Alkaline Earth Element Adsorption onto PAA-Coated Magnetic Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
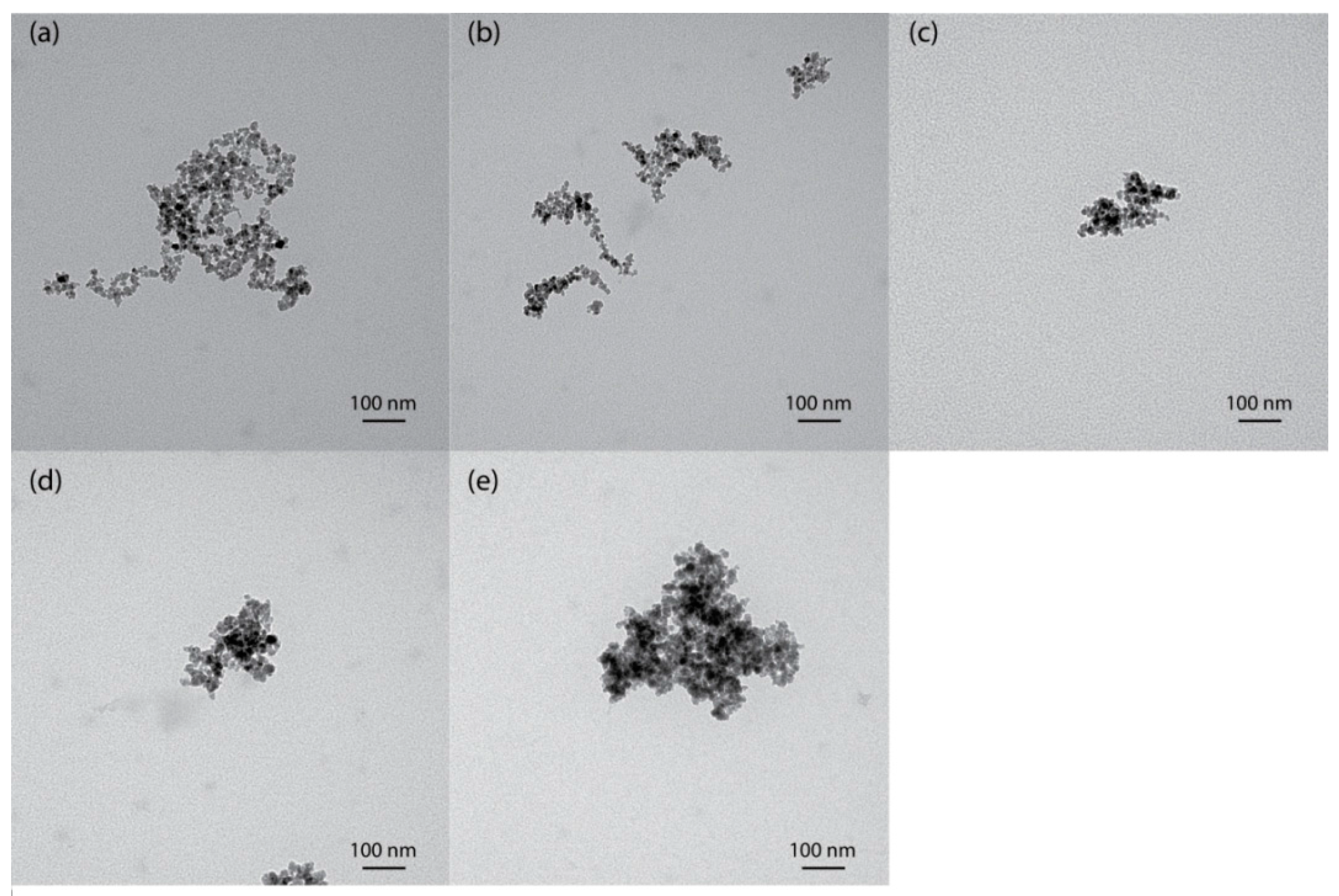
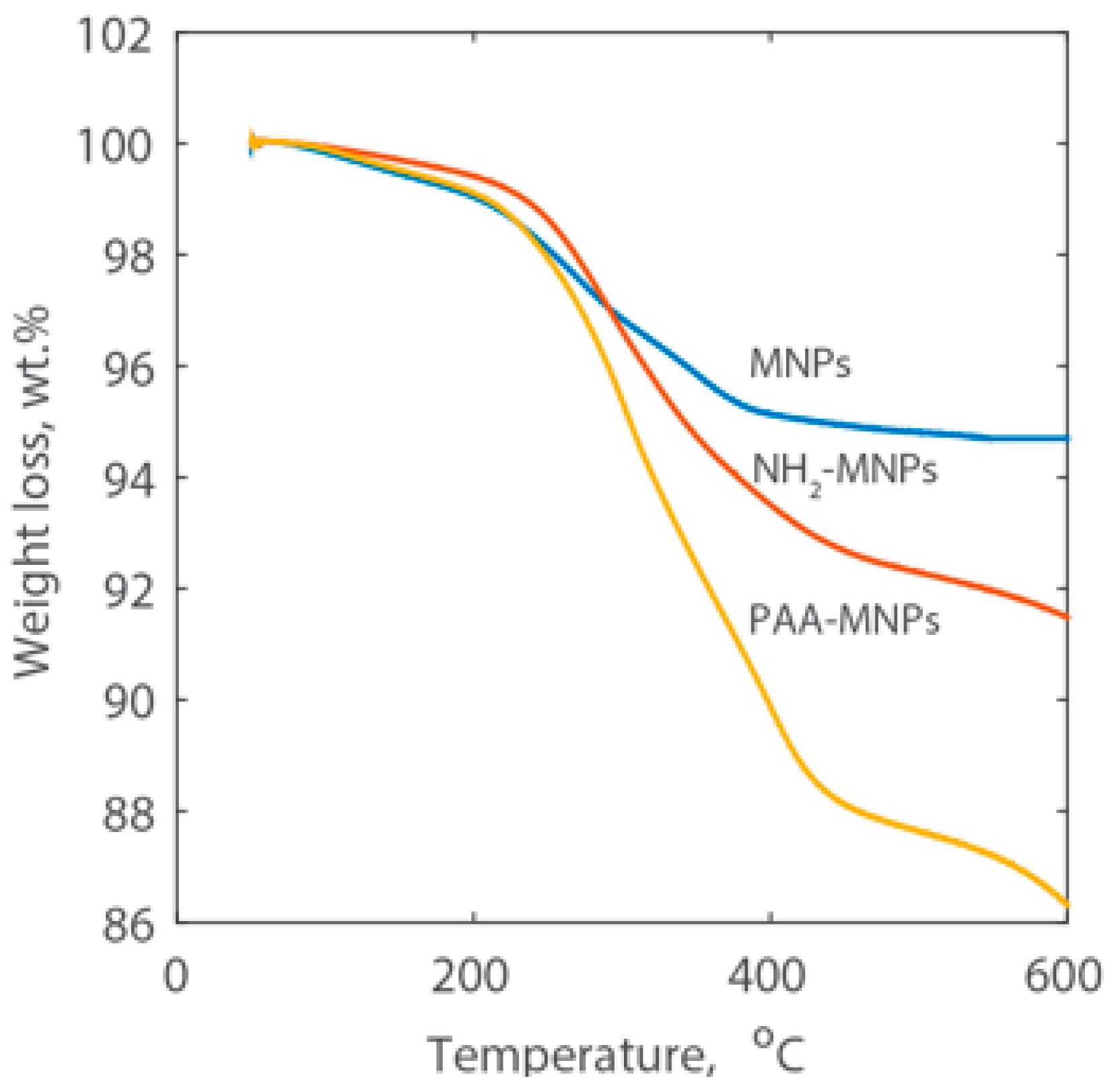
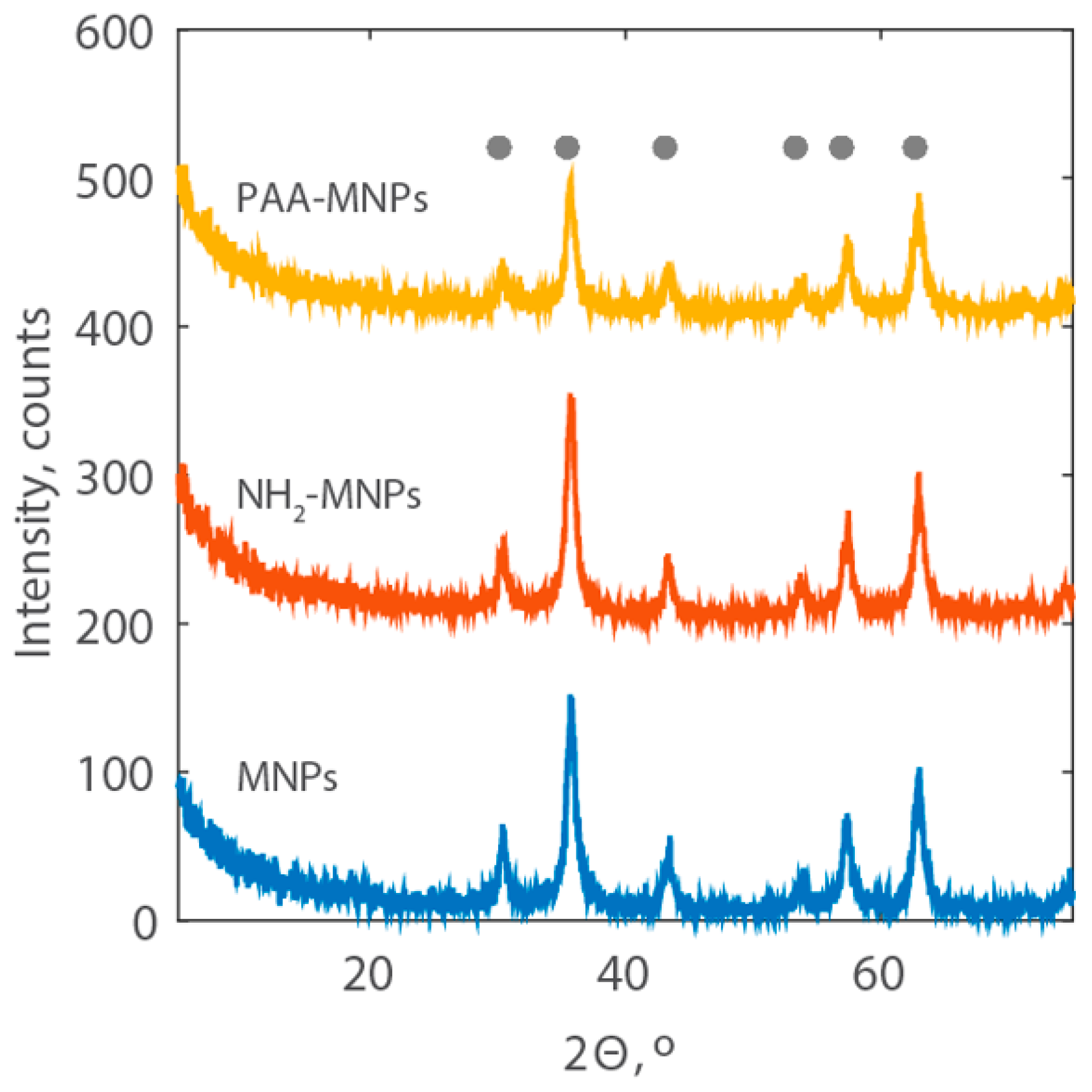
2.1. Characterization of the Synthesized Nanoparticles
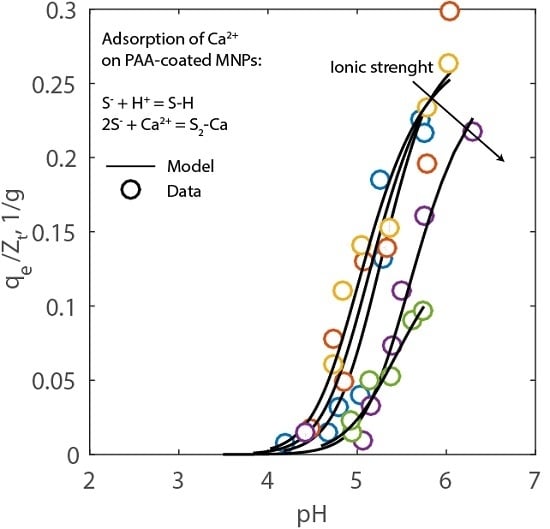
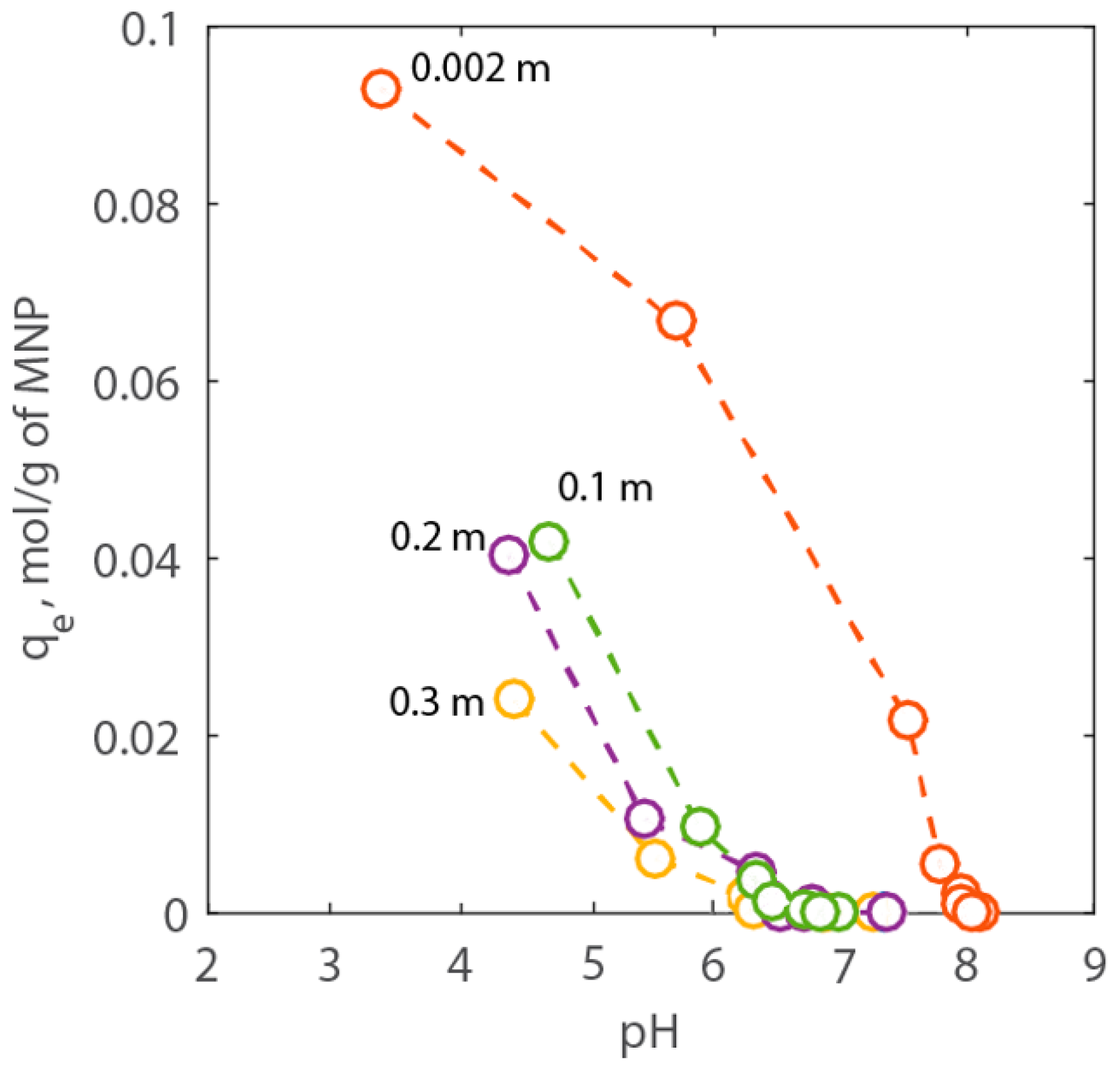
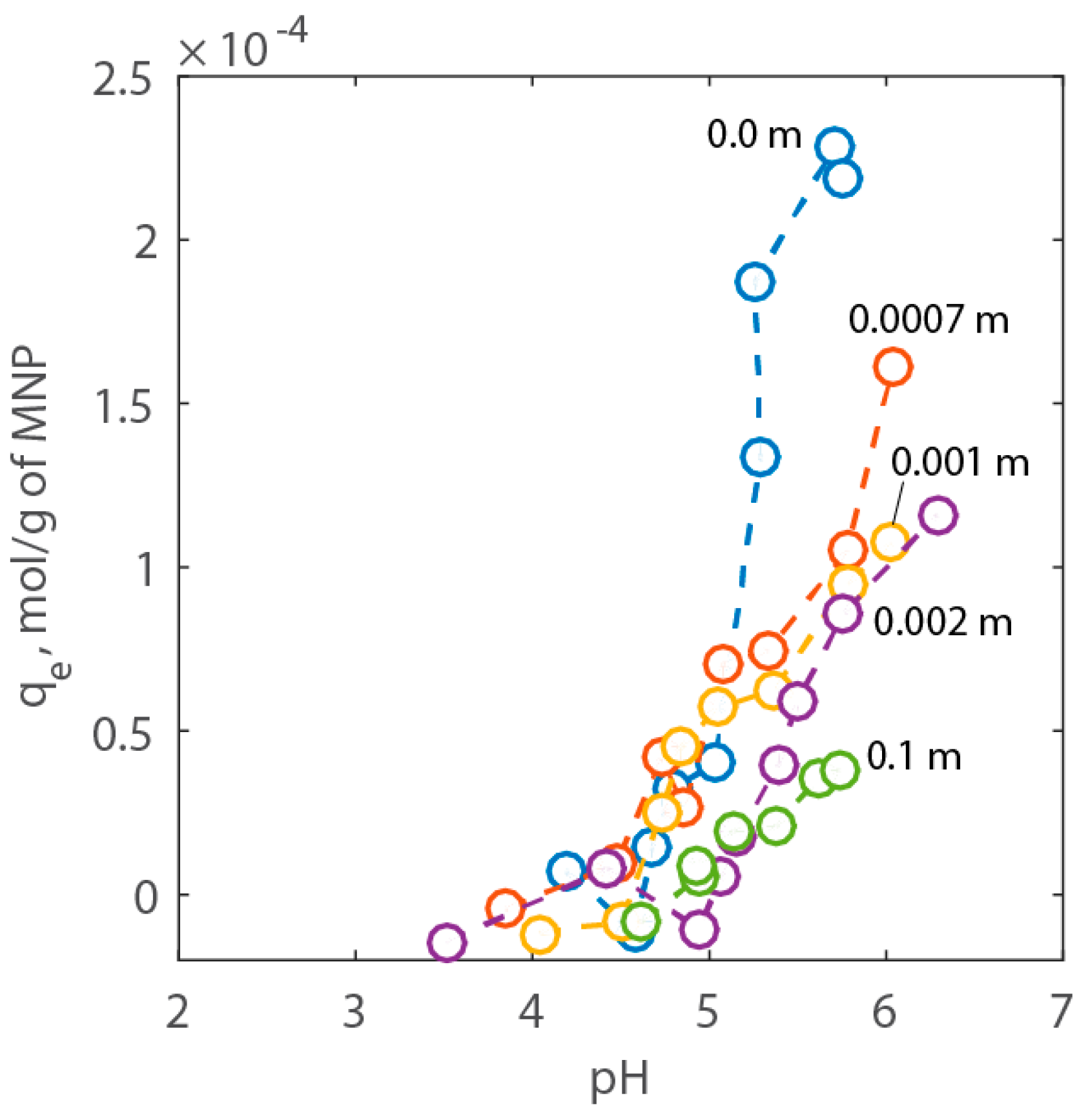
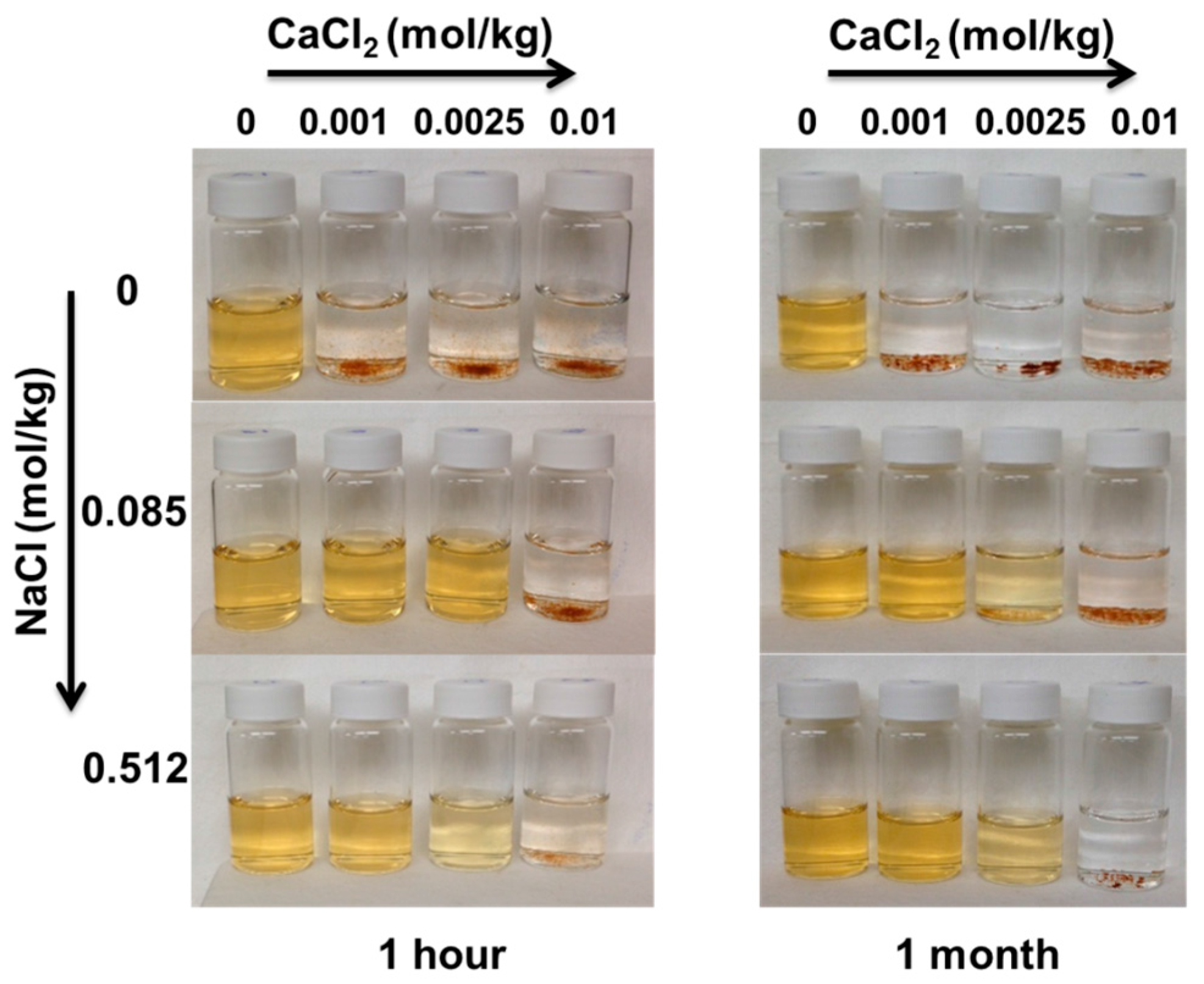
2.2. Adsorption Experiments
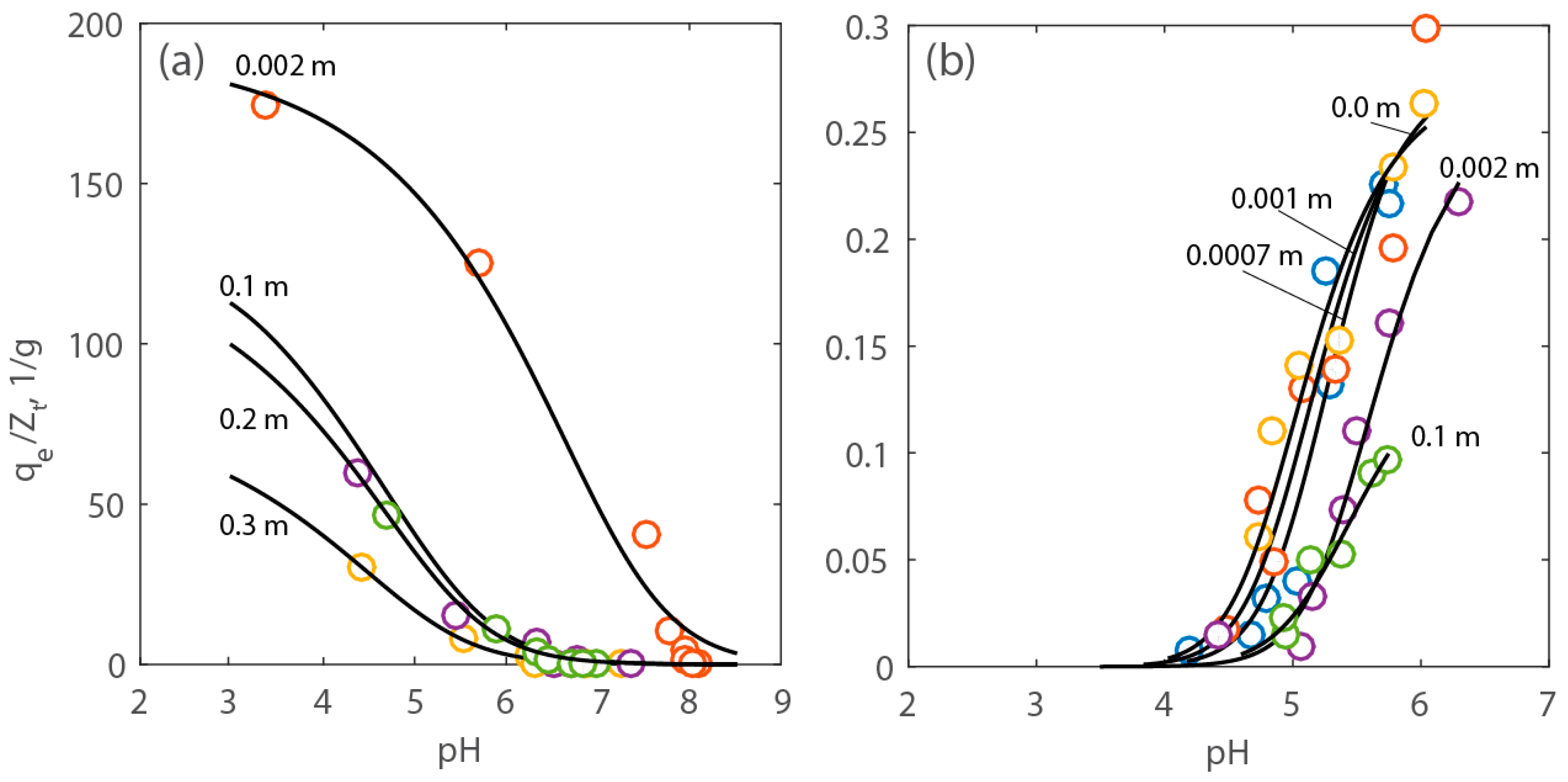
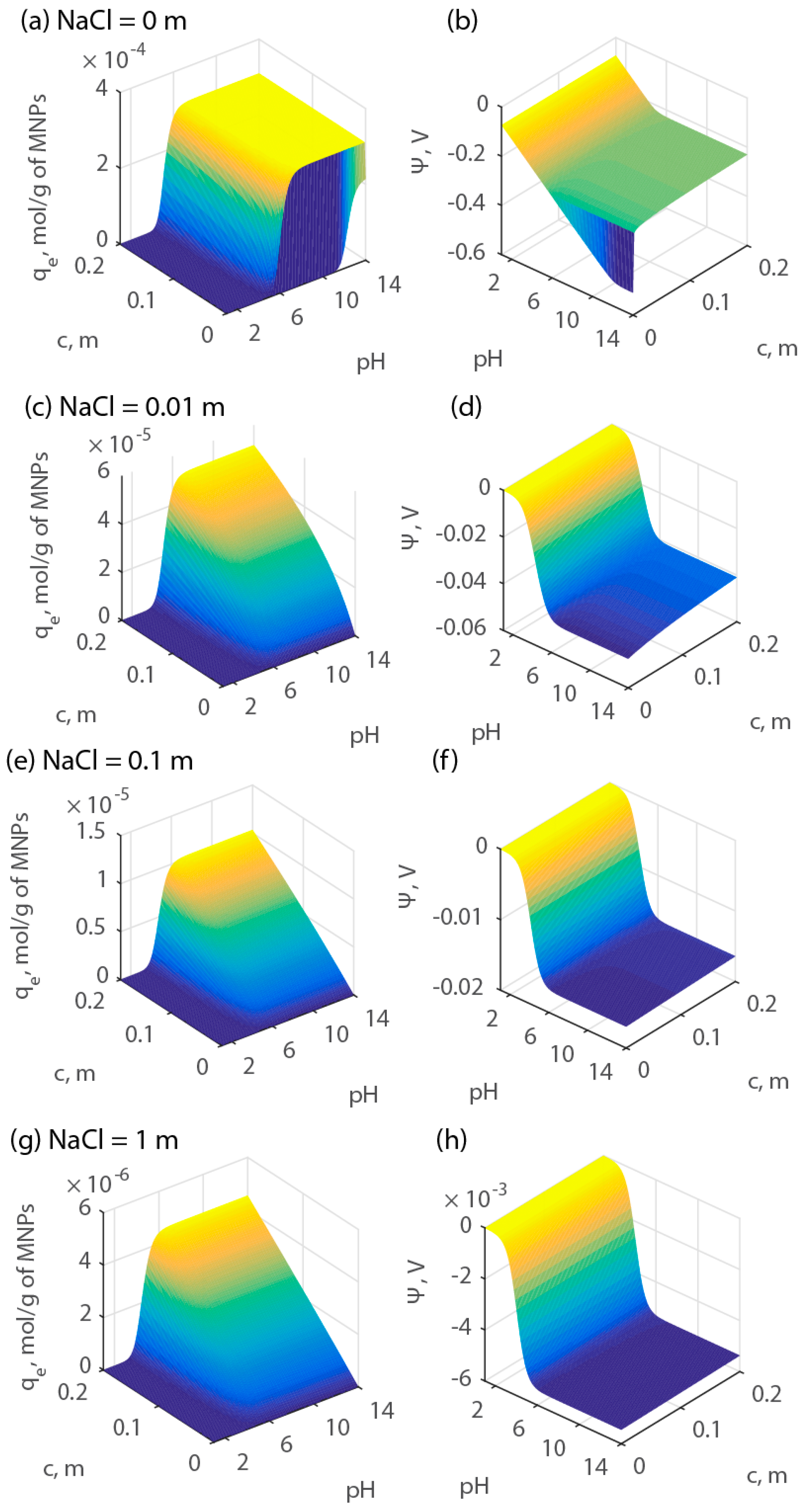
2.3. Simulations
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Iron Oxide Magnetic Nanoparticles
3.3. Functionalization of Iron Oxide Magnetic Nanoparticles
3.4. Adsorption Experiments
3.5. Characterization
3.5.1. Transmission Electron Microscopy
3.5.2. Dynamic Light Scattering Analysis
3.5.3. Surface Area
3.5.4. Zeta Potential
3.5.5. Thermogravimetric Analysis
3.5.6. X-ray Diffraction Analysis
3.5.7. Magnetization
3.5.8. MNP Concentration Analysis
3.6. Adsorption Modeling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, C.; Veil, J. Produced Water Volumes and Management Practices in the United States. Prepared for U.S. Department of Energy, the National Energy Technology Laboratory; 2009. Available online: http://www.ipd.anl.gov/anlpubs/2009/07/64622.pdf (accessed on 21 December 2016). [Google Scholar]
- Thiel, G.P.; Lienhard, J.H. Treating produced water from hydraulic fracturing: Composition effects on scale formation and desalination system selection. Desalination 2014, 346, 54–69. [Google Scholar] [CrossRef]
- Lutz, B.D.; Lewis, A.N.; Doyle, M.W. Generation, transport, and disposal of wastewater associated with Marcellus shale gas development. Water Resour. Res. 2013, 9, 647–656. [Google Scholar] [CrossRef]
- Groundwater Protection Council (GWPC). Modern Shale Gas Development in the United States: A Primer. Available online: https://energy.gov/fe/downloads/modern-shale-gas-development-united-states-primer (accessed on 9 February 2017).
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Castrillin, S.R.-V.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, M.-M.; Ye, H.; Zhao, B.-X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Yongmei, H. Study on the adsorption of Cu (II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2013, 218, 46–54. [Google Scholar] [CrossRef]
- Takafuji, M.; Ide, S.; Ihara, H.; Xu, Z. Preparation of poly (1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem. Mater. 2004, 16, 1977–1983. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb(II) removal of Fe3O4 with SiO2–NH2 core–shell nanomaterials prepared via a controllable sol-gel process. Chem. Eng. J. 2013, 215, 461–471. [Google Scholar] [CrossRef]
- Ko, S.; Prigiobbe, V.; Huh, C.; Bryant, S.L.; Bennetzen, M.V.; Mogensen, K. Accelerated oil droplet separation from produced water using magnetic nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, Amsterdam, The Netherlands, 27–29 October 2014.
- Ling, M.M.; Wang, K.Y.; Chung, T.-S. Highly water-soluble magnetic nanoparticles as novel draw solutes in forward osmosis for water reuse. Ind. Eng. Chem. Res. 2010, 49, 5869–5876. [Google Scholar] [CrossRef]
- Wang, Q.; Prigiobbe, V.; Huh, C.; Bryant, S.L.; Mogensen, K.; Bennetzen, M.V. Removal of divalent cations from brine using selective adsorption onto magnetic nanoparticles. In Proceedings of the International Petroleum Technology Conference, Kuala Lumpur, Malaysia, 10–12 December 2014.
- Huang, S.-H.; Chen, D.-H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhuang, L.; Lin, H.; Shen, H.; Li, J.W. Preparation and characterization of polyacrylic acid coated magnetite nanoparticles functionalized with amino acids. Thin Solid Films 2013, 544, 368–373. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q.; Zheng, S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009, 151, 19–29. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Mirrahimi, M.A.-S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010, 159, 264–271. [Google Scholar] [CrossRef]
- Morales, D.V.; Rivas, B.L. Poly (2-acrylamidoglycolic acid-co-2-acrylamide-2-methyl-1-propane sulfonic acid) and poly (2-acrylamidoglycolic acid-co-4-styrene sodium sulfonate): Synthesis, characterization, and properties for use in the removal of Cd (II), Hg (II), Zn (II), and Pb (II). Polym. Bull. 2015, 72, 339–352. [Google Scholar]
- Moulay, S.; Bensacia, N. Removal of heavy metals by homolytically functionalized poly (acrylic acid) with hydroquinone. Int. J. Ind. Chem. 2016, 7, 369–389. [Google Scholar] [CrossRef]
- Sezgin, N.; Balkaya, N. Adsorption of heavy metals from industrial wastewater by using polyacrylic acid hydrogel. Desalination Water Treat. 2016, 57, 2466–2480. [Google Scholar] [CrossRef]
- Chang, D.M. The binding of free calcium ions in aqueous solution using chelating agents, phosphates and poly (acrylic acid). J. Am. Oil Chem. Soc. 1983, 60, 618–622. [Google Scholar] [CrossRef]
- Bartós, B.; Bilewicz, A. Effect of crown ethers on Sr2+, Ba2+, and Ra2+ uptake by tunnel-structure ion exchangers. Solvent Extr. Ion Exch. 2006, 24, 261–269. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Xue, Z.; Neilson, B.M.; Worthen, A.J.; Yoon, K.Y.; Nayak, S.; Cheng, V.; Lee, J.H.; Bielawski, C.W.; Johnston, K.P. Iron oxide nanoparticles grafted with sulfonated copolymers are stable in concentrated brine at elevated temperatures and weakly adsorb on silica. ACS Appl. Mater. Interfaces 2013, 5, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of very fine maghemite particles. J. Magn. Magn. Mater. 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Xue, Z.; Foster, E.; Wang, Y.; Nayak, S.; Cheng, V.; Ngo, V.W.; Pennell, K.D.; Bielawski, C.W.; Johnston, K.P. Effect of grafted copolymer composition on iron oxide nanoparticle stability and transport in porous media at high salinity. Energy Fuels 2014, 28, 3655–3665. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Li, Z.; Neilson, B.M.; Lee, W.; Huh, C.; Bryant, S.L.; Bielawski, C.W.; Johnston, K.P. Effect of adsorbed amphiphilic copolymers on the interfacial activity of superparamagnetic nanoclusters and the emulsification of oil in water. Macromolecules 2012, 45, 5157–5166. [Google Scholar] [CrossRef]
- Takafuji, M.; Kitaura, K.; Nishiyama, T.; Govindarajan, S.; Gopal, V.; Imamura, T.; Ihara, H. Chemically tunable cationic polymer-bonded magnetic nanoparticles for gene magnetofection. J. Mater. Chem. B 2014, 2, 644–650. [Google Scholar] [CrossRef]
- Ortega, D. Magnetic Nanoparticles: From Fabrication of Clinical Application, 1st ed.; CRC Press: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xiao, J.; Kan, A.T.; Tomson, M.B. Acid−base and metal complexation chemistry of phosphino-polycarboxylic acid under high ionic strength and high temperature. Langmuir 2001, 17, 4661–4667. [Google Scholar] [CrossRef]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Prigiobbe, V.; Ko, S.; Huh, C.; Bryant, S.L. Measuring and modeling the magnetic settling of superparamagnetic nanoparticle dispersions. J. Colloid Interface Sci. 2015, 447, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Prigiobbe, V.; Ko, S.; Wang, Q.; Huh, C.; Bryant, S.L.; Bennetzen, M.V. Magnetic nanoparticles for efficient removal of oilfield “contaminants”: Modeling of magnetic separation and validation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015.
- Hwang, E.-D.; Lee, K.-W.; Choo, K.-H.; Choi, S.-J.; Kim, S.-H.; Yoon, C.-H.; Lee, C.-H. Effect of precipitation and complexation on nano-filtration of strontium-containing nuclear wastewater. Desalination 2002, 47, 289–294. [Google Scholar] [CrossRef]
- Zi, Y.; Prigiobbe, V. Effect of salinity and temperature on pH-dependent transport of heavy metals and radionuclides in reactive porous media. 2017. in preparation. [Google Scholar]
- He, C.; Li, M.; Liu, W.; Barbot, E.; Vidic, R.D. Kinetics and equilibrium of barium and strontium sulfate formation in Marcellus Shale flowback. Water J. Environ. Eng. 2014, 140, B4014001. [Google Scholar] [CrossRef]
- Ferrar, K.J.; Michanowicz, D.R.; Christen, C.L.; Mulcahy, N.; Malone, S.L.; Sharma, R.K. Assessment of effluent contaminants from three facilities discharging Marcellus Shale wastewater to surface waters in Pennsylvania. Environ. Sci. Technol. 2013, 47, 3472–3481. [Google Scholar] [CrossRef] [PubMed]
- Warner, N.R.; Christie, C.A.; Jackson, R.B.; Vengosh, A. Impacts of shale gas wastewater disposal on water quality in western Pennsylvania. Environ. Sci. Technol. 2013, 47, 11849–11857. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Huang, C.P.; Jenkins, S.R. Specific Chemical Interactions Affecting the Stability of Dispersed Systems. Croat. Chem. Acta 1970, 42, 223–245. [Google Scholar]
- Huang, C.-P.; Stumm, W. Specific adsorption of cations on hydrous γ-Al2O3. J. Colloid Interface Sci. 1970, 43, 409–420. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M.M. Surface Complexation Modeling: Hydrous Ferric Oxide; Wiley: New York, NY, USA, 1990. [Google Scholar]
- MATLAB Version 8.6.0; The MathWorks Inc.: Natick, MA, USA, 2015.


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Prigiobbe, V.; Huh, C.; Bryant, S.L. Alkaline Earth Element Adsorption onto PAA-Coated Magnetic Nanoparticles. Energies 2017, 10, 223. https://doi.org/10.3390/en10020223
Wang Q, Prigiobbe V, Huh C, Bryant SL. Alkaline Earth Element Adsorption onto PAA-Coated Magnetic Nanoparticles. Energies. 2017; 10(2):223. https://doi.org/10.3390/en10020223
Chicago/Turabian StyleWang, Qing, Valentina Prigiobbe, Chun Huh, and Steven L. Bryant. 2017. "Alkaline Earth Element Adsorption onto PAA-Coated Magnetic Nanoparticles" Energies 10, no. 2: 223. https://doi.org/10.3390/en10020223
APA StyleWang, Q., Prigiobbe, V., Huh, C., & Bryant, S. L. (2017). Alkaline Earth Element Adsorption onto PAA-Coated Magnetic Nanoparticles. Energies, 10(2), 223. https://doi.org/10.3390/en10020223