The Investigation of High Quality PEDOT:PSS Film by Multilayer-Processing and Acid Treatment
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Azzopardi, B.; Emmott, C.J.M.; Urbina, A.; Krebs, F.C.; Mutale, J.; Nelson, J. Economic assessment of solar electricity production from organic-based photovoltaic modules in a domestic environment. Energy Environ. Sci. 2011, 4, 3741–3753. [Google Scholar] [CrossRef]
- Cui, J.; Wang, A.; Edleman, N.L.; Ni, J.; Lee, P.; Armstrong, N.R.; Marks, T.J. Indium Tin oxide alternatives—High work function transparent conducting oxides as anodes for organic light-emitting diodes. Adv. Mater. 2001, 13, 1476–1480. [Google Scholar] [CrossRef]
- Park, S.; Tark, S.J.; Kim, D. Effect of sorbitol doping in PEDOT:PSS on the electrical performance of organic photovoltaic devices. Curr. Appl. Phys. 2011, 11, 1299–1301. [Google Scholar] [CrossRef]
- Shin, D.; Kim, T.; Ahn, B.T.; Han, S.M. Solution-processed Ag nanowires + PEDOT:PSS hybrid electrode for Cu(In,Ga)Se2 thin-film solar cells. ACS Appl. Mater. Interfaces 2015, 7, 13557–13563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Yang, J.; Fu, Y.; Xie, Z.; Zhang, S.; Wang, L. Performance enhancement of polymer light-emitting diodes by using ultrathin fluorinated polyimide modifying the surface of poly(3,4-ethylene dioxythiophene):poly(styrenesulfonate). J. Phys. Chem. C 2009, 113, 7898–7903. [Google Scholar] [CrossRef]
- Latessa, G.; Brunetti, F.; Reale, A.; Saggio, G.; Di Carlo, A. Piezoresistive behaviour of flexible PEDOT:PSS based sensors. Sens. Actuators B Chem. 2009, 139, 304–309. [Google Scholar] [CrossRef]
- Onorato, A.; Invernale, M.A.; Berghorn, I.D.; Pavlik, C.; Sotzing, G.A.; Smith, M.B. Enhanced conductivity in sorbitol-treated PEDOT–PSS. Observation of an in situ cyclodehydration reaction. Synth. Met. 2010, 160, 2284–2289. [Google Scholar] [CrossRef]
- Ke, J.-C.; Wang, Y.-H.; Chen, K.-L.; Huang, P.-H.; Huang, C.-J. Study of small molecule organic solar cells performance based on boron subphthalocyanine chloride and C60. Int. J. Photoenergy 2013, 2013, 803126. [Google Scholar] [CrossRef]
- Huang, P.-H.; Huang, C.-J.; Chen, K.-L.; Ke, J.-C.; Wang, Y.-H.; Kang, C.-C. Improved reliability of small molecule organic solar cells by double anode buffer layers. J. Nanomater. 2014, 2014, 741761. [Google Scholar] [CrossRef]
- Huang, P.-H.; Wang, Y.-H.; Ke, J.-C.; Huang, C.-J. Investigation of various active layers for their performance on organic solar cells. Materials 2016, 9, 667. [Google Scholar] [CrossRef]
- Havare, A.K.; Can, M.; Demic, S.; Kus, M.; Icli, S. The performance of OLEDs based on sorbitol doped PEDOT:PSS. Synth. Met. 2012, 161, 2734–2738. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; de Kok, M.M.; Vinken, E.; Maturova, K.; Janssen, R.A.J. Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chen, K.-L.; Tsao, Y.-J.; Chou, D.-W.; Chen, W.-R.; Meen, T.-H. Study of solvent-doped PEDOT:PSS layer on small molecule organic solar cells. Synth. Met. 2013, 164, 38–41. [Google Scholar] [CrossRef]
- Huang, J.-H.; Kekuda, D.; Chu, C.-W.; Ho, K.-C. Electrochemical characterization of the solvent-enhanced conductivity of poly(3,4-ethylenedioxythiophene) and its application in polymer solar cells. J. Mater. Chem. 2009, 19, 3704–3712. [Google Scholar] [CrossRef]
- Cruz-Cruz, I.; Reyes-Reyes, M.; Aguilar-Frutis, M.A.; Rodriguez, A.G.; López-Sandoval, R. Study of the effect of DMSO concentration on the thickness of the PSS insulating barrier in PEDOT:PSS thin films. Synth. Met. 2010, 160, 1501–1506. [Google Scholar] [CrossRef]
- Luo, J.; Billep, D.; Waechtler, T.; Otto, T.; Toader, M.; Gordan, O.; Sheremet, E.; Martin, J.; Hietschold, M.; Zahn, D.R.T.; et al. Enhancement of the thermoelectric properties of PEDOT:PSS thin films by post-treatment. J. Mater. Chem. A 2013, 1, 7576–7583. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.K.; Kim, Y.-H.; Kang, J.W.; Han, J.-I. Glycerol-doped poly(3,4-ethylenedioxy-thiophene):poly(styrene sulfonate) buffer layer for improved power conversion in organic photovoltaic devices. J. Electrochem. Soc. 2009, 156, H782–H785. [Google Scholar] [CrossRef]
- Lee, M.-W.; Lee, M.-Y.; Choi, J.-C.; Park, J.-S.; Song, C.-K. Fine patterning of glycerol-doped PEDOT:PSS on hydrophobic PVP dielectric with ink jet for source and drain electrode of OTFTs. Org. Electron. 2010, 11, 854–859. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Hao, Z.; Zhao, Y. Influence of doped PEDOT:PSS on the performance of polymer solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 2763–2767. [Google Scholar] [CrossRef]
- Crispin, X.; Jakobsson, F.L.E.; Crispin, A.; Grim, P.C.M.; Andersson, P.; Volodin, A.; van Haesendonck, C.; Van der Auweraer, M.; Salaneck, W.R.; Berggren, M. The origin of the high conductivity of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT-PSS) plastic electrodes. Chem. Mater. 2006, 18, 4354–4360. [Google Scholar] [CrossRef]
- Yan, H.; Okuzaki, H. Effect of solvent on PEDOT/PSS nanometer-scaled thin films: XPS and STEM/AFM studies. Synth. Met. 2009, 159, 2225–2228. [Google Scholar] [CrossRef]
- Alemu Mengistie, D.; Wang, P.-C.; Chu, C.-W. Effect of molecular weight of additives on the conductivity of PEDOT:PSS and efficiency for ITO-free organic solar cells. J. Mater. Chem. A 2013, 1, 9907–9915. [Google Scholar] [CrossRef]
- Yagci, Ö.; Yesilkaya, S.S.; Yüksel, S.A.; Ongül, F.; Varal, N.M.; Kus, M.; Günes, S.; Icelli, O. Effect of boric acid doped PEDOT:PSS layer on the performance of P3HT: PCBM based organic solar cells. Synth. Met. 2016, 212, 12–18. [Google Scholar] [CrossRef]
- Pettersson, L.A.A.; Ghosh, S.; Inganäs, O. Optical anisotropy in thin films of poly(3,4-ethylenedioxythiophene)-poly(4-styrenesulfonate). Org. Electron. 2002, 3, 143–148. [Google Scholar] [CrossRef]
- Timpanaro, S.; Kemerink, M.; Touwslager, F.J.; De Kok, M.M.; Schrader, S. Morphology and conductivity of PEDOT/PSS films studied by scanning-tunneling microscopy. Chem. Phys. Lett. 2004, 394, 339–343. [Google Scholar] [CrossRef]
- Meen, T.-H.; Chen, K.-L.; Chen, Y.-H.; Chen, W.-R.; Chou, D.-W.; Lan, W.-H.; Huang, C.-J. The Effects of dilute sulfuric acid on sheet resistance and transmittance in poly(3,4-thylenedioxythiophene):poly(styrenesulfonate) films. Int. J. Photoenergy 2013, 2013, 843410. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-Processed metallic conducting polymer films as transparent electrode of optoelectronic devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Garreau, S.; Duvail, J.L.; Louarn, G. Spectroelectrochemical studies of poly(3,4-ethylenedioxythiophene) in aqueous medium. Synth. Met. 2001, 125, 325–329. [Google Scholar] [CrossRef]
- Ha, Y.H.; Nikolov, N.; Pollack, S.K.; Mastrangelo, J.; Martin, B.D.; Shashidhar, R. Towards a transparent, highly conductive poly(3,4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615–622. [Google Scholar] [CrossRef]
- Hsiao, Y.-S.; Whang, W.-T.; Chen, C.-P.; Chen, Y.-C. High-conductivity poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film for use in ITO-free polymer solar cells. J. Mater. Chem. 2008, 18, 5948–5955. [Google Scholar] [CrossRef]
- Crispin, X.; Marciniak, S.; Osikowicz, W.; Zotti, G.; van der Gon, A.W.D.; Louwet, F.; Fahlman, M.; Groenendaal, L.; De Schryver, F.; Salaneck, W.R. Conductivity, morphology, interfacial chemistry, and stability of poly(3,4-ethylene dioxythiophene)-poly(styrene sulfonate): A photoelectron spectroscopy study. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2561–2583. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Highly conductive poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells. Energy Environ. Sci. 2012, 5, 5325–5332. [Google Scholar] [CrossRef]
- Ouyang, J.; Chu, C.W.; Chen, F.C.; Xu, Q.; Yang, Y. High-conductivity poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Lang, U.; Müller, E.; Naujoks, N.; Dual, J. Microscopical investigations of PEDOT:PSS thin films. Adv. Funct. Mater. 2009, 19, 215–1220. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiaansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; van Breemen, A.J.J.M.; de Kok, M.M. Microscopic understanding of the anisotropic conductivity of PEDOT:PSS thin films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Zhang, W.; Bi, X.; Zhao, X.; Zhao, Z.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. Isopropanol-treated PEDOT:PSS as electron transport layer in polymer solar cells. Org. Electr. 2014, 15, 3445–3451. [Google Scholar] [CrossRef]
- Hansen, W.N.; Hansen, G.J. Standard reference surfaces for work function measurements in air. Surf. Sci. 2001, 481, 172–184. [Google Scholar] [CrossRef]
- Sommerhalter, C.; Mattes, T.W.; Glatzel, T.; Jäger-Waldau, A.; Lux-Steiner, M.C. High-sensitivity quantitative Kelvin probe microscopy by noncontact ultra-high-vacuum atomic force microscopy. Appl. Phys. Lett. 1999, 75, 286–288. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.F.; Wilson, J.S.; de Mello, A.J.; de Mello, J.C.; Bradley, D.D.C. Investigation of the effects of doping and post-deposition treatments on the conductivity, morphology, and work function of poly(3,4-ethylenedioxythiophene)/poly(styrene sulfonate) films. Adv. Funct. Mater. 2005, 15, 290–296. [Google Scholar] [CrossRef]
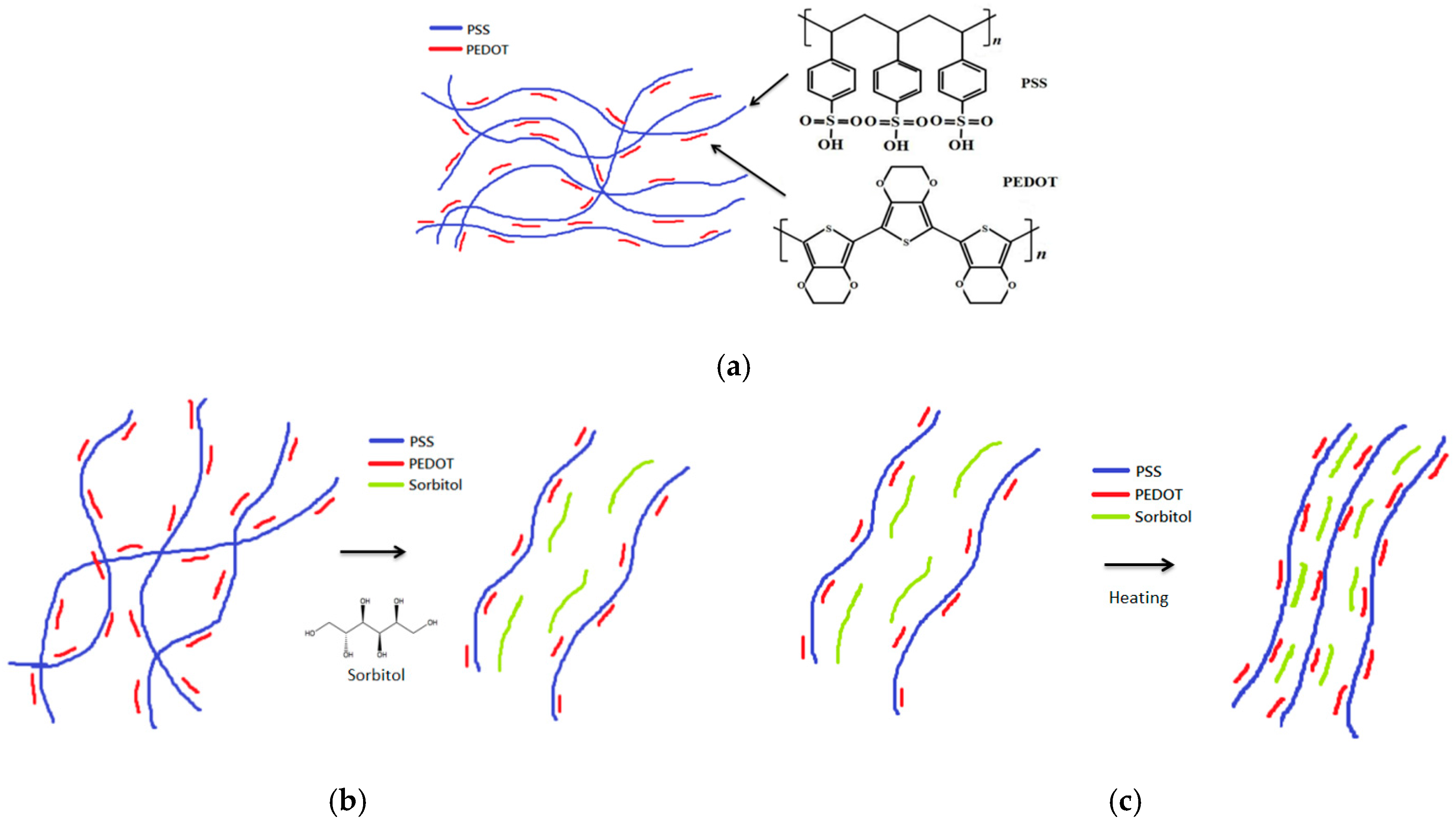
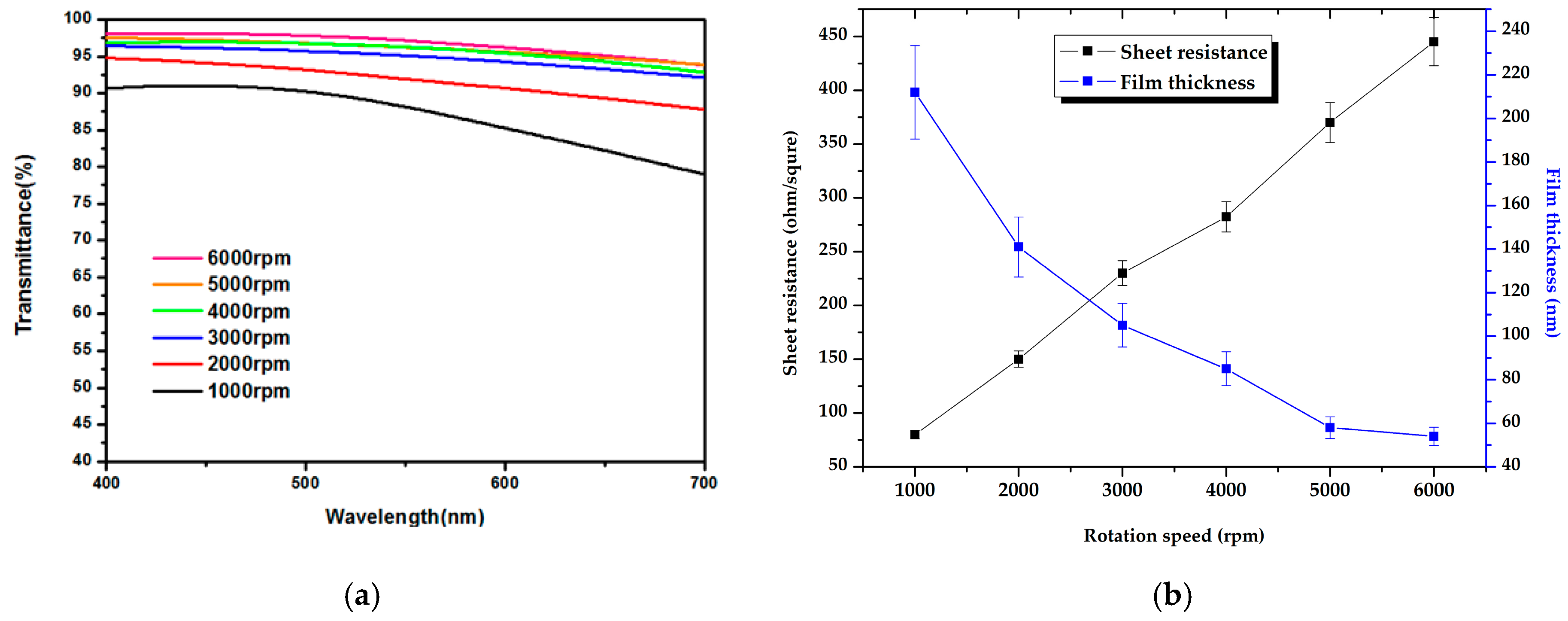
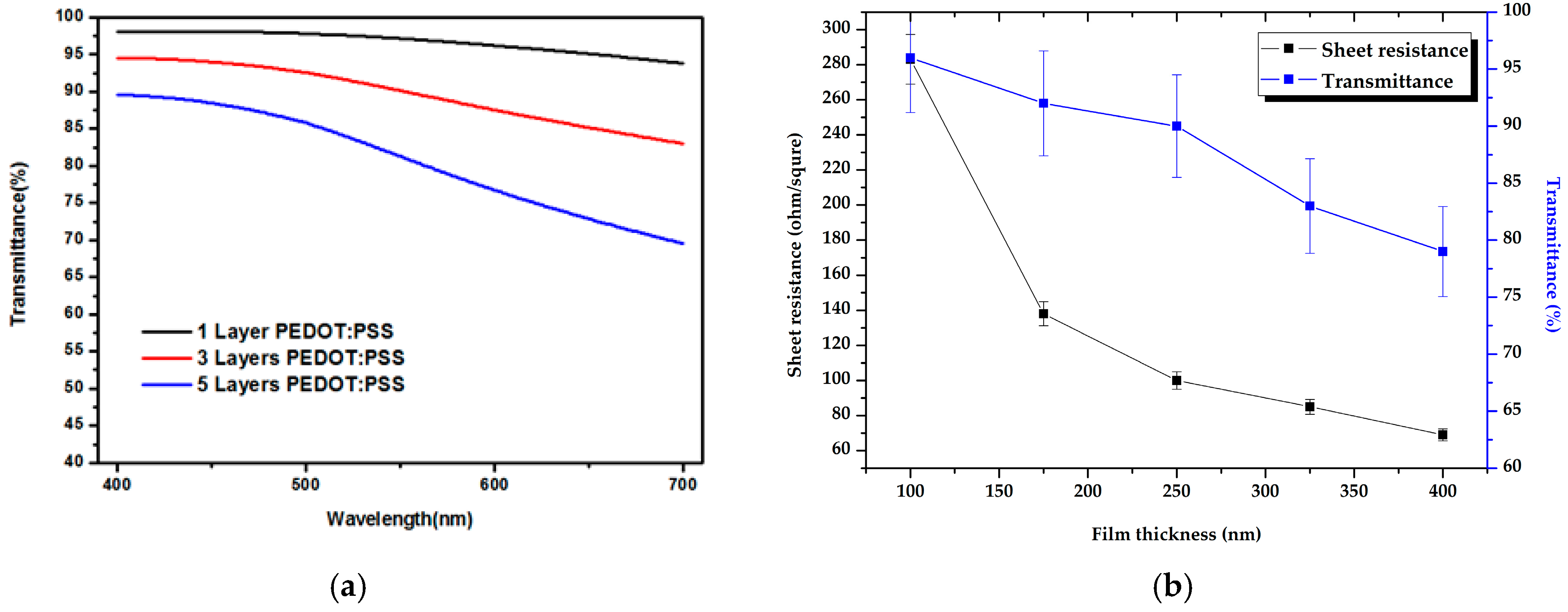
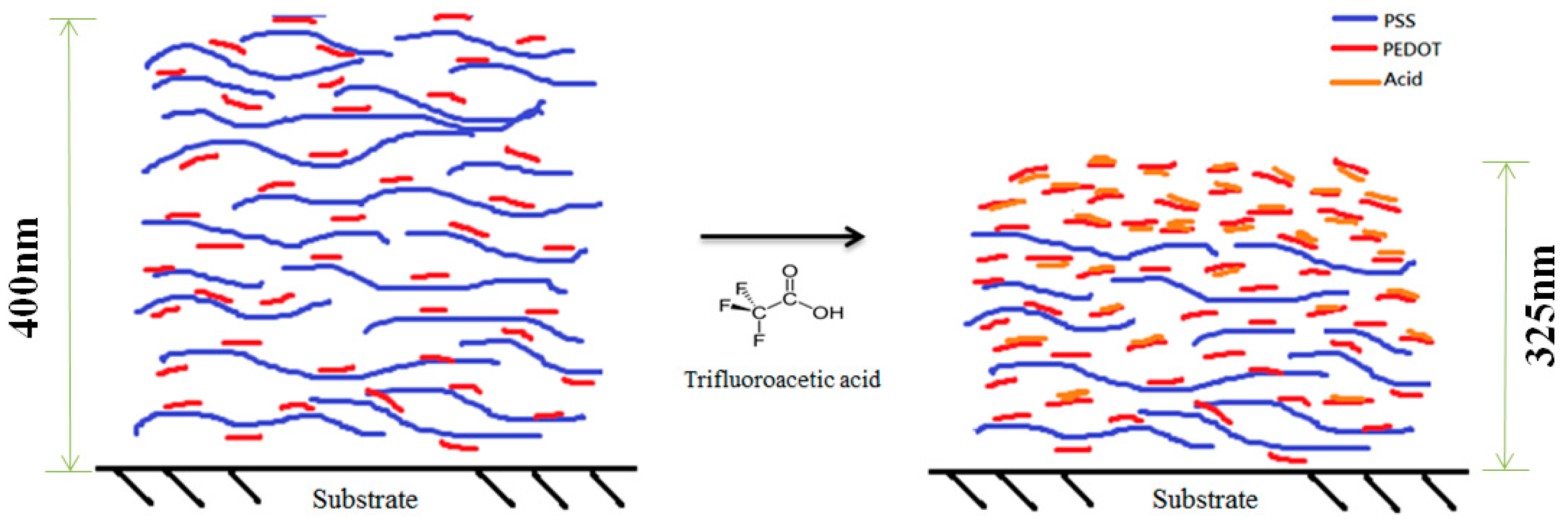
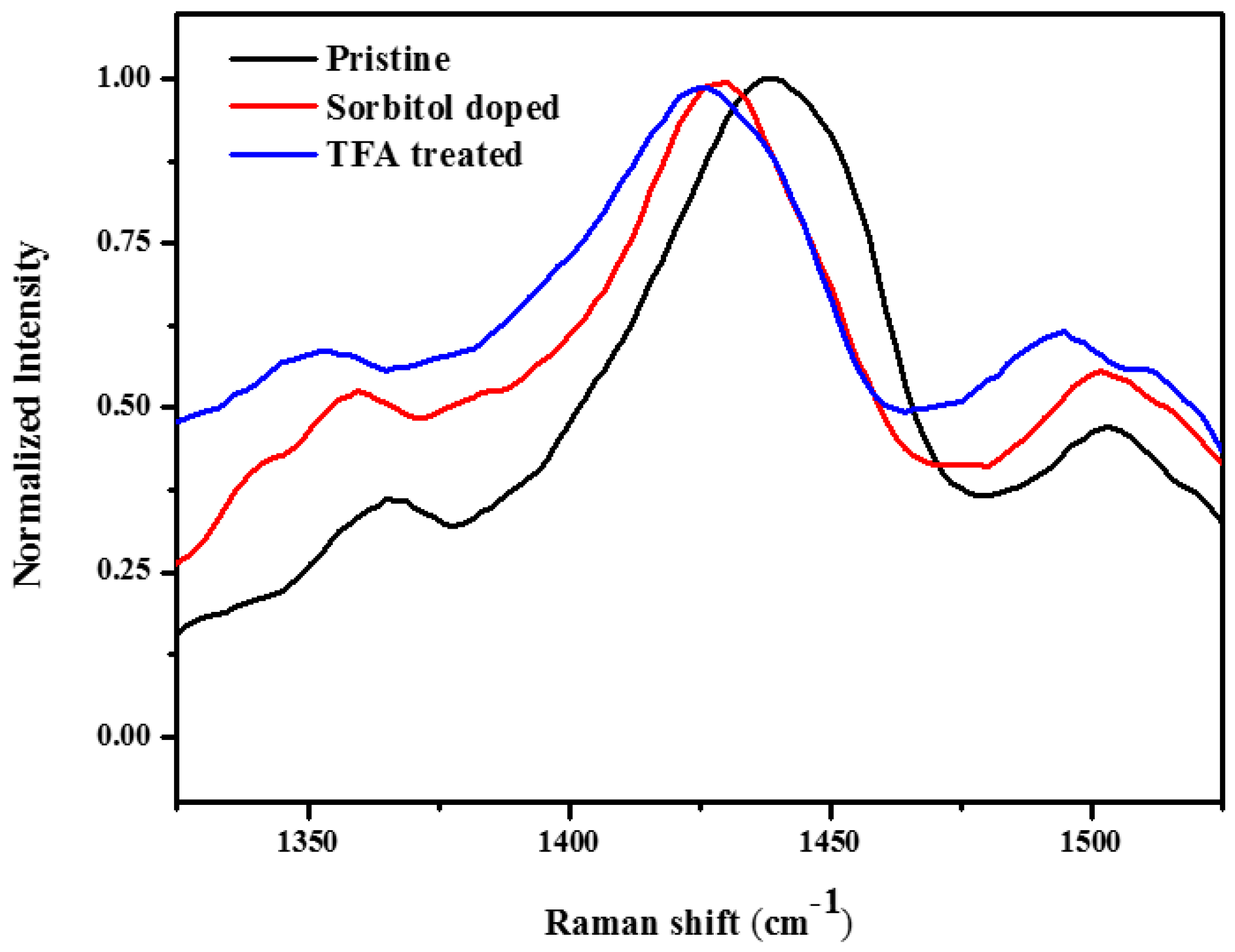
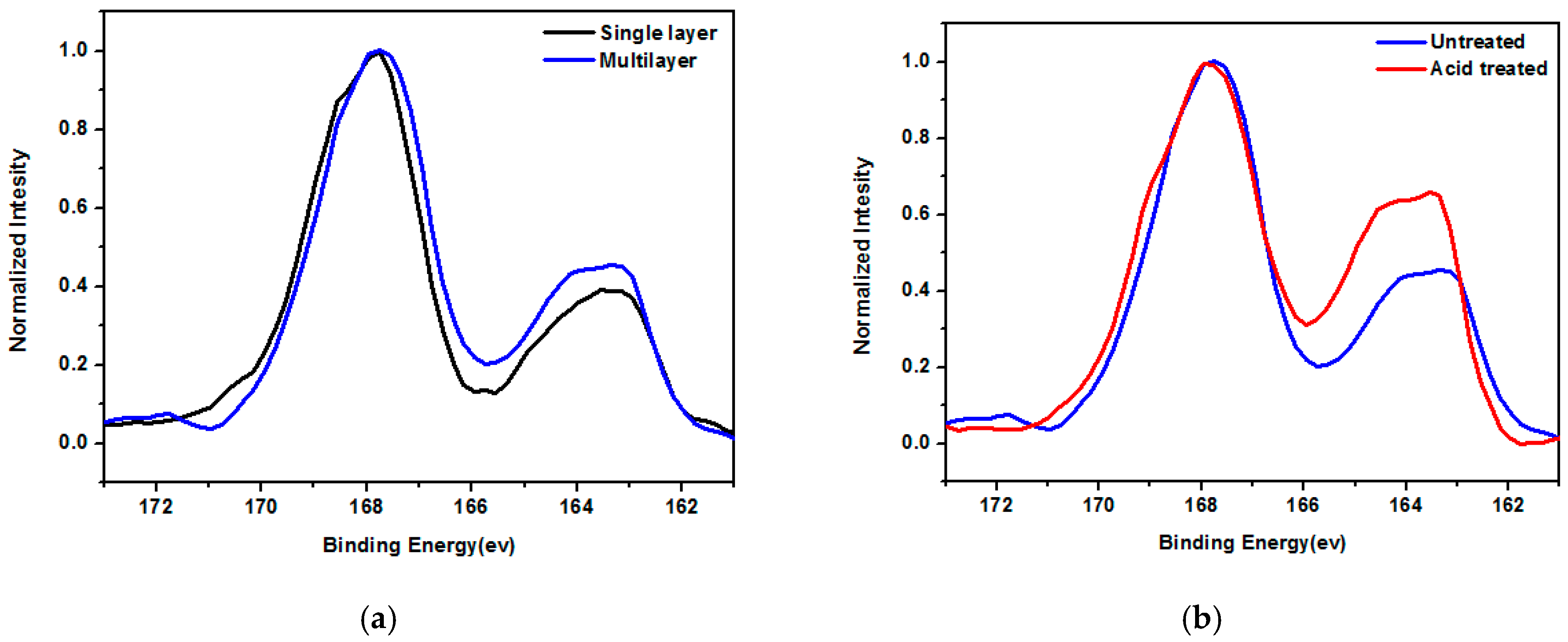
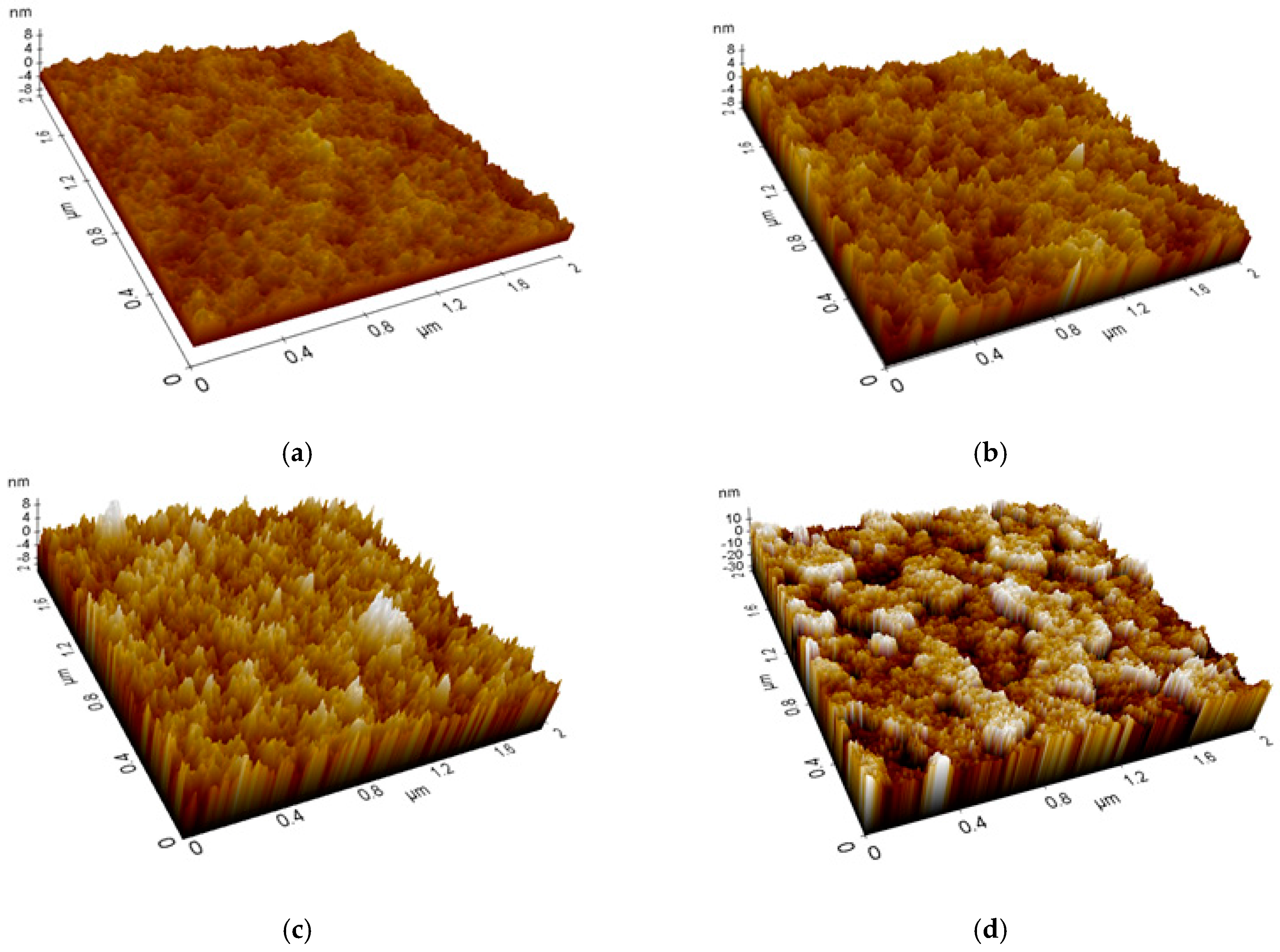
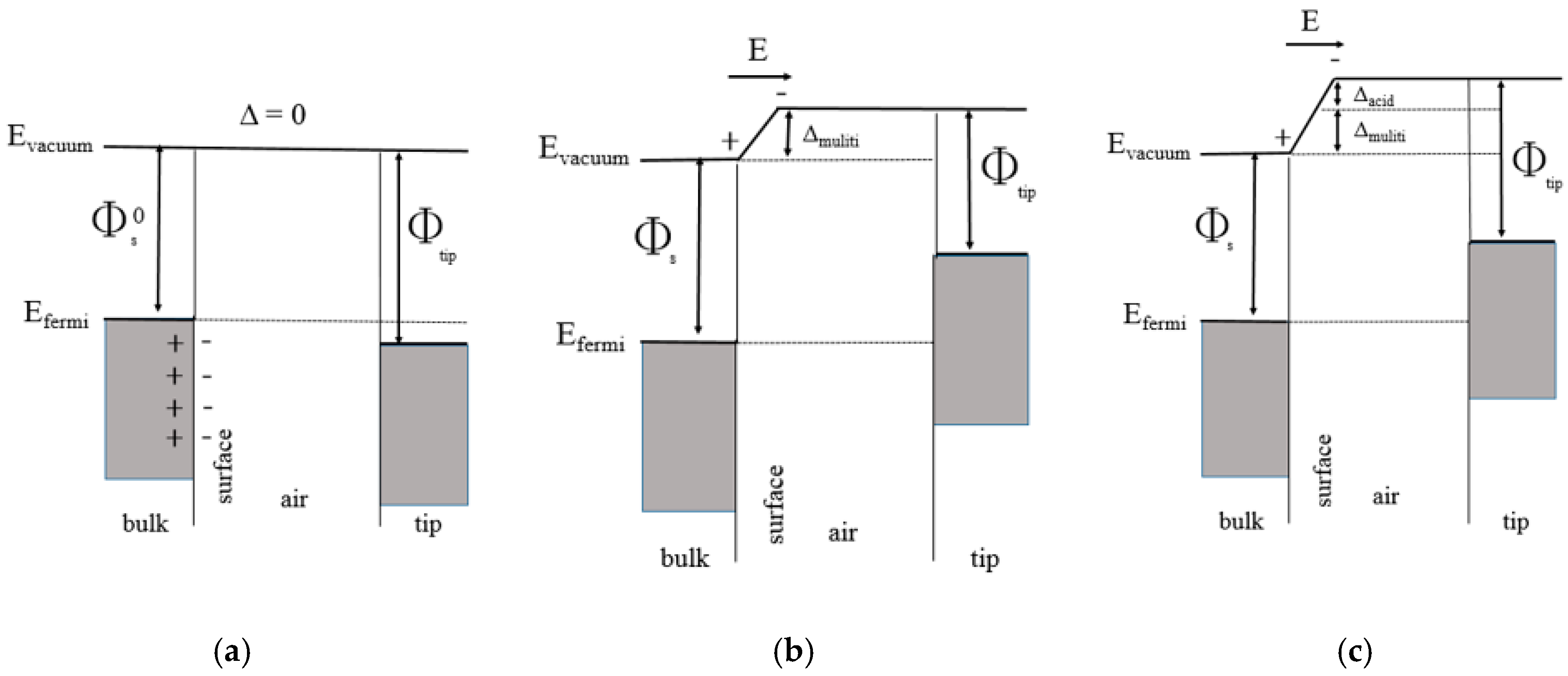
| Layer (s) | d (nm) | Rsq (Ω/sq) | T (%) |
|---|---|---|---|
| 1 | 86 | 283 | 97 |
| 3 | 250 | 90 | 90 |
| 5 | 400 | 69 | 79 |
| 5 with TFA treatment | 325 | 45 | 82 |
| Process | Work Function (eV) |
|---|---|
| PEDOT:PSS filmes with sorbitol | 4.87 |
| Multilayer of PEDOT:PSS films | 5.08 |
| Acid-treated PEDOT:PSS films | 5.12 |
| Typical ITO sample | 4.78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sze, P.-W.; Lee, K.-W.; Huang, P.-C.; Chou, D.-W.; Kao, B.-S.; Huang, C.-J. The Investigation of High Quality PEDOT:PSS Film by Multilayer-Processing and Acid Treatment. Energies 2017, 10, 716. https://doi.org/10.3390/en10050716
Sze P-W, Lee K-W, Huang P-C, Chou D-W, Kao B-S, Huang C-J. The Investigation of High Quality PEDOT:PSS Film by Multilayer-Processing and Acid Treatment. Energies. 2017; 10(5):716. https://doi.org/10.3390/en10050716
Chicago/Turabian StyleSze, Po-Wen, Kuan-Wei Lee, Pin-Chiao Huang, Dei-Wei Chou, Bing-Siang Kao, and Chien-Jung Huang. 2017. "The Investigation of High Quality PEDOT:PSS Film by Multilayer-Processing and Acid Treatment" Energies 10, no. 5: 716. https://doi.org/10.3390/en10050716








