Coupled Model of Heat and Mass Balance for Droplet Growth in Wet Steam Non-Equilibrium Homogeneous Condensation Flow
Abstract
:1. Introduction
2. Water Droplet Growth Model
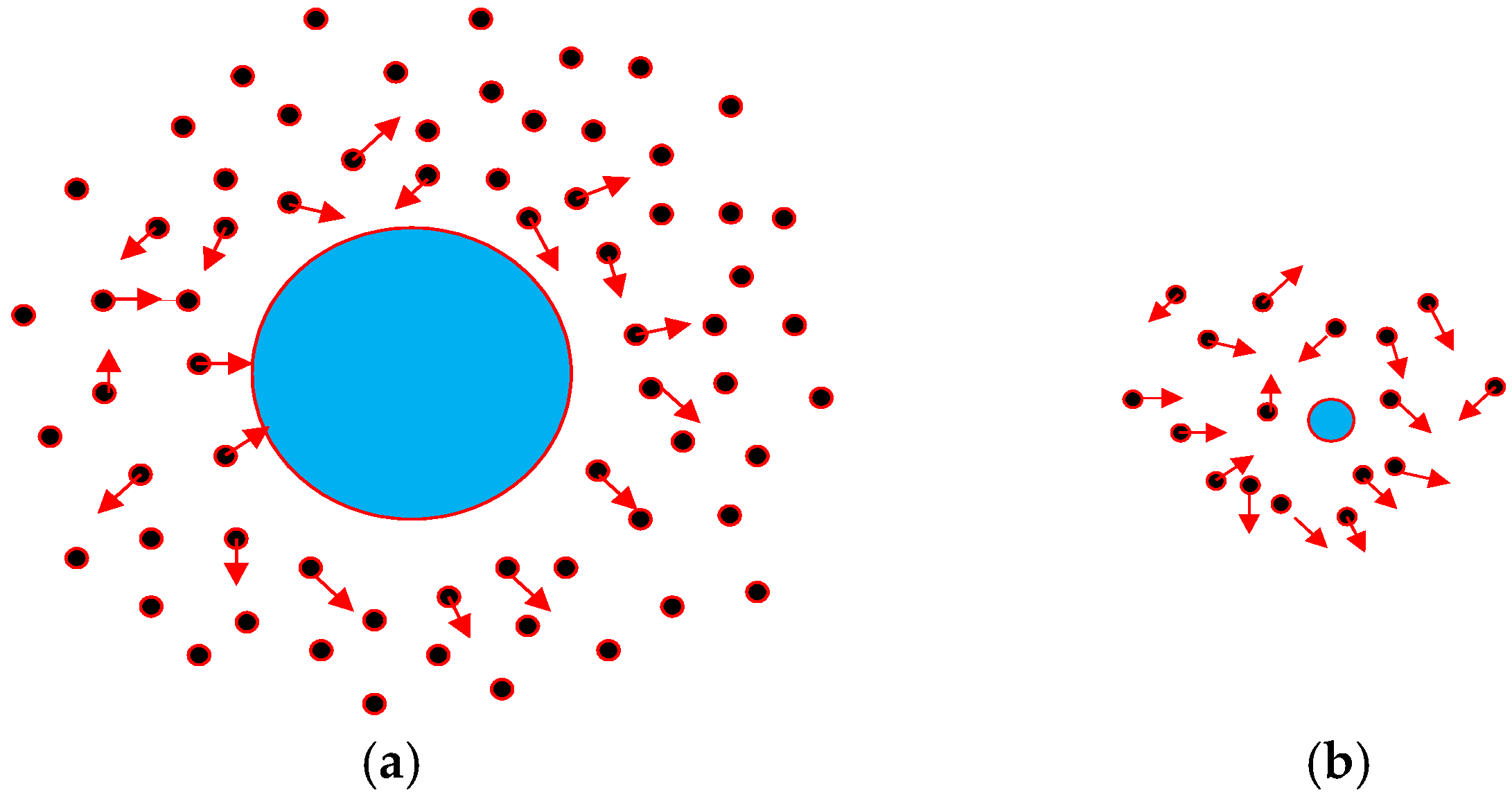
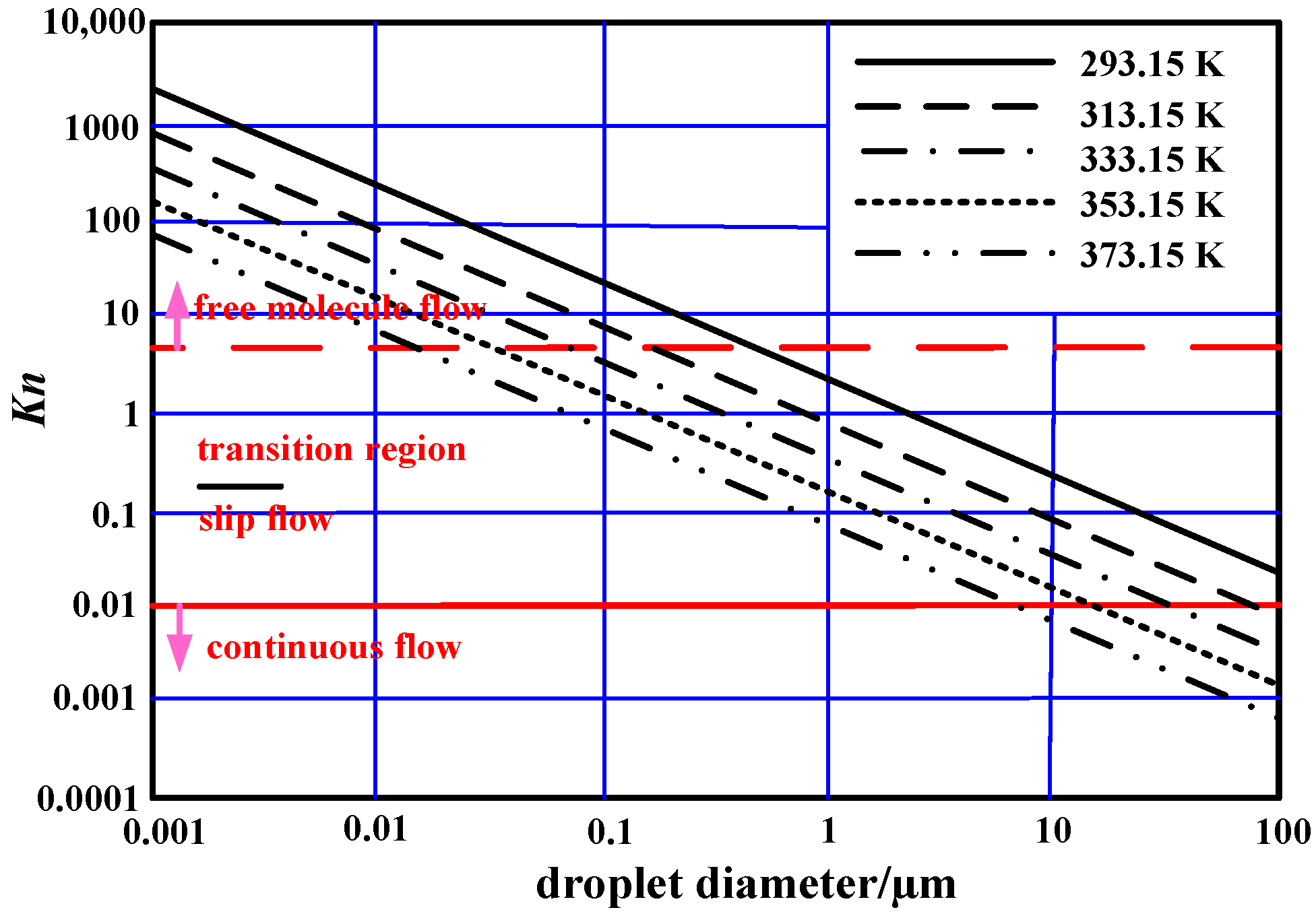
2.1. Droplet Growth Theory
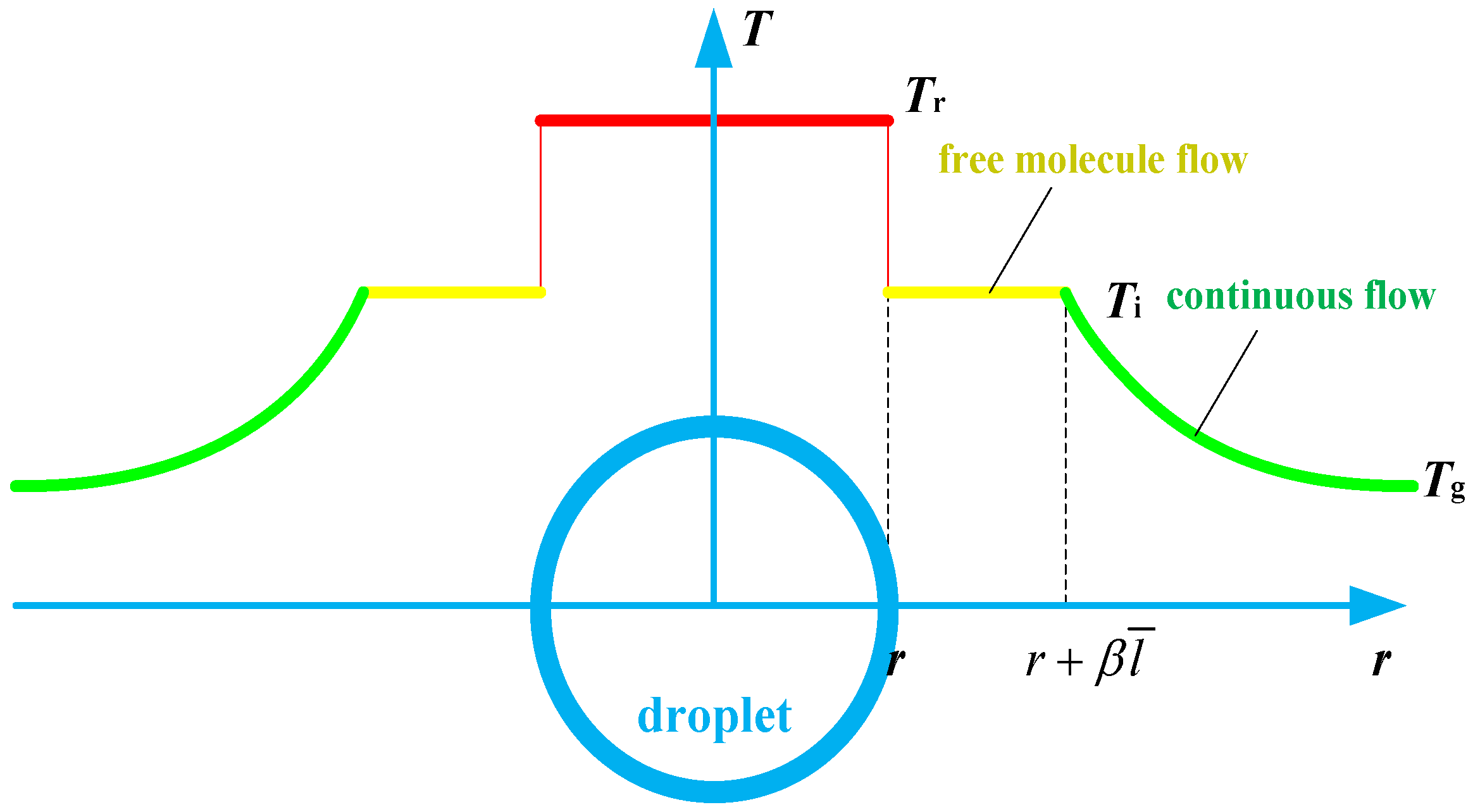
2.2. Coupled Model of Heat and Mass Balance
2.3. Droplet Surface Tension Correction
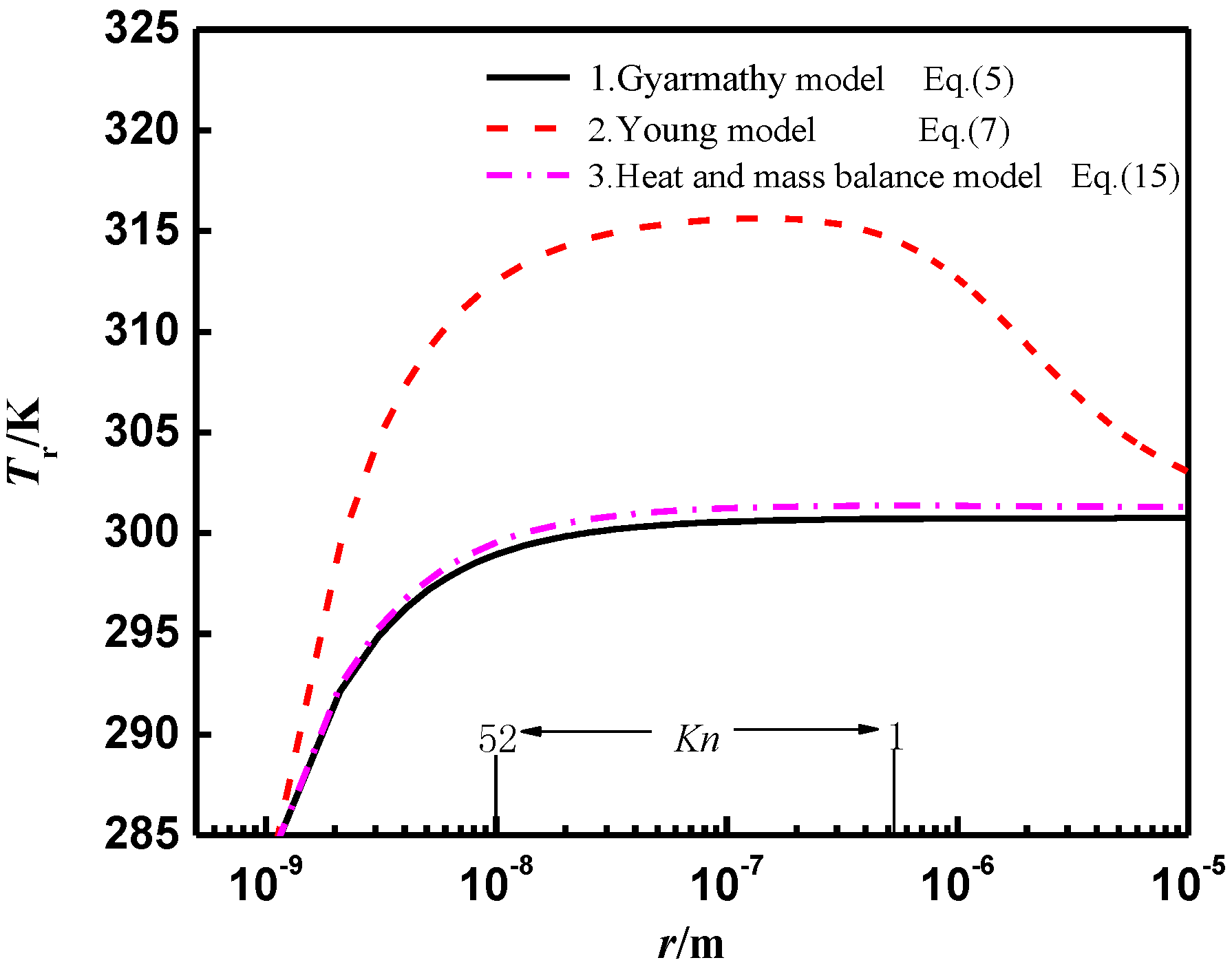
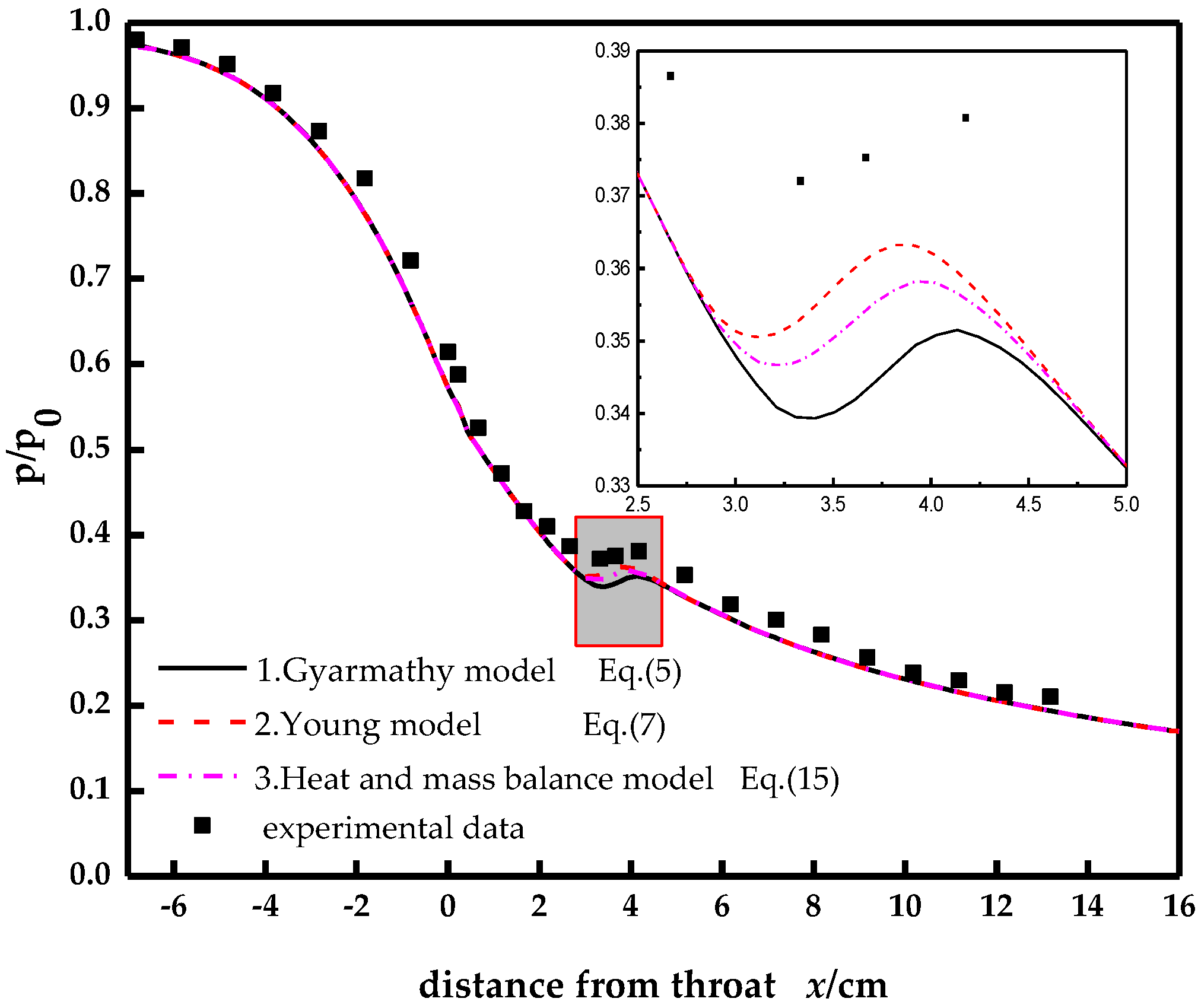
3. Calculation Results and Analysis
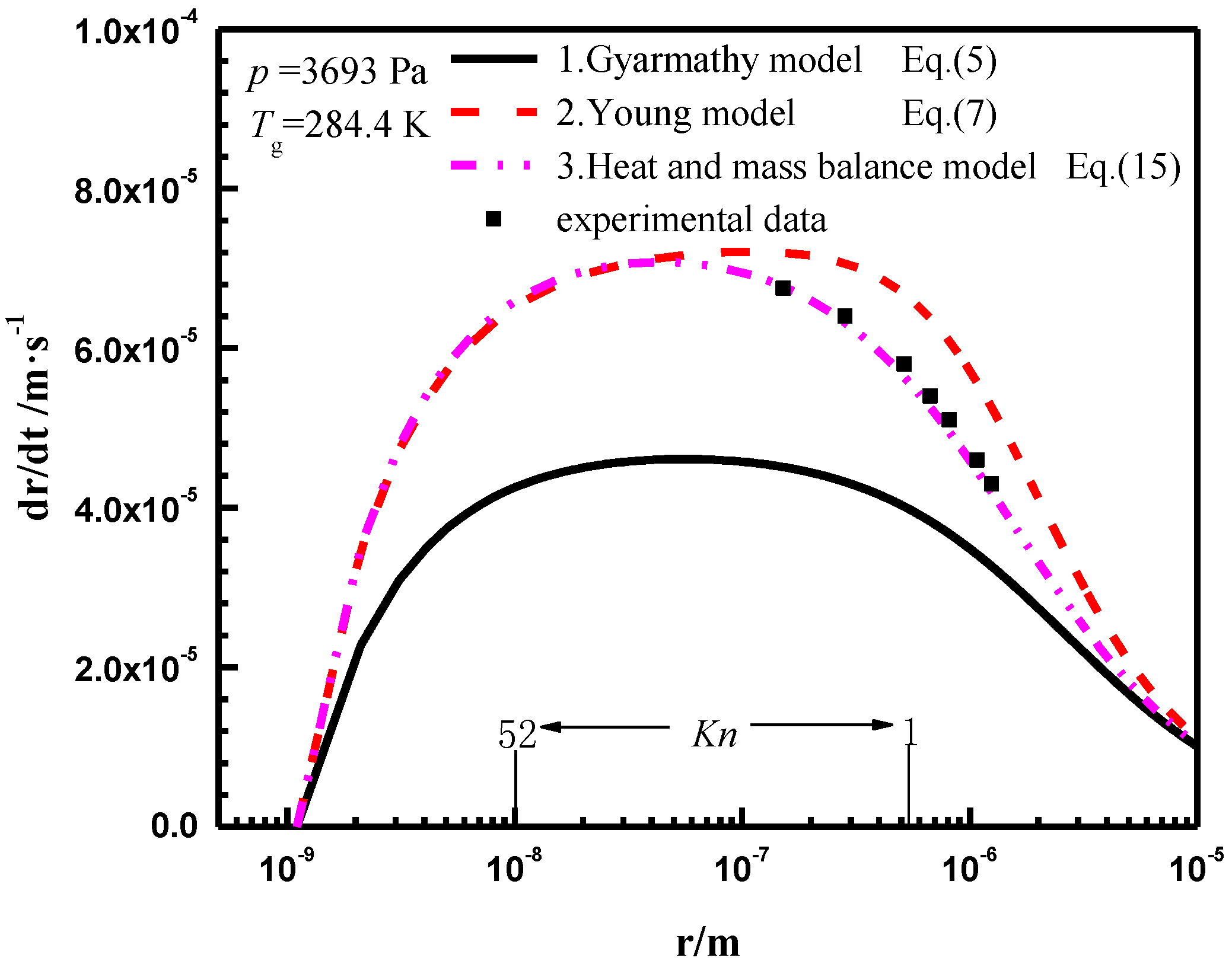
3.1. Modelling Verification
3.2. Wet Steam Condensation Flow Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanak, D.P.; Kolios, A.J.; Biliyok, C.; Manovic, V. Probabilistic performance assessment of a coal-fired power plant. Appl. Energy 2015, 141, 350–364. [Google Scholar] [CrossRef]
- Yousefi Rad, E.; Mahpeykar, M.R. A Novel Hybrid Approach for Numerical Modeling of the Nucleating Flow in Laval Nozzle and Transonic Steam Turbine Blades. Energies 2017, 10, 1285. [Google Scholar] [CrossRef]
- Yang, Y.; Walther, J.H.; Yan, Y.; Wen, C. CFD modelling of condensation process of water vapor in supersonic flows. Appl. Therm. Eng. 2017, 115, 1357–1362. [Google Scholar] [CrossRef]
- Han, Z.; Han, X.; Li, H.; Li, P. Comparative study of homogeneous nucleation rate models for wet steam condensing flows. Korean J. Chem. Eng. 2016, 33, 3487–3492. [Google Scholar] [CrossRef]
- Han, Z.; Han, X.; Wang, Z. Numeric simulation of wet-steam two-phase condensing flow in a steam turbine cascade. J. Braz. Soc. Mech. Sci. Eng. 2017, 39, 1189–1199. [Google Scholar] [CrossRef]
- Gyarmathy, G. The spherical droplet in gaseous carrier streams: Review and synthesis. Multiph. Sci. Technol. 1982, 1, 99–279. [Google Scholar] [CrossRef]
- Hill, P.G. Condensation of water vapor during supersonic expansion in nozzles. J. Fluid Mech. 1966, 25, 593–620. [Google Scholar] [CrossRef]
- White, A.J.; Young, J.B.; Walters, P.T. Experimental validation of condensing flow theory for a stationary cascade of steam turbine blades. Philos. Trans. R. Soc. Lond. 1996, 354, 59–88. [Google Scholar] [CrossRef]
- Bakhtar, F.; Young, J.B.; White, A.J.; Simpson, D.A. Classical nucleation theory and its application to condensing steam flow calculations. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2005, 219, 1315–1333. [Google Scholar] [CrossRef]
- Heiler, M. Instationäre Phänomene in Homogen/Heterogen Kondensierenden Düsen und Turbinenströmungen; Universität Karlsruhe: Karlsruhe, Germany, 1999. [Google Scholar]
- Delale, C.F.; Meier, G.E.A. A semi phenomenological droplet model of homogeneous nucleation from the vapor phase. J. Chem. Phys. 1993, 98, 9850–9858. [Google Scholar] [CrossRef]
- Bakhtar, F.; Zamri, M. On the Performance of a Cascade of Improved Nozzle Blades in Nucleating Steam. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2011, 225, 1649–1671. [Google Scholar] [CrossRef]
- Bakhtar, F.; Mamat, Z.A.; Jadayel, O.C. On the performance of a cascade of improved turbine nozzle blades in nucleating steam. Part 2: Wake traverses. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2009, 223, 1915–1929. [Google Scholar] [CrossRef]
- Yang, Y.; Li, A.; Wen, C. Optimization of static vanes in a supersonic separator for gas purification. Fuel Process. Technol. 2016, 156, 265–270. [Google Scholar] [CrossRef]
- Young, J.B. Two-dimensional, nonequilibrium, wet-steam calculation for nozzles and turbines cascade. J. Turbomach. 1992, 114, 567–579. [Google Scholar] [CrossRef]
- Wen, C.; Yang, Y.; Walther, J.H.; Pang, K.M.; Feng, Y. Effects of delta wing on the particle flow in a novel gas supersonic separator. Powder Technol. 2016, 304, 261–267. [Google Scholar] [CrossRef]
- Bakhtar, F.; Mashmoushy, H.; Jadayel, O.C. Calibration characteristics of a three-hole probe and a static tube in wet steam. Int. J. Heat Fluid Flow 2001, 22, 537–542. [Google Scholar] [CrossRef]
- Chandler, K.; White, A.; Young, J. Non-equilibrium wet-steam calculations of unsteady low-pressure turbine flows. Proc. Inst. Mech. Eng. Part A J. Power Energy 2014, 228, 143–152. [Google Scholar] [CrossRef]
- Luo, X.; Prast, B.; Dongen, M.V.; Hoeijmakers, H.W.M.; Yang, J. On phase transition in compressible flows: modelling and validation. J. Fluid Mech. 2006, 548, 403–430. [Google Scholar] [CrossRef]
- Gerber, A.G. Two-phase eulerian/lagrangian model for nucleating steam flow. J. Fluids Eng. 2002, 124, 465–475. [Google Scholar] [CrossRef]
- Xu, T.; Huang, Y. Development of experimental device for spontaneous condensation of supersaturated water vapor and determination of Wilson position of actual flow. J. Xi'an Jiaotong Univ. 1984, 4, 56–68. [Google Scholar]
- Tolman, R.C. Consideration of the Gibbs theory of surface tension. J. Chem. Phys. 1948, 16, 758–774. [Google Scholar] [CrossRef]
- Kashchiev, D. The kinetic approach to nucleation. Cryst. Res. Technol. 1984, 19, 1413–1423. [Google Scholar] [CrossRef]
- Benson, G.C.; Shuttleworth, R. The surface energy of small nuclei. J. Chem. Phys. 1951, 19, 130–131. [Google Scholar] [CrossRef]
- Peters, F.; Meyer, K.A.J. Measurement and interpretation of growth of mon dispersed water droplets suspended in pure vapour. Int. J. Heat Mass Transf. 1995, 38, 3285–3293. [Google Scholar] [CrossRef]
- Yang, Y.; Wen, C. CFD modeling of particle behavior in supersonic flows with strong swirls for gas separation. Sep. Purif. Technol. 2017, 174, 22–28. [Google Scholar] [CrossRef]
- Wölk, J.; Strey, R.; Heath, C.H.; Wyslouzil, B.E. Empirical function for homogeneous water nucleation rates. J. Chem. Phys. 2002, 117, 4954–4960. [Google Scholar] [CrossRef]
- Han, X.; Han, Z.; Li, P. Influence of external particles on heterogeneous condensation flow in cascades. Trans. Can. Soc. Mech. Eng. 2017, 41, 265–280. [Google Scholar]



| Year | Total Power Generation/108 kW·h | Thermal Power Generation/108 kW·h | Nuclear Power Generation/108 kW·h |
|---|---|---|---|
| 2010 | 42,280 | 34,145 (80.76%) | 768 (1.82%) |
| 2011 | 47,217 | 38,975 (82.54%) | 874 (1.85%) |
| 2012 | 49,774 | 39,108 (78.60%) | 982 (1.97%) |
| 2013 | 53,474 | 41,900 (78.36%) | 1121 (2.10%) |
| 2014 | 56,801 | 43,030 (75.76%) | 1332 (2.35%) |
| 2015 | 57,399 | 42,307 (73.71%) | 1714 (2.99%) |
| 2016 | 59,111 | 43,958 (74.37%) | 2127 (3.60%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Han, Z.; Zeng, W.; Qian, J.; Wang, Z. Coupled Model of Heat and Mass Balance for Droplet Growth in Wet Steam Non-Equilibrium Homogeneous Condensation Flow. Energies 2017, 10, 2033. https://doi.org/10.3390/en10122033
Han X, Han Z, Zeng W, Qian J, Wang Z. Coupled Model of Heat and Mass Balance for Droplet Growth in Wet Steam Non-Equilibrium Homogeneous Condensation Flow. Energies. 2017; 10(12):2033. https://doi.org/10.3390/en10122033
Chicago/Turabian StyleHan, Xu, Zhonghe Han, Wei Zeng, Jiangbo Qian, and Zhi Wang. 2017. "Coupled Model of Heat and Mass Balance for Droplet Growth in Wet Steam Non-Equilibrium Homogeneous Condensation Flow" Energies 10, no. 12: 2033. https://doi.org/10.3390/en10122033





