Lithium-Sulfur Battery Technology Readiness and Applications—A Review
Abstract
:1. Introduction
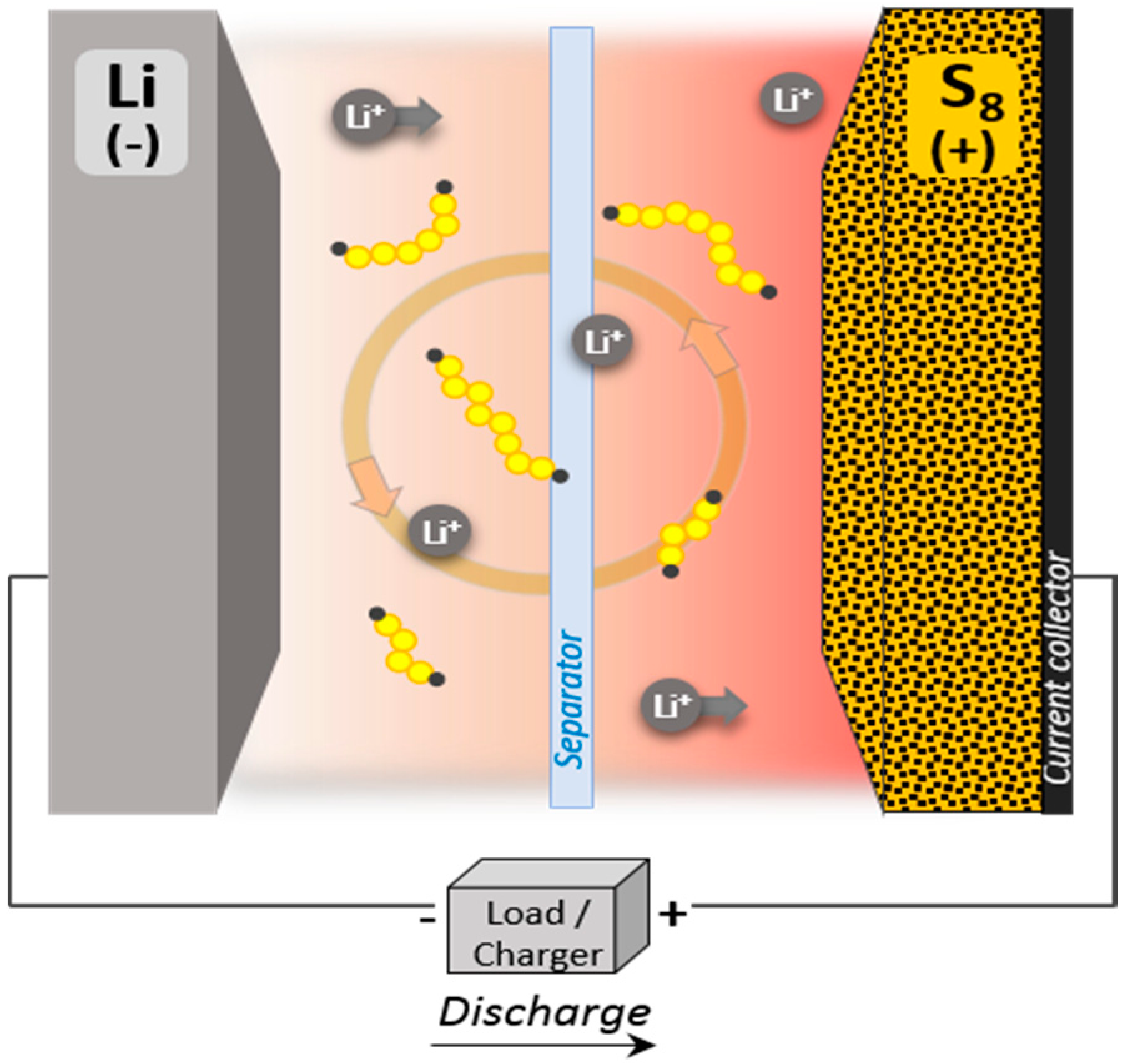
2. Li-S Battery Technology: Advantages and Limitations
2.1. Advantages of Lithium-Sulfur System
2.2. Limitations of Lithium-Sulfur
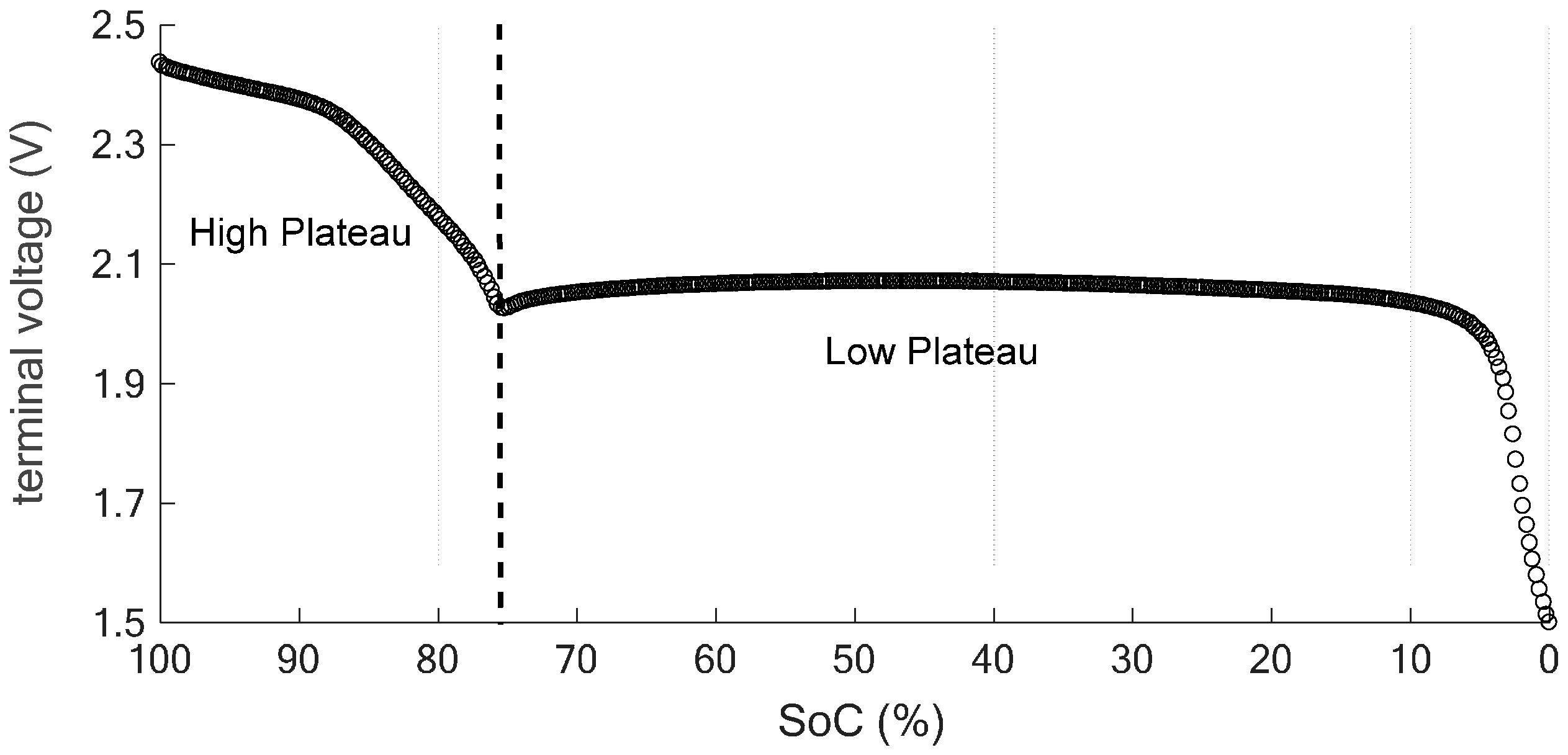
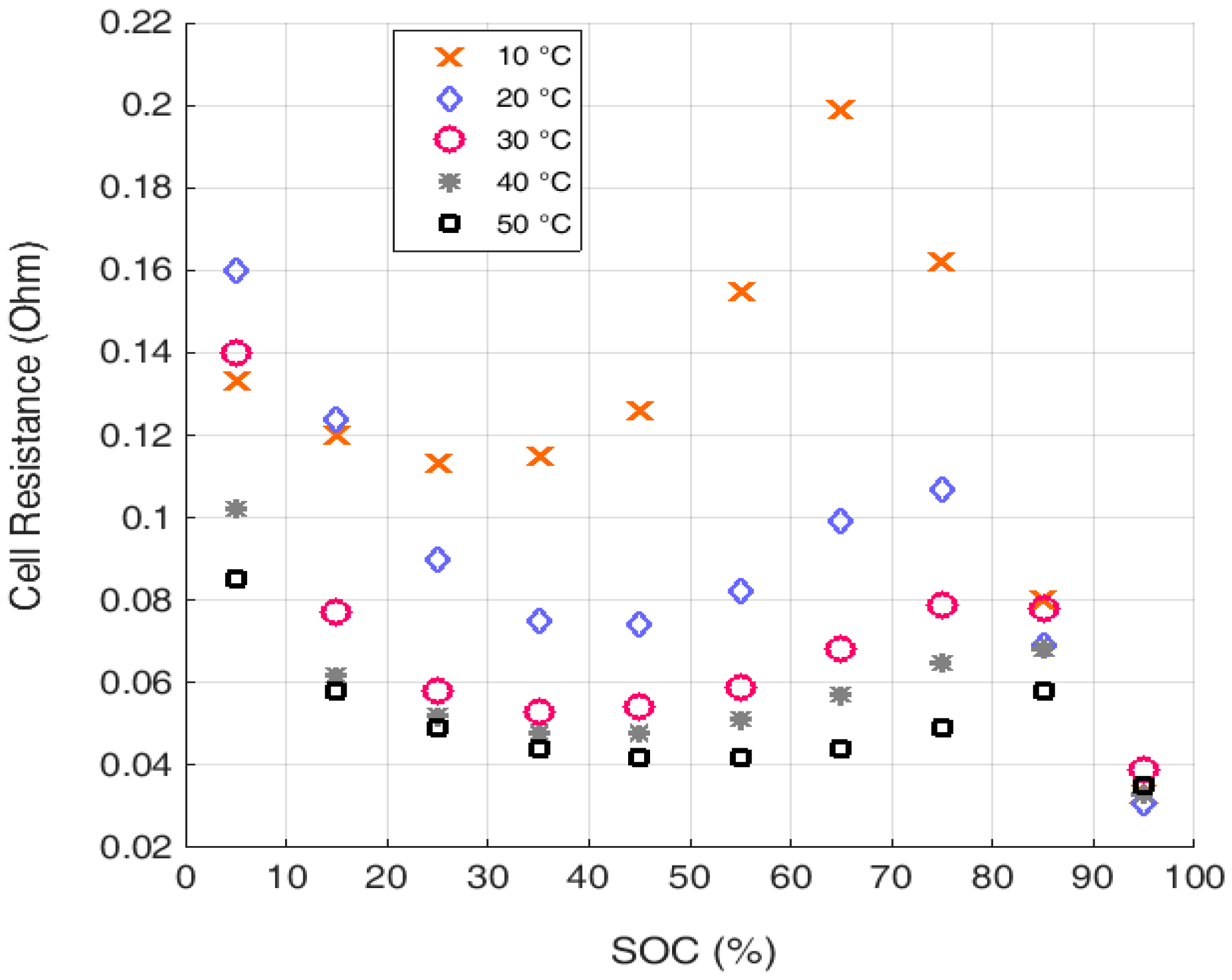
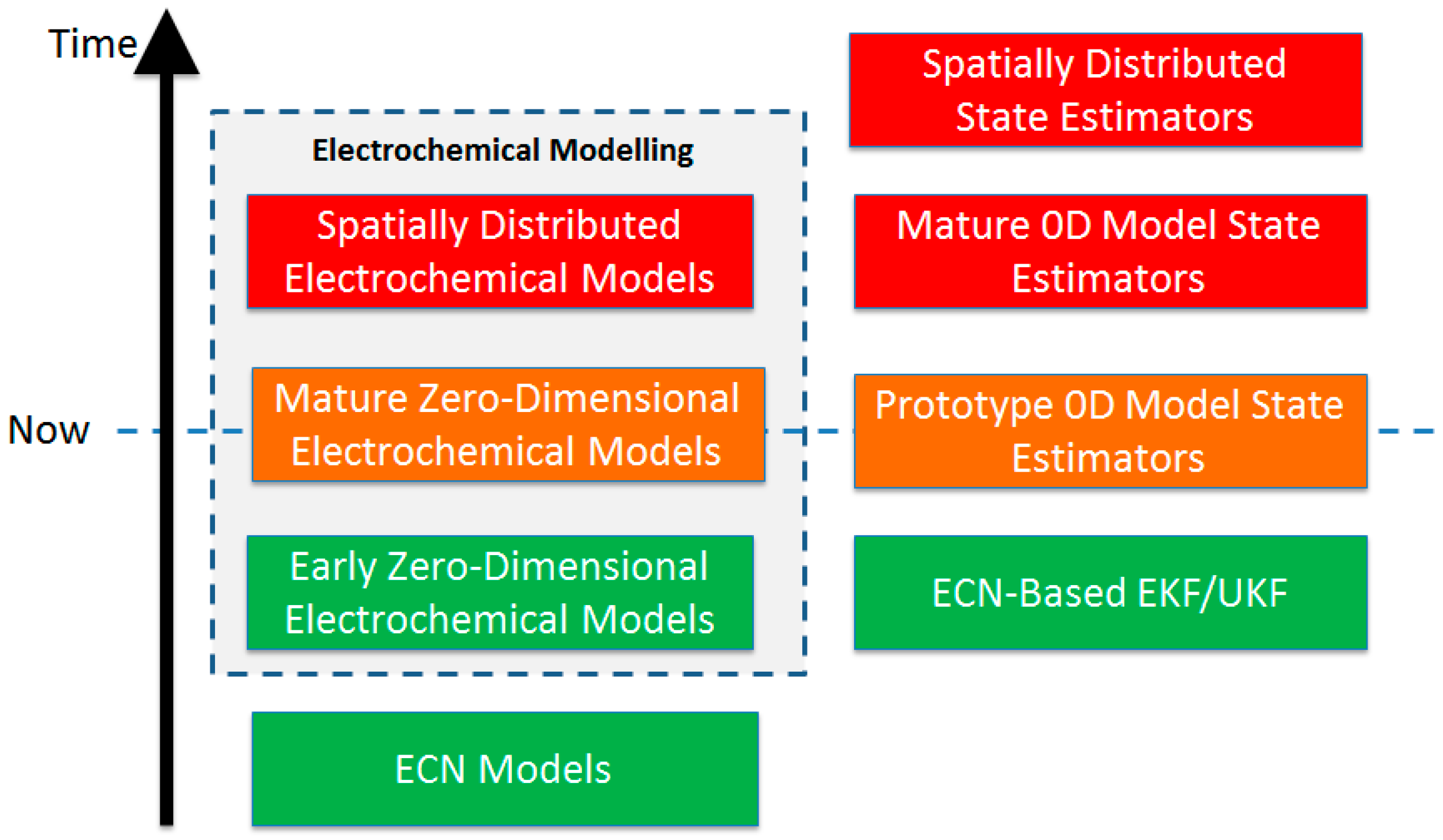
3. State-of-the-Art Li-S Cell Modelling and State Estimation Techniques
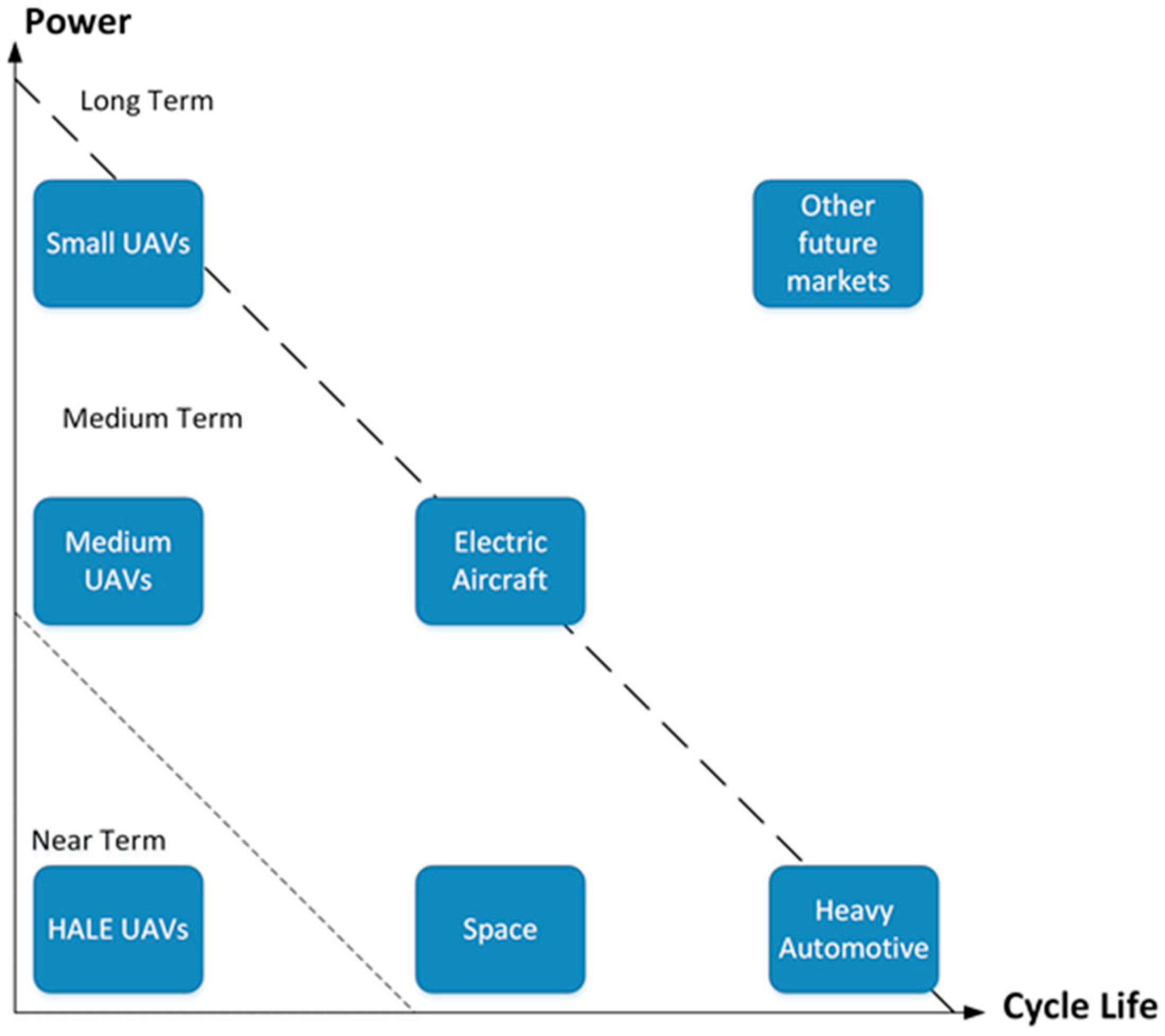
4. Current and Future Applications of Li-S Battery
4.1. Current Applications
4.2. Future Applications
4.2.1. Li-S with Improved Cycle Life
4.2.2. Li-S with Improved Power
4.2.3. Li-S with Improved Cycle Life and Power
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.; Xie, H.; Kim, C.-S.; Zaghib, K.; Mauger, A.; Juliend, C.M. Advances in Lithium-Sulfur Batteries. Mater. Sci. Eng. R Rep. 2017, 121, 1–29. [Google Scholar] [CrossRef]
- Chen, L.; Shaw, L.L. Recent advances in lithium–sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.; Zu, C.; Su, Y. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. J. Nanoscale 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Z.W.; Peng, H.J. A Toolbox for Lithium–Sulfur Battery Research: Methods and Protocols. J. Small Methods 2017, 1. [Google Scholar] [CrossRef]
- Kawase, A.; Shirai, S.; Yamoto, Y. Electrochemical reactions of lithium–sulfur batteries: An analytical study using the organic conversion technique. Phys. Chem. Chem. Phys. 2014, 16, 9344. [Google Scholar] [CrossRef] [PubMed]
- Diaoz, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425. [Google Scholar]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Minton, G.; Purkayastha, R.; Lue, L. A Non-Electroneutral Model for Complex Reaction-Diffusion Systems Incorporating Species Interactions. J. Electrochem. Soc. 2017, 164, E3276–E3290. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Electric Vehicle Battery Parameter Identification and SOC Observability Analysis: NiMH and Li-S Case Studies. IET Power Electron. 2017. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Purkayastha, R.; O’Neill, L.; Walus, S. Lithium-Sulfur Cell Equivalent Circuit Network Model Parameterization and Sensitivity Analysis. IEEE Trans. Veh. Technol. 2017, 66, 7711–7721. [Google Scholar] [CrossRef]
- OXIS Energy. Available online: http://www.oxisenergy.com (accessed on 29 September 2017).
- Sion Power. Available online: http://www.sionpower.com (accessed on 29 September 2017).
- LG Chem and Sion Power Partnership. Available online: http://pushevs.com/2016/10/07/lg-chem-sion-power-partnership/ (accessed on 29 September 2017).
- Sony Developing High-Capacity Rechargeable Battery. Available online: http://techon.nikkeibp.co.jp/atclen/news_en/15mk/121800252/?n_cid=nbptec_tecrs (accessed on 29 September 2017).
- Agostini, M.; Hassoun, J.; Yamada, T.; Aihara, Y.; Scrosati, B. All Solid-State Lithium–Sulfur Battery Using a Glass-Type P2S5–Li2S Electrolyte: Benefits on Anode Kinetics. J. Electrochem. Soc. 2015, 162, A646–A651. [Google Scholar]
- Jia, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010. [Google Scholar] [CrossRef]
- Hagen, M.; Fanz, P.; Tübke, J. Cell energy density and electrolyte/sulfur ratio in Li–S cells. J. Power Sources 2014, 264, 30–34. [Google Scholar] [CrossRef]
- Hagen, M.; Hanselmann, D.; Ahlbrecht, K.; Maça, R.; Gerber, D.; Tübke, J. Lithium-sulfur cells: The Gap between the state-of-the-art and the requirements for high energy battery cells. Adv. Energy Mater. 2015, 5, 1401986. [Google Scholar] [CrossRef]
- Hunt, I.; Patel, Y.; Szczygielski, M.; Kabacik, L.; Offer, G. Lithium sulfur battery nail penetration test under load. J. Energy Storage 2015, 2, 25–29. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Fronczek, D.N.; Bessler, W.G. Mechanistic modeling of polysulfide shuttle and capacity loss in lithium-sulfur batteries. J. Power Sources 2014, 259, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Walus, S.; Barchasz, C.; Colin, J.F.; Martin, J.F.; Elkaïm, E.; Leprêtre, J.C.; Alloin, F. New insight into the working mechanism of lithium–sulfur batteries: In situ and operando X-ray diffraction characterization. Chem. Commun. 2013, 49, 7899–7901. [Google Scholar] [CrossRef] [PubMed]
- Waluś, S.; Barchasz, C.; Bouchet, R. Lithium/Sulfur Batteries upon Cycling: Structural Modifications and Species Quantification by In Situ and Operando X-ray Diffraction Spectroscopy. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Barghamadi, M.; Best, A.S.; Bhatt, A.I.; Hollenkamp, A.F.; Musameh, M.; Ress, R.J. Lithium-sulfur batteries—The solution is in the electrolyte, but is the electrolyte a solution? Energy Environ. Sci. 2014, 7, 3902–3920. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Shuttle phenomenon the irreversible oxidation mechanism of sulfur active material in Li-S battery. J. Power Sources 2013, 235, 181–186. [Google Scholar] [CrossRef]
- Zoski, C.G. Handbook of Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Luo, J.; Lee, R.; Jin, J.; Weng, Y.; Fang, C.; Wu, N.L. A dual-functional polymer coating on a lithium anode for suppressing dendrite growth and polysulfide shuttling in Li–S batteries. Chem. Commun. 2017, 53, 963. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Seh, Z.W.; Yan, K.; Fu, Z.; Tang, P.; Lu, Y.; Zhang, R.; Legut, D.; Cui, Y.; Zhang, Q. Theoretical Investigation of 2D Layered Materials as Protective Films for Lithium and Sodium Metal Anodes. Adv. Energy Mater. 2017, 7, 1602528. [Google Scholar] [CrossRef]
- McCloskey, B. Attainable Gravimetric and Volumetric Energy Density of Li–S and Li Ion Battery Cells with Solid Separator-Protected Li Metal Anodes. J. Phys. Chem. Lett. 2015, 6, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Otaegui, L. Estimation of energy density of Li-S batteries with liquid and solid Electrolytes. J. Power Sources 2016, 326, 1–5. [Google Scholar] [CrossRef]
- Scrosati, B.; Abraham, K.M.; van Schalkwijk, W.A.; Hassoun, J. Lithium Batteries: Advanced Technologies and Applications; Wiley: New York, NY, USA, 2013; Chapter 8. [Google Scholar]
- Pressure Tolerant Lithium Sulfur Battery. Available online: http://noc.ac.uk/projects/pressure-tolerant-lithium-sulfur-battery (accessed on 29 September 2017).
- Jeschull, F.; Brandell, D.; Edströma, K.; Lacey, M. A stable graphite negative electrode for the lithium–sulfur battery. Chem. Commun. 2015, 51, 17100–17103. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Dörfler, S.; Piwko, M.; Wisser, F.M.; Jaumann, T.; Ahrens, E.; Giebeler, L.; Althues, H.; Schädlich, S.; Grothe, J.; et al. High Area Capacity Lithium-Sulfur Full-cell Battery with Prelitiathed Silicon Nanowire-Carbon Anodes for Long Cycling Stability. Sci. Rep. 2016, 6, 27982. [Google Scholar] [CrossRef] [PubMed]
- Piwko, M.; Kuntze, T.; Winkler, S.; Straach, S.; Härtel, P.; Althues, H.; Kaskela, S. Hierarchical columnar silicon anode structures for high energy density lithium sulfur batteries. J. Power Sources 2017, 351, 183–191. [Google Scholar] [CrossRef]
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.G. Anodes for Rechargeable Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.; Hou, T.; Huang, J.; Chen, C.; Wang, D.; Cheng, X.; Wei, F.; Zhang, Q. Powering Lithium–Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, G.; Yang, Y.; Seh, Z.W.; Liu, N.; Cui, Y. High-performance hollow sulfur nanostructured battery cathode through a scalable, room temperature, one-step, bottom-up approach. Proc. Natl. Acad. Sci. USA 2013, 110, 7148–7153. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S.; Wild, M. A review on electric vehicle battery modelling: From lithium-ion toward lithium-sulphur. Renew. Sustain. Energy Rev. 2016, 56, 1008–1021. [Google Scholar] [CrossRef]
- Ghaznavi, M.; Chen, P. Sensitivity analysis of a mathematical model of lithium-sulfur cells part I. J. Power Sources 2014, 257, 394–401. [Google Scholar] [CrossRef]
- Ghaznavi, M.; Chen, P. Sensitivity analysis of a mathematical model of lithium-sulfur cells part II. J. Power Sources 2014, 257, 402–411. [Google Scholar] [CrossRef]
- Neidhardt, J.P.; Fronczek, D.N.; Jahnke, T.; Danner, T.; Horstmann, B.; Bessler, W.G. A Flexible Framework for Modeling Multiple Solid, Liquid and Gaseous Phases in Batteries and Fuel Cells. J. Electrochem. Soc. 2012, 159, A1528–A1542. [Google Scholar] [CrossRef]
- Marinescu, M.; Zhang, T.; Offer, G.J. A zero dimensional model of lithium–sulfur batteries during charge and discharge. Phys. Chem. Chem. Phys. 2016, 18, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Knap, V.; Stroe, D.; Swierczynski, M.; Teodorescu, R.; Schaltz, E. Investigation of the Self-Discharge Behavior of Lithium-Sulfur Batteries. J. Electrochem. Soc. 2016, 163, A911–A916. [Google Scholar] [CrossRef]
- Propp, K.; Marinescu, M.; Auger, D.; O’Neill, L.; Fotouhi, A.; Somasundaram, K.; Offer, G.; Minton, G.; Longo, S.; Wild, M.; et al. Multi-temperature state-dependent equivalent circuit discharge model for lithium-sulfur batteries. J. Power Sources 2016, 328, 289–299. [Google Scholar] [CrossRef]
- Aström, K.J.; Murray, R.M. Feedback Systems: An Introduction for Scientists and Engineers; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Vinnicombe, G. Uncertainty and Feedback: ℌ∞ Loop-Shaping and the υ-Gap Metric; Imperial College Press: London, UK, 2000. [Google Scholar]
- Kalman, R.E. A New Approach to Linear Filtering and Prediction Problems. Trans. AMSE J. Basic Eng. 1960, 82, 35–45. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs—Part 1. Background. J. Power Sources 2004, 134, 252–261. [Google Scholar] [CrossRef]
- Plett, G.L. ‘Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs—Part 2. Modelling and Identification’. J. Power Sources 2004, 134, 262–276. [Google Scholar] [CrossRef]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs—Part 3. State and Parameter Estimation. J. Power Sources 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Propp, K.; Auger, D.J.; Fotouhi, A.; Longo, S.; Knap, V. Kalman-variant estimators for state of charge in lithium-sulfur batteries. J. Power Sources 2017, 343, 254–267. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.J.; Propp, K.; Longo, S. Lithium-Sulfur Battery State-of-Charge Observability Analysis and Estimation. IEEE Trans. Power Electron. 2017. [Google Scholar] [CrossRef]
- Bizeray, A.M.; Zhao, S.; Duncan, S.R.; Howey, D.A. Lithium-ion battery thermal-electrochemical model-based state estimation using orthogonal collocation and a modified extended Kalman filter. J. Power Sources 2015, 296, 400–412. [Google Scholar] [CrossRef]
- Auger, D.J. Using Ideas from Control Theory in the Modelling and Management of Lithium-Sulfur Batteries; University of Oxford: Oxford, UK, 6 February 2017. [Google Scholar]
- Recommendations on the TRANSPORT OF DANGEROUS GOODS. Available online: http://www.batteryspace.com/prod-specs/UN_Test_Manual_Lithium_Battery_Requirements%205.1.pdf (accessed on 20 November 2017).
- OXIS Energy Advances its Lithium Sulfur (Li-S) Cell Technology to 400 Wh/kg. Available online: https://oxisenergy.com/oxis-energy-advances-lithium-sulfur-li-s-cell-technology-400whkg/ (accessed on 20 November 2017).
- Sion Power Delivers Next Generation Battery Performance through Patented Licerion® Technology. Available online: http://www.sionpower.com/media-center.php?code=sion-power-delivers-next-generation-battery-perfor (accessed on 20 November 2017).
- What is Project Loon. Available online: https://x.company/loon/ (accessed on 20 November 2017).
- The Technology behind Aquila. Available online: https://www.facebook.com/notes/mark-zuckerberg/the-technology-behind-aquila/10153916136506634/ (accessed on 20 November 2017).
- Zephyr, the High Altitude Pseudo-Satellite. Available online: http://defence.airbus.com/portfolio/uav/zephyr/ (accessed on 20 November 2017).
- And the 2016 Golden Balloon Goes to…. Available online: https://plus.google.com/+ProjectLoon/posts/2vGeQAXF4Qo (accessed on 20 November 2017).
- ‘Eternal’ Solar Plane’s Records Are Confirmed. Available online: http://www.bbc.co.uk/news/science-environment-12074162 (accessed on 20 November 2017).
- Advanced Space Transportation Program: Paving the Highway to Space. Available online: https://www.nasa.gov/centers/marshall/news/background/facts/astp.html (accessed on 20 November 2017).
- Elon Musk: Tesla Has ‘No Plans’ For an Electric Plane—Yet. Available online: https://www.inverse.com/article/32623-tesla-ceo-elon-musk-plane-yet (accessed on 20 November 2017).
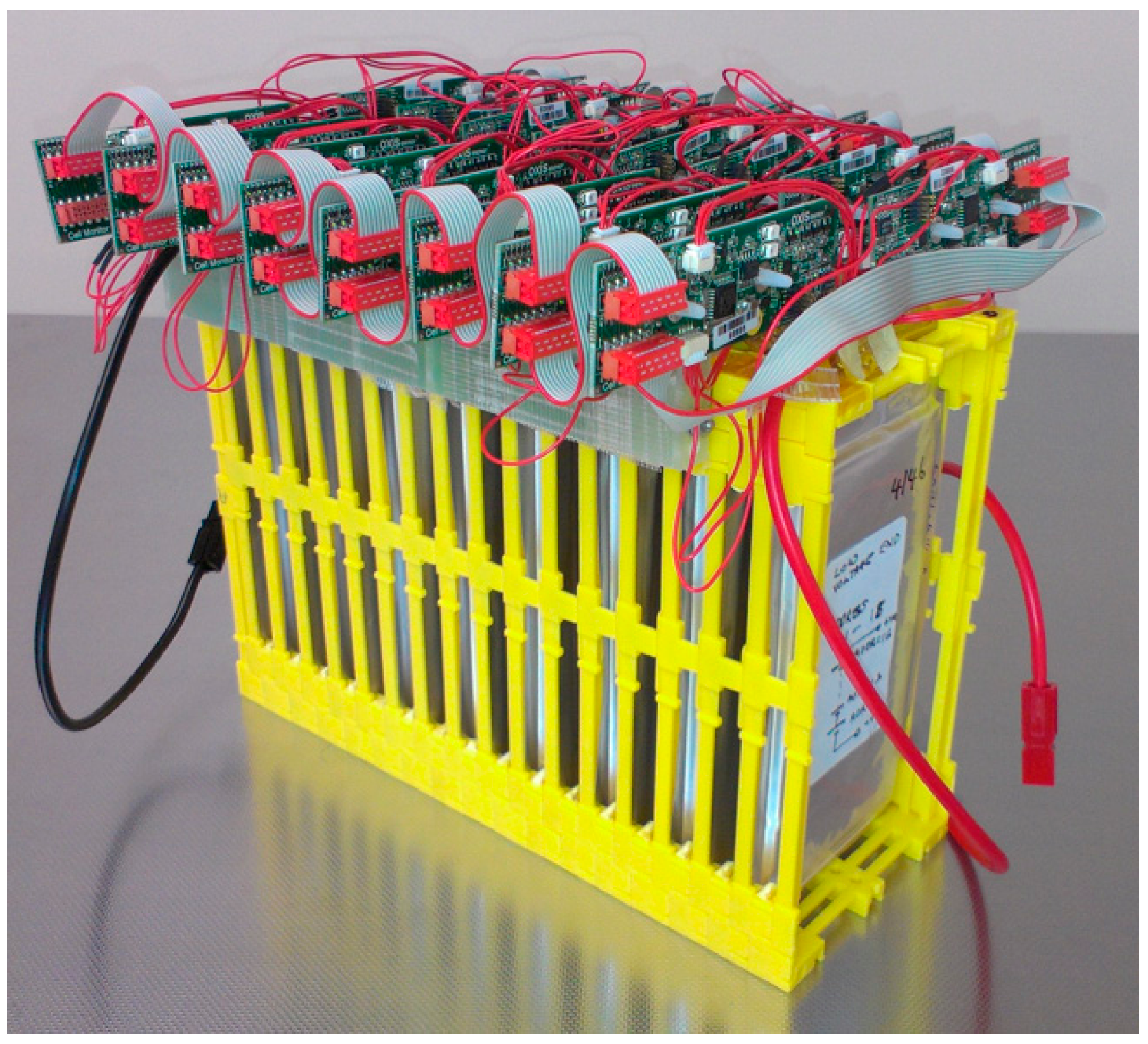
| Type | Rechargeable Lithium-Sulfur Pouch Cell Remarks: Li Metal Anode |
|---|---|
| cell dimension | 176 mm × 114 mm × 13.7 mm |
| maximum voltage | 2.45 V |
| nominal voltage | 2.05 V |
| capacity | 21 Ah (at 0.2 C, 30 °C) |
| weight | 233 ± 5 g |
| volume | 0.252 L |
| recommended charging condition in applications | 2.1 A constant current (C/10) at 30 °C (charge stop at 2.45 V or 11 h max charge time) |
| recommended discharging condition in applications | peak discharge current: 2 C maximum continuous discharge current (at 30 °C): 1.5 C |
| maximum continuous power | 60 W (at 30 °C) |
| optimum operating temperature | 30 °C |
| Cycle number before getting 80% of the beginning-of-life capacity | 180 cycles (cycled at 30 °C in a standard life test regime) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotouhi, A.; Auger, D.J.; O’Neill, L.; Cleaver, T.; Walus, S. Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies 2017, 10, 1937. https://doi.org/10.3390/en10121937
Fotouhi A, Auger DJ, O’Neill L, Cleaver T, Walus S. Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies. 2017; 10(12):1937. https://doi.org/10.3390/en10121937
Chicago/Turabian StyleFotouhi, Abbas, Daniel J. Auger, Laura O’Neill, Tom Cleaver, and Sylwia Walus. 2017. "Lithium-Sulfur Battery Technology Readiness and Applications—A Review" Energies 10, no. 12: 1937. https://doi.org/10.3390/en10121937
APA StyleFotouhi, A., Auger, D. J., O’Neill, L., Cleaver, T., & Walus, S. (2017). Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies, 10(12), 1937. https://doi.org/10.3390/en10121937






