1. Introduction
Microalgae are of considerable interest for the production of next-generation biofuels [
1,
2] that are indistinguishable from petroleum fuels based on their properties [
3]. This is because using microalgae for biofuel production would have fewer adverse effects on food supply and other agriculture [
4,
5] as they can be cultivated on non-arable land using fresh, waste, or saline water sources and enable more efficient nutrient recycling and achieve higher productivities [
6,
7].
A range of conversion techniques are under development to generate biofuels from microalgae. These include solvent extraction followed by trans-esterification to produce fatty acid methyl esters (FAME), fermentation to alcohols, as well as thermochemical conversion pathways, such as pyrolysis and hydrothermal liquefaction (HTL) [
8,
9], to produce bio-crude oils. HTL efficiently converts wet microalgae biomass feedstock into bio-crude oil [
10,
11,
12] as it eliminates the need for expensive pre-drying of the raw material. Compared to trans-esterifying lipids obtained from microalgae by solvent extraction, HTL has the potential to require less energy for the conversion process which would improve production costs [
13]. The high solvent losses associated with FAME production means its financial viability is directly related to the lipid content (high lipid content being better) whereas HTL can make use of more highly productive microalgae species (i.e., more tonnes per hectare) which have higher carbohydrate content, lower lipid content and the potential for additional co-products [
14]. These advantages of HTL make it a competitive route for the conversion of raw microalgae biomass to fuels [
10,
11,
12].
HTL converts microalgae biomass into bio-crude oil, as well as aqueous, gas, and solid phase products at elevated pressures (5–24 MPa) and moderate temperatures (250–400 °C) [
15,
16]. A wide range of microalgae biomass feedstocks has been explored using HTL, including laboratory and commercially grown strains
Botryococcus braunii [
17],
Arthrospira (
Spirulina),
Scenedesmus sp. [
18], and
Tetraselmis sp. [
2]. Among the green microalgae the most common
Scenedesmus sp. has high productivity although the lipid yield can be optimised to reach over 60% [
19], which makes this strain attractive for biofuel production. In general, the variation in the biochemical composition, particularly the carbon chain length and the degree of saturation, affects the conversion rate and the chemical-physical properties of the HTL bio-crude [
20,
21].
The effect of operating conditions such as residence time, temperature, slurry concentration, and catalyst, on physical and chemical properties of microalgae HTL bio-crude have been investigated previously in limited studies [
22,
23,
24,
25]. In this study, three temperatures and slurry concentrations were investigated in the range 280–350 °C and 15–30%, respectively; temperature and concentration, as well as reaction time of 60 min was based loosely on the literature [
10,
23]. Higher temperatures were not explored so as to remain below water’s supercritical point. Jena et al. [
23] demonstrated that among the various process parameters the temperature had the strongest influence on the higher heating value (HHV), viscosity and chemical composition. HTL produces bio-crude oil that, by weight, can have 6–8 times higher oxygen and nitrogen content than heavy fuel oil (HFO) [
26]. The inorganic salts in many types of HTL bio-crude oil require modifications to be compatible with a traditional refining process [
2,
10,
27,
28]. Bio-crude oil may also need subsequent upgrading to improve quality and reduce these undesired components.
Most experimental research in HTL continues to use small batch reactors (less than 1000 mL), while scale-up reactors are usually continuous. The kinetics of small size reactors or autoclaves may differ from large ones, both batch and continuous, which lead to different chemical reactions. Therefore, the results from these reactors are not fully reliable in terms of scale-up. Batch reactors are commonly used to validate the process conditions for further commercial scale. Although industry tends to prefer continuous reactors to batch reactors because of higher energy efficiency, the steady production rate and uniformity of product, large batch reactors still have several key advantages [
29]:
Pumping the biomass slurry into a continuous reactor at high pressure and temperature remains a technological challenge at the industrial scale;
Ability to easily switch between feedstocks; and
For a factory, individual reactors can be taken out-of-service for maintenance.
Due to the lack of bio-crude for testing, most groups only reported values at optimised parameters. The aim of this study was to conduct more comprehensive experimental analyses with three key objectives: (i) to empirically investigate the relationship between the reaction parameters and experimentally determined values physical properties of bio-crude; (ii) to evaluate how the chemical composition varies with operating conditions; and (iii) to identify the best possible fuel physical and chemical properties of microalgae HTL bio-crude. Both physical and chemical properties are needed to meet legislated diesel and FAME biodiesel standards. In further analysis, the physical properties of the best bio-crude were compared with other fuel standards to determine the similarities and reveal areas for improvement.
2. Materials and Methods
2.1. Hydrothermal Liquefaction (HTL)
The experiments were performed in a 1.8 L batch reactor system (Parr Instruments Co., Moline, IL, USA) and data were collected across three physical variables: temperature, slurry concentration, and solvents for recovering the bio-crude from the liquid mixture. The experimental temperatures used were 280 °C, 300 °C, and 350 °C, and solid concentrations in the slurry were 15%, 25%, and 30% by weight. When loading the reactor, the headspace was purged thoroughly using nitrogen to remove oxygen, and pre-pressurised to 2 bar with nitrogen gas. The reactor was heated to the desired temperature (heating rate ~3.3 °C/min) and held constant for one hour which is consistent with the literature [
23,
30,
31,
32,
33,
34]. At the end of the reaction time, the reactor was cooled by passing water through an internal cooling tube until room temperature was reached. Experiments were performed in duplicate and the average yield of two runs is reported.
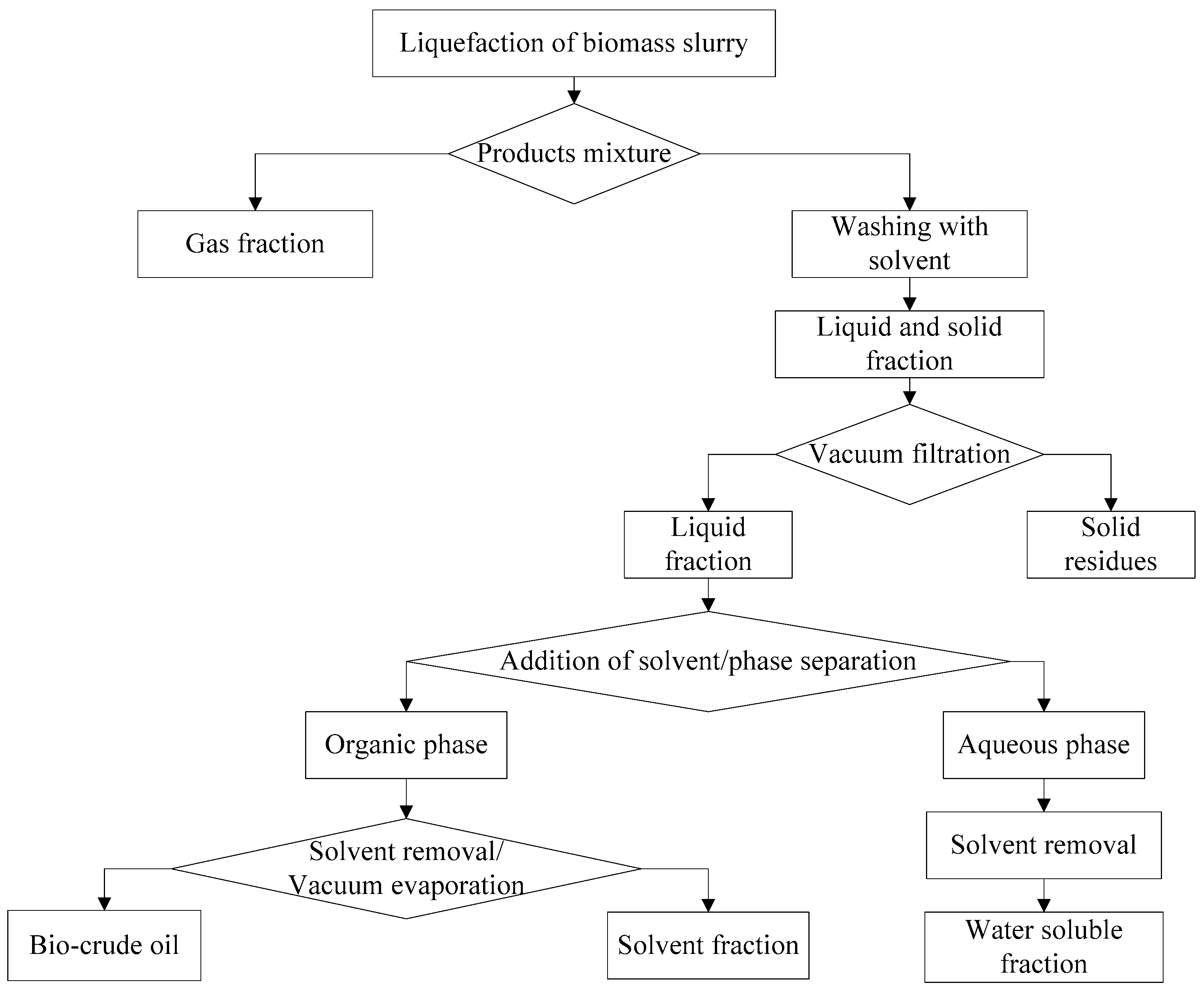
After the gas was vented from the reactor, the vessel was opened and the mixture was separated (following the steps in
Figure 1). The walls of the reactor were washed thoroughly with solvent (dichloromethane or
n-hexane) and mixed with the liquid phase. The amount of solvent added was determined as the volume of the solvent per mass dry weight of algae. The solid phase was removed from the liquid mixture by vacuum filtration before being washed with the remaining solvent. The solids were then oven-dried at 105 °C overnight.
The liquid phase mixture was poured into a separation funnel and the water-insoluble components separated (organic phase) were separated from the water soluble (aqueous) phase. The solvent was evaporated from the bio-crude by a rotary vacuum evaporator. Due to the volatile nature of dichloromethane (DCM) we have used 40 °C (760 Tor) and placed an additional trap between the vacuum source and the condenser unit, whereas for n-hexane the temperature was set at 68 °C (760 Tor). The evaporation was continued until no further yield was obtained. The time for the DCM was shorter than that for n-hexane.
2.2. Raw Materials
A robust and fast growing green freshwater microalga
Scenedesmus sp. which had been isolated as part of previous work [
35] was used for these experiments. The
Scenedesmus sp. bulk biomass was produced at the University of Queensland’s Solar Biofuels Research Centre facility at Pinjarra Hills near Brisbane, Australia. High density slurry was frozen and stored at −20 °C, directly after harvest to prevent degradation during the course of the HTL experiments. Proximate analyses were performed according to American Society for Testing and Materials (ASTM) standards and using a thermo-gravimetric analyzer (TGA–NETZSCH thermal analyser, NETZSCH Australia Pty Ltd., New South Wales, Australia). Ultimate analyses were carried out using a LECO TruSpec Micro CHNS elemental analyser (LECO Corporation, Saint Joseph, MI, USA) (
Table 1). The HHV of the dried microalgae sample was calculated according to the formula used by Demirbas [
36] and Friedl [
37], which were 18.07 MJ·kg
−1 and 19.5 MJ·kg
−1, respectively, for
Scenedesmus species microalgae. The lipid content for the samples used in this study was typically 15–20%.
2.3. Analytical Methods
The conversion and product yields were defined as the mass fraction of the respective product (i.e., bio-crude and solid residue) as a function of the initial mass of biomass. For approximation, the total yield of gas + aqueous products were determined by difference according to the approach commonly used in the literature (e.g., [
38]).
2.4. Bio-Crude Properties Measurements
The chemical and physical bio-crude properties such as HHV, viscosity, density, and chemical composition were measured experimentally. HHV of the bio-crude was measured with a Parr 6200 compensated jacket calorimeter (Moline, IL, USA). For comparative purposes, the correlations which are used for fossil fuels (liquid, gas and coal) form the basis of calculation applied in other research articles. Boie’s and Dulong’s formulae are shown Equations (3) and (4), respectively [
36,
39].
A Brookfield DV-III ultra-programmable rheometer (Brookfield engineering laboratories, Inc., Middleboro, MA, USA) was used to measure the viscosity of the bio-crude at constant temperature. The accuracy of the rheometer for viscosity measurements is ±1.0% of full scale range for a specific spindle running at a specific speed. The density of the bio-crude was measured using German industrial standard (DIN) 1306.
Gas chromatography with mass spectroscopy (GC-MS) was used to identify the chemical compositions in the bio-crude samples. GC-MS analyses were performed using a Thermoscientific, Trace 1310 system (Thermo Scientific™, Milan, Italy), equipped with a single quadrupole mass selective detector (ISQ). Each sample was dissolved and diluted in dichloromethane. The injector was set to 250 °C and a Thermo TG-5MS (30 m long, 0.25 mm ID, 0.25 mm film) column was used. The oven was programmed at an initial temperature at 50 °C (held 1/min) then heated at a constant rate of 10 °C·min−1 until a temperature of 250 °C was reached, and then held for 9 min with a split ratio of 1:25 and a column flow of 1.4 mL·min−1. The MS detector scanned from 40 to 400 m/z with a solvent cut time of 1.8 min and the ion source and transfer line temps were both set at 250 °C. The carrier mode was set to constant flow.
4. Conclusions
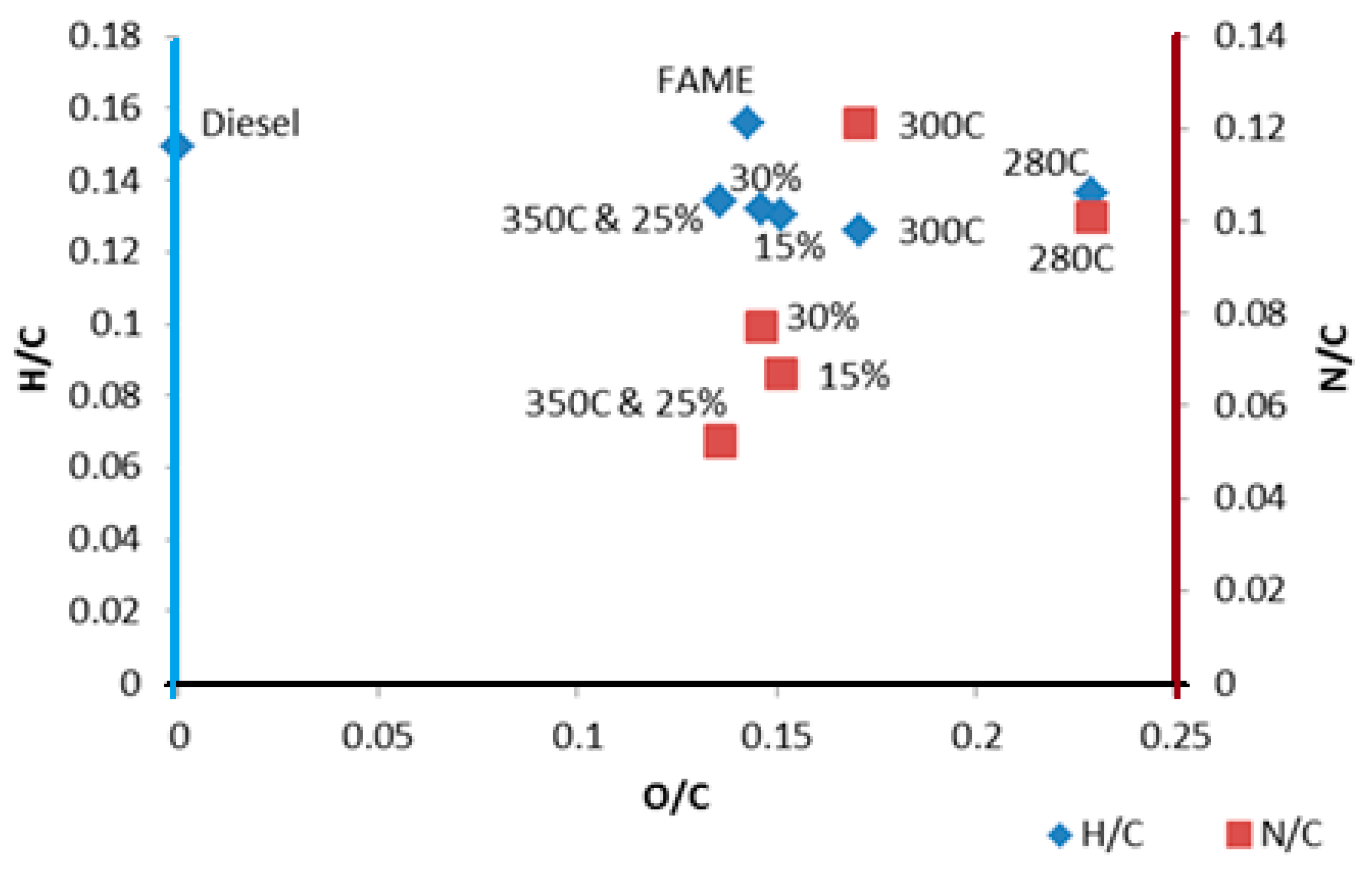
This paper studied the relationship of HTL operating conditions (reaction temperature and slurry concentration) on the chemical pomposities and physical and chemical properties of the microalgae bio-crude. Physical properties were able to be measured experimentally because of the larger scale of the reactor rather than being derived using correlations based on elemental analysis. We note a significant difference in the values for calculated and measured values, which is consistent with the literature. The highest bio-crude oil yield (33.6%) was produced at 350 °C and at 25% solids concentration. The aliphatic, aromatic, nitrogen containing hetero-cyclic, oxygenated compounds were the major group of components of HTL microalgae bio-crude. These conditions also produced the lowest O:C and N:C. The bio-crude had higher density and lower HHV than diesel and biodiesel and was closer in character to heavy fuel oil where it could be used directly. Future work will focus on further improving chemical and physical properties, such as HHV, density, and decreasing N and O percentages, via catalytic upgrading, or via reactions in situ, followed by engine testing.