Gasification under CO2–Steam Mixture: Kinetic Model Study Based on Shared Active Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Char Preparation
2.2. Isothermal Gasification Tests
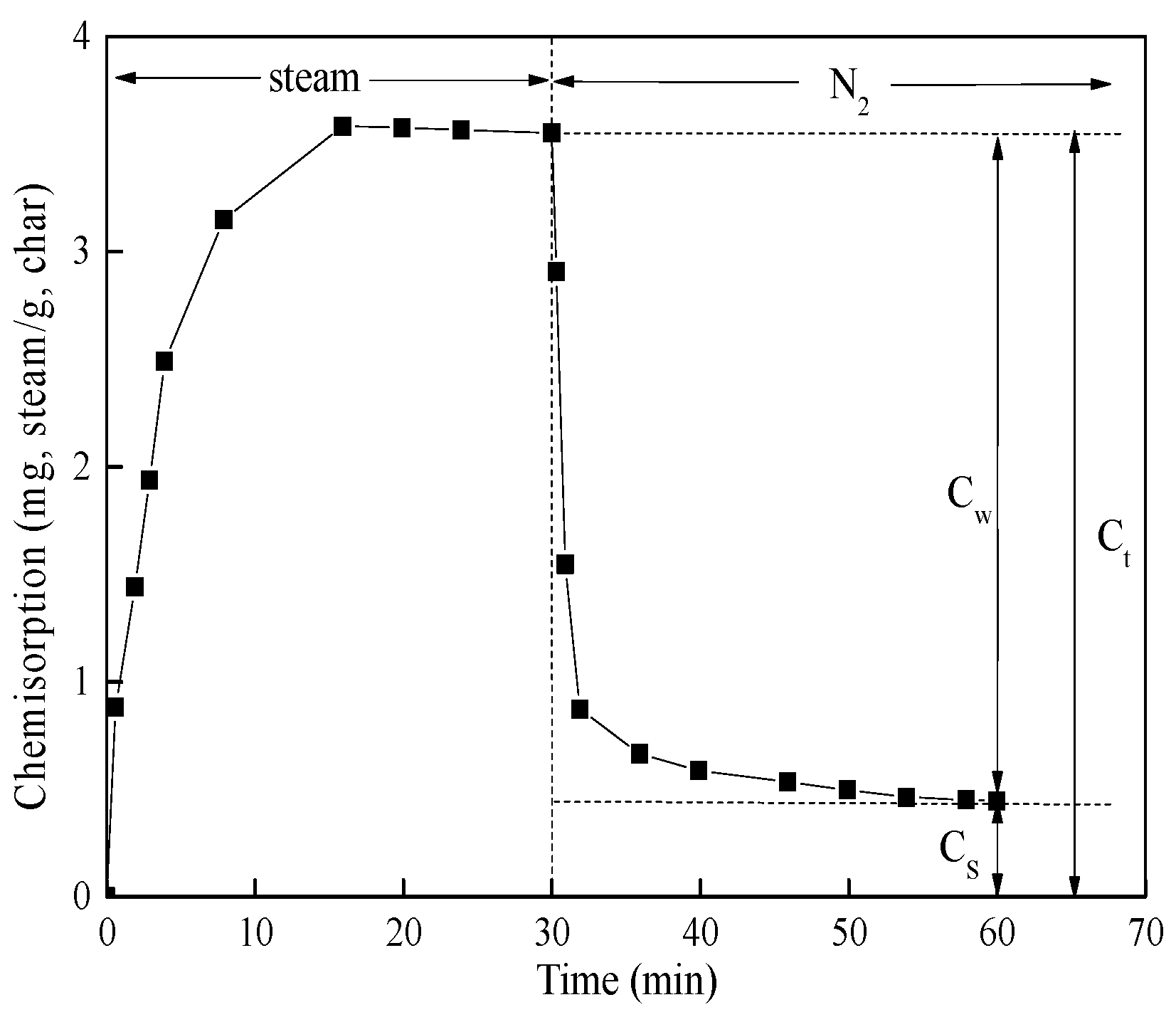
2.3. CO2/Steam Chemisorption
3. Results and Discussion
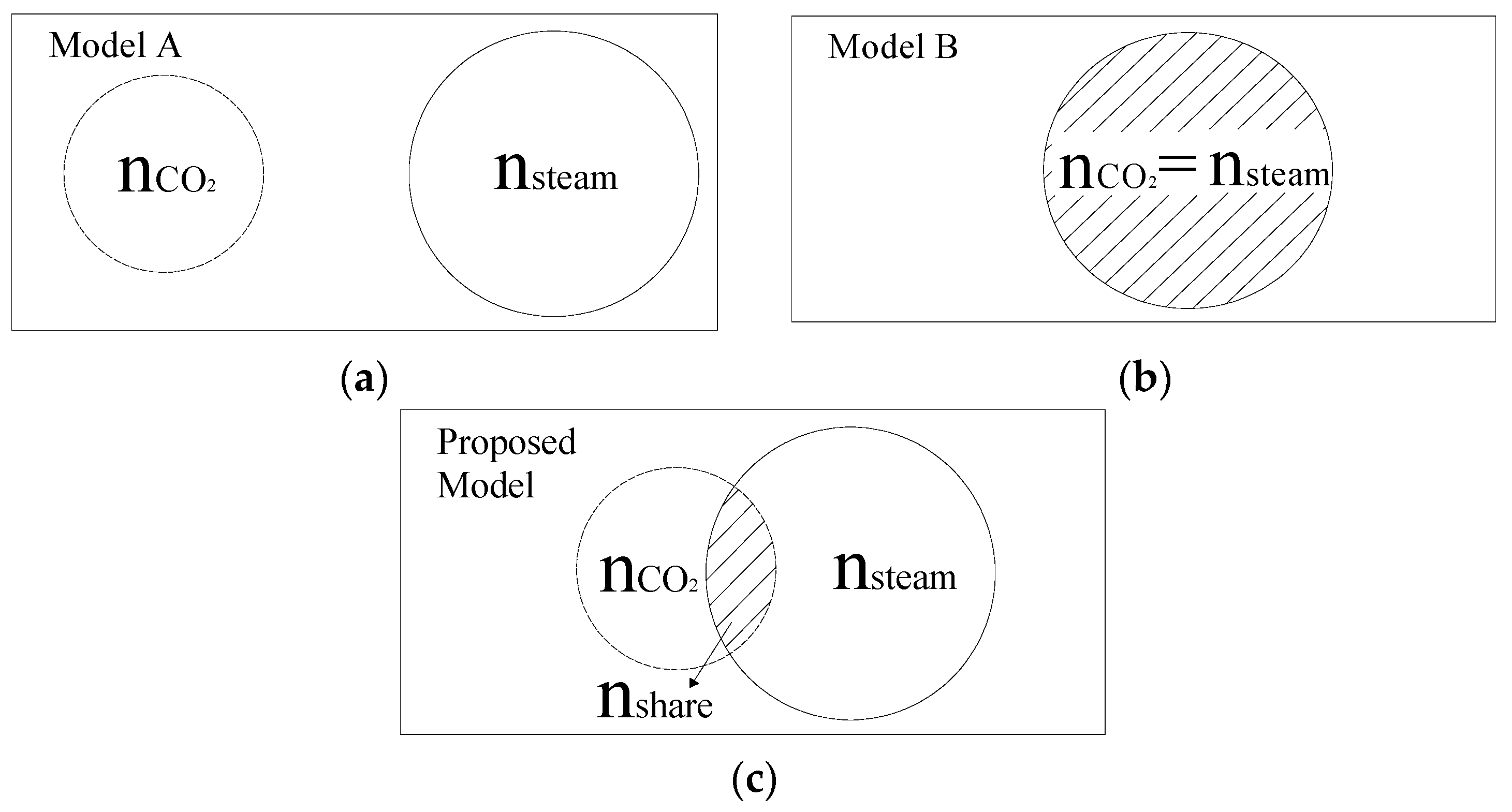
3.1. Choice of Kinetic Model of Char–CO2–Steam Gasification
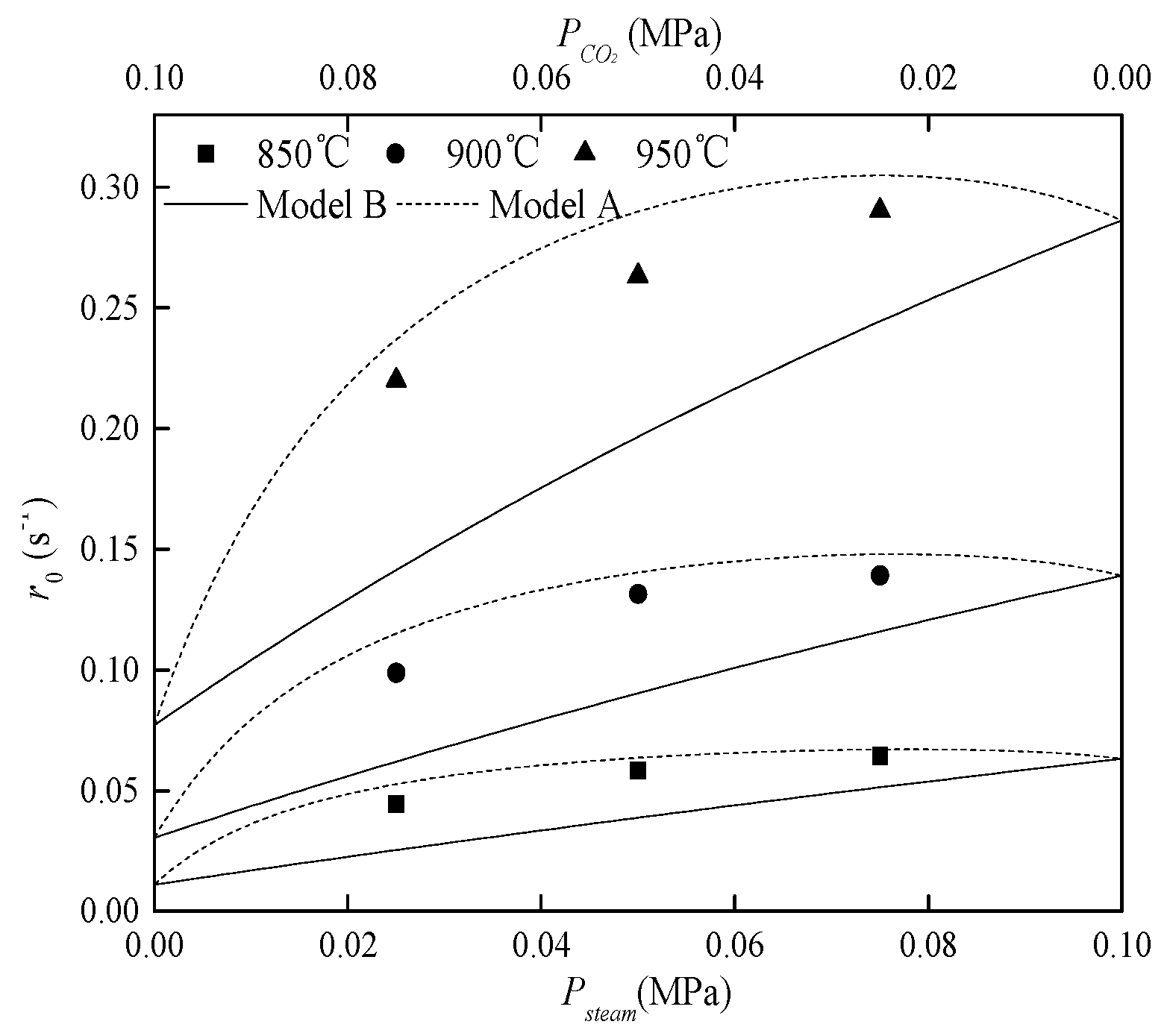
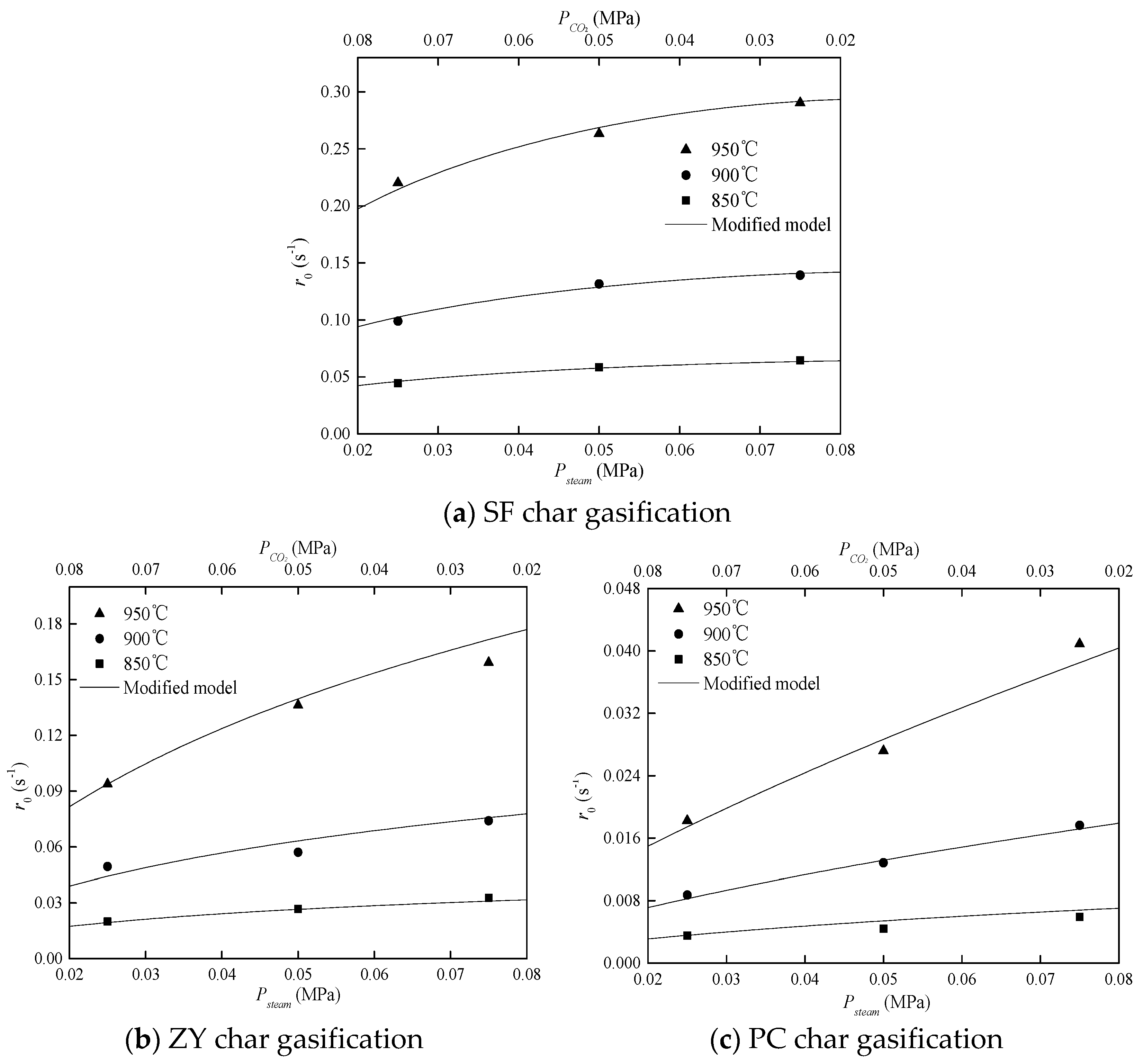
3.2. Validation of the Modified Model
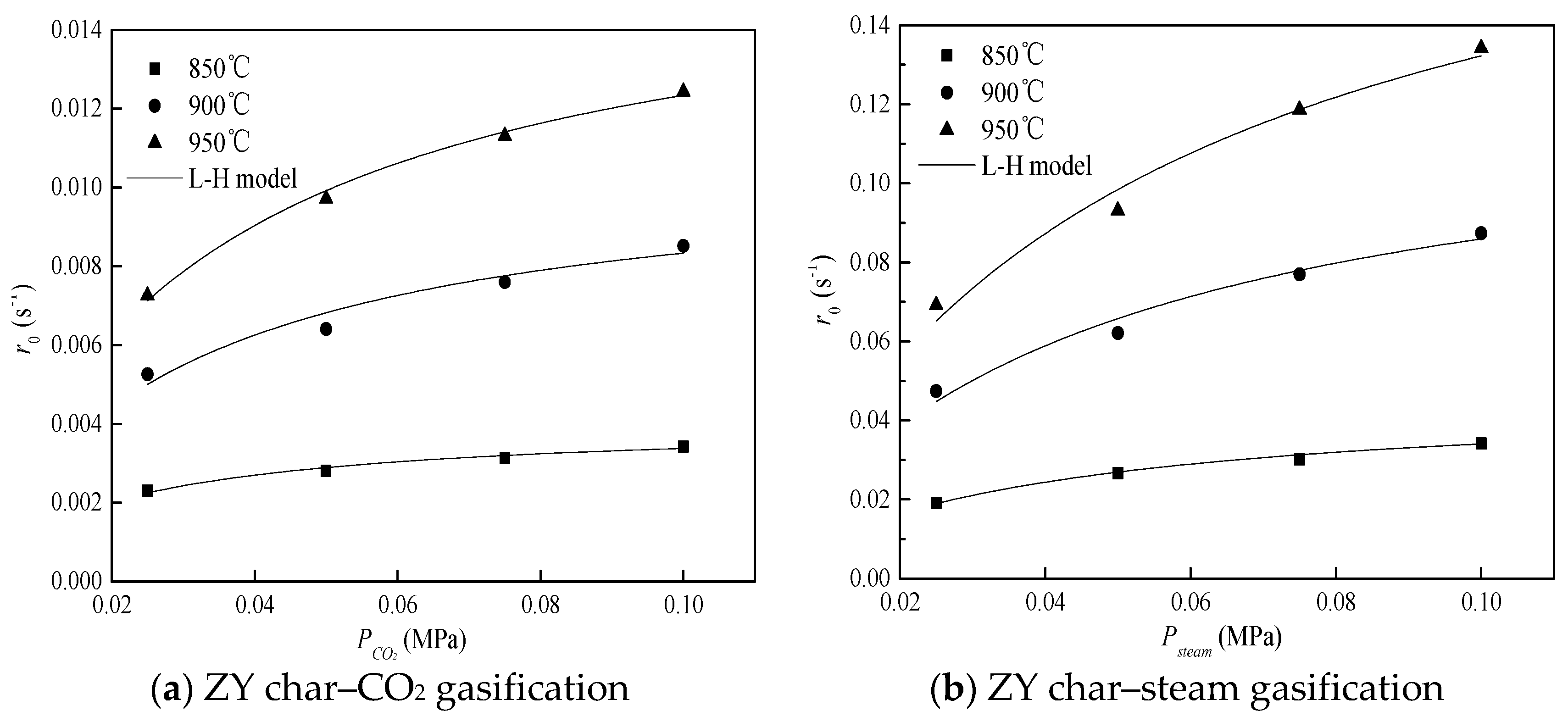
3.2.1. Kinetic Parameters of Char Gasification with Sole Gasifying Agent
3.2.2. Kinetic Parameters of Char Gasification with Gasifying Agent Mixture
3.3. Correlations between Chemisorption Quantity and Model Parameters [a,b]
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| Ai | frequency factor for L–H model, s−1MPa−1 (I = a,d), MPa−1 (I = b,c,e,f) |
| A | parameter for the modified model, dimensionless |
| b | parameter for the modified model, dimensionless |
| Cs | the quantity of strong chemisorption, mg/mg-char |
| Ct | the total quantity of chemisorption, mg/mg-char |
| Cw | the quantity of weak chemisorption, mg/mg-char |
| Ei | activation energy for L–H model, kJ/mol |
| Ki | adsorption constant for L–H model, s−1MPa−1 (I = a,d), MPa−1 (I = b,c,e,f) |
| nCO2 | the total amount of active sites for char–CO2 reaction |
| nshare | the amount of shared active sites for char–steam and char–CO2 reactions |
| nsteam | the total amount of active sites for char–steam reaction |
| P | the partial pressure of H2/CO/steam/CO2, MPa |
| r0 | initial gasification rate, s−1 |
| T | the thermodynamic temperature, K |
| w0 | the initial mass of char, wt. % |
| wash | the sample mass at the end of char gasification, wt. % |
| wt | the sample mass at the gasification time of t, wt. % |
| x | carbon conversion |
References
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Zaccariello, L.; Mastellone, M.L. Fluidized-bed gasification of plastic waste, wood, and their blends with coal. Energies 2015, 8, 8052–8068. [Google Scholar] [CrossRef]
- Roberts, D.G.; Hodge, E.M.; Harris, D.J.; Stubington, J.F. Kinetics of Char Gasification with CO2 under Regime II Conditions: Effects of Temperature, Reactant, and Total Pressure. Energy Fuels 2010, 24, 5300–5308. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: I. Isothermal, kinetic control. AIChE J. 1980, 26, 379–385. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Perlmutter, D.D. A random pore model for fluid-solid reactions: II. Diffusion and transport effects. AIChE J. 1981, 27, 247–254. [Google Scholar] [CrossRef]
- Gavals, G.R. A random capillary model with application to char gasification at chemically controlled rates. AIChE J. 1980, 26, 577–585. [Google Scholar] [CrossRef]
- Wen, C.Y. Noncatalytic heterogeneous solid-fluid reaction models. Ind. Eng. Chem. 1968, 60, 34–54. [Google Scholar] [CrossRef]
- Bliek, A.; Lont, J.C.; Van Swaaij, W.P.M. Gasification of coal-derived chars in synthesis gas mixtures under intraparticle mass-transfer-controlled conditions. Chem. Eng. Sci. 1986, 41, 1895–1909. [Google Scholar] [CrossRef]
- Kirtania, K.; Joshua, J.; Kassim, M.A.; Bhattacharya, S. Comparison of CO2 and steam gasification reactivity of algal and woody biomass chars. Fuel Process. Technol. 2014, 117, 44–52. [Google Scholar] [CrossRef]
- Ren, L.; Yang, J.; Gao, F.; Yan, J. Laboratory study on gasification reactivity of coals and petcokes in CO2/steam at high temperatures. Energy Fuels 2013, 27, 5054–5068. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I.; Jeyakumar, S. Estimation of synergetic effects of CO2 in high ash coal-char steam gasification. Appl. Therm. Eng. 2017, 110, 991–998. [Google Scholar] [CrossRef]
- Koba, K.; Ida, S. Gasification reactivities of metallurgical cokes with carbon dioxide, steam and their mixtures. Fuel 1980, 59, 59–63. [Google Scholar] [CrossRef]
- Muhlen, H.J.; Van Heek, K.H.; Juntgen, H. Kinetic studies of steam gasification of char in the presence of H2, CO2 and CO. Fuel 1985, 64, 944–949. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J. Char gasification in the mixtures of CO2 and H2O: Competition and inhibition. Fuel 2007, 86, 2672–2678. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, J.S.; Zhao, Y.; Zhang, H.; Yue, G.X.; Suda, T.; Narukawa, M. Kinetic studies of char gasification by steam and CO2 in the presence of H2 and CO. Fuel Process. Technol. 2010, 91, 843–847. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.J.P.; Kasaini, H.; Njapha, D. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam. Fuel 2006, 85, 1076–1082. [Google Scholar] [CrossRef]
- Li, F.H.; Yan, Q.X.; Huang, J.J.; Zhao, J.T.; Fang, Y.T.; Wang, J.F. Lignite-char gasification mechanism in mixed atmospheres of steam and CO2 at different pressures. Fuel Process. Technol. 2015, 138, 555–563. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Liu, W.; Zhang, S.; Yin, J.S.; Luo, G.Q.; Yao, H. Effect of pyrolysis conditions on the char gasification with the mixtures of CO2 and H2O. Proc. Combust. Inst. 2013, 34, 2453–2460. [Google Scholar] [CrossRef]
- Umemoto, S.; Kajitani, S.; Hara, S. Modeling of coal char gasification in coexistence of CO2 and H2O considering sharing of active sites. Fuel 2013, 103, 14–21. [Google Scholar] [CrossRef]
- Wei, J.T.; Guo, Q.H.; Ding, L.; Yoshikawa, K.; Yu, G.S. Synergy mechanism analysis of petroleum coke and municipal solid waste (MSW)-derived hydrochar co-gasification. Appl. Energy 2017, 206, 1354–1363. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass chars: The effects of pyrolysis conditions on their morphology, structure, chemical properties and reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Jing, X.L.; Wang, Z.Q.; Zhang, Q.; Yu, Z.L.; Li, C.Y.; Huang, J.J.; Fang, Y.T. Evaluation of CO2 gasification reactivity of different coal rank chars by physicochemical properties. Energy Fuels 2013, 27, 7287–7293. [Google Scholar] [CrossRef]
- Molina, A.; Montoya, A.; Mondragon, F. CO2 strong chemisorption as an estimate of coal char gasification reactivity. Fuel 1999, 78, 971–977. [Google Scholar] [CrossRef]
- Xu, K.; Hu, S.; Su, S.; Xu, C.F.; Sun, L.S.; Shuai, C.; Jiang, L.; Xiang, J. Study on char surface active sites and their relationship to gasification reactivity. Energy Fuels 2013, 27, 118–125. [Google Scholar] [CrossRef]
- Takarada, T.; Ida, N.; Hioki, A.; Kanbara, S.; Yamamoto, M.; Kato, K. Estimation of gasification of coal chars in steam-nitrogen and carbon dioxide–nitrogen atmospheres. J. Fuel Soc. Jpn. 1988, 67, 1061–1069. [Google Scholar] [CrossRef]
- Huttinger, K.J.; Nill, J.S. A method for the determination of active sites and true activation energies in carbon gasification: (II) Experimental results. Carbon 1990, 4, 457–465. [Google Scholar] [CrossRef]
- Miura, K.; Hashimoto, K.; Silveston, P.L. Factors affecting the reactivity of coal chars during gasification, and indexes representing reactivity. Fuel 1989, 68, 1461–1475. [Google Scholar] [CrossRef]
| Samples | Proximate Analysis/d, % | Ultimate Analysis/daf, % | Specific Surface Area/m2·g−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VM | FC | A | C | H | N | S | O | ||
| SF char | 8.60 | 81.33 | 10.07 | 95.96 | 1.02 | 1.40 | 0.40 | 1.22 | 2.05 |
| ZY char | 5.50 | 73.48 | 21.02 | 96.11 | 0.95 | 1.68 | 1.04 | 0.22 | 1.52 |
| PC char | 4.11 | 90.86 | 5.03 | 95.95 | 0.73 | 1.19 | 2.08 | 0.05 | 0.41 |
| Ki | SF Char | ZY Char | PC Char | |||
|---|---|---|---|---|---|---|
| Ai | Ei (kJ/mol) | Ai | Ei (kJ/mol) | Ai | Ei (kJ/mol) | |
| Ka | 2.02 × 107 | 161.9 | 1.69 × 104 | 105.4 | 1.28 × 106 | 149.8 |
| Kb | 9.50 × 10−3 | −78.6 | 0.14 | −54.3 | 4.88 | −32.0 |
| Kd | 4.78 × 106 | 131.1 | 6.07 × 107 | 165.0 | 1.08× 107 | 168.6 |
| Ke | 0.21 | −51.3 | 0.11 | −52.0 | 1.90 × 10−4 | −101.1 |
| Samples | CO2 Chemisorption | Steam Chemisorption | Cw(H2O)/Cw(CO2) | Cs(H2O)/Cs(CO2) | Ct(H2O)/Ct(CO2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cw | Cs | Ct | Cw | Cs | Ct | ||||
| SF char | 1.127 | 1.298 | 2.425 | 2.069 | 0.463 | 2.531 | 1.84 | 0.36 | 1.04 |
| ZY char | 0.979 | 3.646 | 4.625 | 4.295 | 0.567 | 4.862 | 4.39 | 0.16 | 1.05 |
| PC char | 0.506 | 1.735 | 2.241 | 3.556 | 0.446 | 4.002 | 7.03 | 0.28 | 1.78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wei, J.; Huo, W.; Yu, G. Gasification under CO2–Steam Mixture: Kinetic Model Study Based on Shared Active Sites. Energies 2017, 10, 1890. https://doi.org/10.3390/en10111890
Liu X, Wei J, Huo W, Yu G. Gasification under CO2–Steam Mixture: Kinetic Model Study Based on Shared Active Sites. Energies. 2017; 10(11):1890. https://doi.org/10.3390/en10111890
Chicago/Turabian StyleLiu, Xia, Juntao Wei, Wei Huo, and Guangsuo Yu. 2017. "Gasification under CO2–Steam Mixture: Kinetic Model Study Based on Shared Active Sites" Energies 10, no. 11: 1890. https://doi.org/10.3390/en10111890




