett. 2014, 6, 250–256. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, W.H.; Lee, S.H.; Lee, S. Positive and negative effects of dielectric breakdown in transformer oil based magnetic fluids. Mater. Res. Bull. 2012, 47, 2984–2987. [Google Scholar] [CrossRef]
- Viali, W.R.; Alcantara, G.B.; Sartoratto, P.P.C.; Soler, M.A.G.; Mosiniewiczszablewska, E.; Andrzejewski, B. Investigation of the molecular surface coating on the stability of insulating magnetic oils. J. Phys. Chem. C 2010, 114, 179–188. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Zhang, S.N.; Du, Y.F.; Chen, M.T.; Li, C.R. Effect of Oleic Acid Surface Modification on Dispersibility of TiO2 Nanoparticles in Transformer Oils. J. Inorg. Mater. 2013, 28, 594–598. [Google Scholar] [CrossRef]
- Thorat, N.D.; Khot, V.M.; Salunkhe, A.B.; Prasad, A.I.; Ningthoujam, R.S.; Pawar, S.H. Surface functionalized lsmo nanoparticles with improved colloidal stability for hyperthermia applications. J. Phys. D Appl. Phys. 2013, 46, 105003–105013. [Google Scholar] [CrossRef]
- Veriansyah, B.; Chun, M.S.; Kim, J. Surface-modified cerium oxide nanoparticles synthesized continuously in supercritical methanol: Study of dispersion stability in ethylene glycol medium. Chem. Eng. J. 2011, 168, 1346–1351. [Google Scholar] [CrossRef]
- Zhou, J.; Song, B.; Zhao, G.; Han, G. Effects of acid on the microstructures and properties of three-dimensional TiO2, hierarchical structures by solvothermal method. Nanoscale Res. Lett. 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.F.; Lv, Y.Z.; Wang, F.C.; Li, X.X.; Li, C.R. Effect of TiO2 nanoparticles on the breakdown strength of transformer oil. In Proceedings of the Conference Record of the 2010 IEEE International Symposium on Electrical Insulation (ISEI’10), San Diego, CA, USA, 6–9 June 2010; pp. 1–3. [Google Scholar]
- O’Sullivan, F.; Hwang, J.G.; Zahn, M.; Hjortstam, O.; Pettersson, L.; Liu, R.; Biller, P. A model for the initiation and propagation of positive streamers in transformer oil. In Proceedings of the IEEE International Symposium on Electrical Insulation (ISEI’08), Vancouver, BC, Canada, 9–12 June 2008; pp. 210–214. [Google Scholar]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.A.; Hjortstam, O.; Liu, R. Electron scavenging by conductive nanoparticles in oil insulated power transformers. In Proceedings of the Electrostatics Joint Conference, Boston, MA, USA, 16–18 June 2009. [Google Scholar]
- Blaise, G. The space charge physics and the breakdown process. J. Appl. Phys. 1995, 77, 2916–2927. [Google Scholar] [CrossRef]
- Wang, X.; Yoshimura, N.; Murata, K.; Tanaka, Y.; Tanaka, T. Space-charge characteristics in polyethylene. J. Appl. Phys. 1998, 84, 1546. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Li, C.R.; Chen, M.T.; Zhong, Y.X. Effect of nanoparticles on electrical characteristics of transformer oil-based nanofluids impregnated pressboard. J. Appl. Phys. 2012, 111, 124322. [Google Scholar] [CrossRef]
- Sartoratto, P.P.C.; Neto, A.V.S.; Lima, E.C.D.; de Sá, A.L.C.R.; Morais, P.C. Preparation and electrical properties of oil-based magnetic fluids. J. Appl. Phys. 2005, 97, 10Q917. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zou, P.; Grzybowski, S.; Zahn, M. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, F.; Sima, W.; Zahn, M. Space charge inhibition effect of nano-Fe3O4 on improvement of impulse breakdown voltage of transformer oil based on improved Kerr optic measurements. AIP Adv. 2015, 5, 097207. [Google Scholar] [CrossRef]
- International Council on Large Electric Systems (CIGRE). Effect of Particles on Dielectric Strength; Working Group of 17 Committee: Paris, France, 2002. [Google Scholar]
- Lv, Y.Z.; Li, X.X.; Du, Y.F. Preparation and breakdown strength of TiO2 fluids based on transformer oil. In Annual Report Conference on Electrical Insulation and Dielectric Phenomena; IEEE: West Lafayette, IN, USA, 2010; pp. 1–3. [Google Scholar]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Selim, K.M.K.; Ha, Y.S.; Kim, S.J.; Chang, Y.; Kim, T.J.; Lee, G.H.; Kang, I.K. Surface modification of magnetite nanoparticles using lactobionic acid and their interaction with hepatocytes. Biomaterials 2007, 28, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Bakandritsos, A.; Georgakilas, V.; Petridis, D. Surface Modification of ultrafine magnetic iron oxide particles. Chem. Mater. 2002, 14, 3226–3228. [Google Scholar] [CrossRef]
- Tsai, T.; Kuo, L.; Chen, P.; Lee, D.; Yang, C. Applications of ferronanofluid on a micro-transformer. Sensors 2010, 10, 8161–8172. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Li, C.; Wang, X.; Yu, D.; Peng, Q.; Li, Y. Magnetic Monodisperse Fe3O4 Nanoparticles. Cryst. Growth Des. 2005, 5, 391–393. [Google Scholar] [CrossRef]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.; Pettersson, L.; Hjortstam, O.; Liu, R. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Li, C.R.; Chen, M.T.; Zhong, Y.X.; Zhou, J.Q.; Li, X.X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770. [Google Scholar]
- OECD: Principles and Strategies Related to the Testing of Degradation of Organic Chemicals. Available online: http://www.oecd.org/chemicalsafety/testing/5598432.pdf (accessed on 1 April 2005).
- Methods for the Determination of the Lighting Imulse Breakdown Voltage of Insulating Liquids; IEC Standard 60897; IEC: Geneva, Switzerland, 1987.
- Metwally, I.A. Influence of Specimen Size on the Dielectric Strength of Transformer Oil. IEEE Trans. Electr. Insul. 1977, EI-12, 281–292. [Google Scholar]
- Maksjejewski, L.J.; Tropper, H. Some Factors Affecting the Measurement of the Electric Strength of Organic Liquids. Proc. IEEE 1954, 101, 183–190. [Google Scholar]
- Bartinikas, R. Electrical Insulating Liquids; ASTM Publication: Philadelphia, PA, USA, 1994. [Google Scholar]
- Zahn, M.; Ohki, Y.; Rhoads, K.; Lagasse, M.; Matsuzawa, H. Electro-optic charge injection and tramsport measurements in highly purified water and water/ethylene glycol mixtures. IEEE Trans. Dielectr. Electr. Insul. 1985, 20, 199. [Google Scholar] [CrossRef]
- Zhang, X.; Zahn, M. Kerr electro-optic field mapping study of the effect of charge injection on the impulse breakdown strength of transformer oil. Appl. Phys. Lett. 2013, 103, 162906. [Google Scholar] [CrossRef]
- Zahn, M.; Ohki, Y.; Fenneman, D.B.; Gripshover, R.J.; Gehman, V.H. Dielectric properties of water and water/ethylene glycol mixtures for use in pulsed power system design. Proc. IEEE 1986, 74, 1182. [Google Scholar] [CrossRef]
- Lewis, T.J. Interfaces: Nanometric dielectrics. J. Phys. D Appl. Phys. 2005, 38, 202. [Google Scholar] [CrossRef]
- Smith, R.C.; Liang, C.; Landry, M. The mechanisms leading to the useful electrical properties of polymer nanodielectrics. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 187. [Google Scholar] [CrossRef]
- Danikas, M.G. On two nanocomposite models: Differences, similarities and interpretational possibilities regarding Tsagaropoulos’ model and Tanaka’s Model. J. Electr. Eng. 2010, 61, 241. [Google Scholar] [CrossRef]
- Du, Y.F.; Lv, Y.Z.; Li, C.R.; Chen, M.T.; Zhou, J.Q.; Li, X.X.; Zhou, Y.; Zhong, Y.X. Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Coll. Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.; Gillies, G.; Papastavrou, G.; Borkovec, A. Attractive and repulsive electrostatic forces between positively charged latex particles in the presence of anionic linear polyelectrolytes. J. Phys. Chem. B 2010, 114, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Kudelcik, J.; Bury, P.; Kopcansky, P.; Timko, M. Dielectric breakdown in mineral oil ITO100 based magnetic fluid. Phys. Proced. 2010, 9, 78–81. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef]
- Wang, L.S.; Hong, R.Y. Synthesis, surface modification and characterization of nanoparticles. In Advances in Nanocomposites—Synthesis, Characterization and Industrial Applications; InTech: Suzhou, China, 2011; pp. 289–323. [Google Scholar]
- Peppas, G.D.; Bakandritsos, A.; Charalampakos, V.P.; Pyrgioti, E.C.; Tucek, J.; Zboril, R.; Gonos, I.F. Ultrastable Natural Ester-Based Nanofluids for High Voltage Insulation Applications. ACS Appl. Mater. Interface 2016, 8, 25202–25209. [Google Scholar] [CrossRef] [PubMed]
- Tijerina, J.T.; Narayanan, T.N.; Gao, G.; Rohde, M.; Tsentalovich, D.A.; Pasquali, M.; Ajayan, P.M. Electrically Insulating Thermal Nano-Oils Using 2D Fillers. ACS Nano 2012, 6, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.G.; O’Sullivan, F.; Zahn, M.; Hjortstam, O.; Pettersson, L.A.A.; Liu, R. Modeling of streamer propagation in transformer oil-based nanofluids. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP’08), Quebec City, QC, Canada, 26–29 October 2008; pp. 361–366. [Google Scholar]
- Mergos, J.A.; Athanassopoulou, M.D.; Argyropoulos, T.G.; Dervos, C.T. Dielectric properties of nanopowder dispersions in paraffin oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1502–1507. [Google Scholar] [CrossRef]
- Krajnik, P.; Pusavec, F.; Rashid, A. Nanofluids: Properties, applications and sustainability aspects in materials processing technologies. In Advances in Sustainable Manufacturing: Proceedings of the 8th Global Conference on Sustainable Manufacturing; Springer: Berlin, Germany, 2011; pp. 107–113. [Google Scholar]
- Kochetov, R.; Morshuis, P.H.F.; Smit, J.J.; Andritsch, T.; Krivda, A. Precautionary remarks regarding synthesis of nanocomposites. In Proceedings of the 32nd Electrical Insulation Conference (EIC’14), Philadelphia, PA, USA, 8–11 June 2014; pp. 51–54. [Google Scholar]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schürch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.W.; Kulur, V.N.; King, N.C.; von Turkovich, B.F. An experimental investigation of air quality in wet and dry turning. CIRP Ann. Manuf. Technol. 2000, 49, 61–64. [Google Scholar] [CrossRef]
- Baroli, B.; Ennas, M.G.; Loffredo, F.; Isola, M.; Pinna, R.; López-Quintela, M.A. Penetration of metallic nanoparticles in human full-thickness skin. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef] [PubMed]

| Samples | Gap Distance (mm) (+/−) | BDV (kV) | Time to BD (µs) | Streamer Velocity (km/s) | Remarks | |||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||
| U-60 a | 25.4/25.4 | 86 | 170 | 12 | 27 | 2.12 | 0.94 | Ref. [9] |
| U-60-Fe3O4 nanofluid | 25.4/25.4 | 157 | 154 | 26 | 15 | 0.98 | 1.70 | |
| Nytro b | 25.4/25.4 | 88 | 177 | 16 | 23 | 1.58 | 1.10 | |
| Nytro-Fe3O4 nanofluid | 25.4/25.4 | 156 | 173 | 25 | 17 | 1.016 | 1.49 | |
| U-60 a | 55/55 | 225 | 340 | 25 | 28 | 2.2 | 1.96 | |
| U-60-Fe3O4 nanofluid | 55/55 | 390 | 321 | 46 | 32 | 1.19 | 1.71 | |
| Mineral oil | 5 | 51.84 | 83.10 | 4.44 | 4.02 | 1.126 | 1.244 | Ref. [30] |
| Fe3O4 nanofluid | 5 | 62.86 | 78.54 | 5.24 | 3.54 | 0.954 | 1.412 | |
| Vegetable oil | 15/15 | 73.9 | 83.8 | 9.9 | 11.1 | 1.51 | 1.35 | Ref. [45] |
| Fe3O4 nanofluid | 15/15 | 101.5 | 93.7 | 12.0 | 12.7 | 1.25 | 1.18 | |
| Mineral oil | 3 | 99.1 | - | 306 | - | 0.0098 | - | Ref. [46] |
| Fe3O4 nanofluid | 3 | 110.2 | - | 188 | - | 0.0159 | - | |
| Properties | Transformer Oil | Magnetic Nanoparticles (Fe3O4) |
|---|---|---|
| Conductivity (S/m) [37,54] | 10−12 | 104–105 |
| Relaxation time (s) [37,54] | - | 7.47 × 10−14 |
| Relative permittivity [37,54] | 2.2 | 80 |
| Density (g/cm3) | 0.89 | 5.18 |
| Surface modification | - | Oleic Acid |
| Material type | Dielectric | Conductive |
| NPs Loadings (% w/v) | Time to BD( µs) | Mean Streamer Velocity (km/s) | Improvement in BDV (%) |
|---|---|---|---|
| 0 | 12.87 | 1.94 | 0 |
| 5 | 14.82 | 1.68 | 5.40 |
| 10 | 18.32 | 1.36 | 6.21 |
| 20 | 21.19 | 1.17 | 18.38 |
| 40 | 25.33 | 0.98 | 36.64 |
| 60 | 25.08 | 0.99 | 29.95 |
| 80 | 21.98 | 1.13 | 11.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Rafiq, M.; Li, C.; Shan, B. Study of Dielectric Breakdown Performance of Transformer Oil Based Magnetic Nanofluids. Energies 2017, 10, 1025. https://doi.org/10.3390/en10071025
Lv Y, Rafiq M, Li C, Shan B. Study of Dielectric Breakdown Performance of Transformer Oil Based Magnetic Nanofluids. Energies. 2017; 10(7):1025. https://doi.org/10.3390/en10071025
Chicago/Turabian StyleLv, Yuzhen, Muhammad Rafiq, Chengrong Li, and Bingliang Shan. 2017. "Study of Dielectric Breakdown Performance of Transformer Oil Based Magnetic Nanofluids" Energies 10, no. 7: 1025. https://doi.org/10.3390/en10071025





