Immunological and Biochemical Markers in Oral Carcinogenesis: The Public Health Perspective
Abstract
: Oral health is an integral component of general health and well being and a basic human right. Dental public health is probably the most challenging specialty of dentistry. Because of the lack of adequate resources among other factors, many people are likely to suffer from dental diseases. Despite great improvements in the oral health status of populations across the world, the burden and impact of dental diseases are still high. This is particularly true among underprivileged groups in both developed and developing communities. Oral diseases and conditions, including oral cancer, oral manifestations of HIV/AIDS, dental trauma, craniofacial anomalies, and noma, all have broad impacts on health and well-being. Oral cancer, the sixth most common cancer worldwide continues to be most prevalent cancer related to the consumption of tobacco, alcohol and other carcinogenic products. Nevertheless, significant reduction in mortality can be achieved by advances in early diagnosis and implementation of multidisciplinary treatment programs leading to improvement of survivorship and better quality of life. The present study was designed to evaluate the immunologic and biochemical markers in oral carcinogenesis using circulating immune complexes (CIC), copper, iron, and selenium concentrations as assessment endpoints. Study results indicated an increase in CIC and copper levels, and a decrease in iron and selenium concentrations in oral cancer patients compared to controls. The implications of these findings for public health are discussed.Introduction
Oral diseases and conditions, including oral cancer, oral manifestations of HIV/AIDS, dental trauma, craniofacial anomalies, and noma (cancrum oris), have broad impacts on health and well-being [1]. In industrialized countries oral cancer is highly related to use of tobacco and excessive consumption of alcohol. The incidence of oral cancer is particularly high among men, the eighth most common cancer worldwide. In south-central Asia, consumption of tobacco in various forms is particularly high and cancer of the oral cavity ranks amongst the three most common types of cancer. The variation in oral cancer incidence rate across the world primarily reflects different risk profiles and access and availability to health services [2]. The assessment of immunological and biochemical alterations in the sera of oral cancer patients can help not only in the early diagnosis and appropriate treatment of the disease, but also in prognosis, as the disease progresses [3].
Dental Public health is the science and art of preventing and controlling dental diseases and promoting dental health through organized community efforts [4]. Dental public health professionals are responsible for the oral health of a population or a group of individuals, in contrast to the private practitioner who is primarily responsible for the oral health of the individual patient sophisticated enough to seek care and who has the resources to pay for services.
The dental public health infrastructure has a major responsibility to promote, protect and enhance the oral health of the community. The dental public health professionals need to emphasize on vulnerable or high risk population groups such as children, the elderly, the low income, the developmentally disabled, the medically compromised, persons with HIV/AIDS, institutionalized individuals and racial cultural and linguistic minorities. [5]
Research should be targeted to include oral disease-systemic disease interrelationships, HIV/AIDS related oral disease, craniofacial anomalies, oral cancer, health outcomes measurement such as quality of life indicators, and health promotion. It is considered highly relevant to ensure integration of oral health research into other health research projects at a community level that should enable efficient linkages of oral health measures with biological, social and environmental health determinants.
Meanwhile, people in deprived communities, certain ethnic minorities, homebound or disabled individuals and older people are not sufficiently covered by oral health care. Many developing countries have a shortage of oral health personnel, services are mostly offered from regional or central hospitals of urban centers and little importance is given to preventive or restorative dental care. Clinical and public health research has shown that a number of individual, professional and community measures are effective in preventing most oral diseases [6]. However, optimal intervention in relation to oral disease is not universally available or affordable because of escalating costs and limited resources in many countries.
Demographic changes in Indian society will have increasingly important effects on the oral health and the practice of dentistry. As the French philosopher, Augustic Comte stated, ‘Demography is destiny’. One such demographic trend affecting dental practice is “graying” of India. The impact of dental practice resulting from these growing numbers of elders has become well recognized. The geriatric oral health scenario in India is changing for the better [7].
According to the Population Reference Bureau’s 2000 World Data Sheets, life expectancy at birth for Indians is between 60 and 61 years. This was also confirmed by the most recent Census of India in 2001[8].
The population of the elderly in India in the year 2021 will be 137 million. According to World Health Organization report 2005 life expectancy has increased both for males and females. It is 64.1 yrs for males and 65.8 yrs for females [1]. This has revealed the decrease in death rate and better improvement of quality health services in India [9].
However, there are variations in life expectancy in rural and urban populations due to variations in availability of health services, literacy levels, states and difference in rural and urban areas population due differential income levels and socio-economic status [10].
Health disparities are well documented in minority populations. Individuals in these groups bear a disproportionate burden of disease and disability and these disparities result in lower life expectancy, decreased quality of life, loss of economic opportunities, and perception of injustice [11].
Epidemiological studies indicate that intervention at an early stage might reduce oral carcinoma related deaths. The discovery of immunological markers at a clinical, histological and molecular level has marked the end of an era of groping in the dark for clues to the basis of cancer.
The development of oral cancer is a multistep process arising from pre-existing potentially malignant lesions. Leukoplakia (L) is the most common precancer representing 85% of such lesions [12]. Histologically, over 95% of oral cancers are squamous cell carcinomas [13, 14]. It has been suggested that a vast majority of Oral squamous cell carcinomas (OSCC) in India arise from preexisting Leukoplakia [15].
Likewise, the incidence of oral submucous fibrosis (OSMF) is increasing like an epidemic, targeting the younger generation. The etiology for OSMF is still obscure and a varied number of factors have been proposed. Of these, areca nut use is the most important and persistent finding in history taking [16].
Intensive studies have documented the role of immune complexes as modulators of both cellular and humoral immune response. The occurrence of circulating immune complexes (CIC) as a marker for tumor burden and prognosis in the sera of patients with oral precancer and cancer is now well established. Recent advances in the fields of CIC, tumor progression, drug resistance, tumor cell heterogeneity and metastasis have resulted in a renewal of interest in the development of non- specific immunotherapeutic modalities [17].
CIC occurrence in cancer cells, especially malignant melanoma, breast carcinoma, ovarian carcinoma, testicular carcinoma and sarcomas has been reported in the literature. Predictive value depends mainly on prevalence and it is known that increased incidence of CIC occurs in other chronic diseases e.g. Rheumatoid Arthritis.
Although further work on composition of CIC will enhance the usefulness of CIC determination in malignant disease, antigenic makeup of CIC in cancer patients reflects the host’s immune response to a variety of often overlapping antigenic stimuli and hence paves the way for further studies [18].
Trace elements have been extensively studied in recent years to assess whether they have any modifying effects in the etiology of cancer. Copper, iron and selenium are essential for numerous enzymes and therefore it is reasonable to assume that variations in serum level of these biochemical markers maybe associated with the pathogenesis of oral cancer. Molybdenum acts as a cofactor for xanthine oxidase, aldehyde oxidase, sulfite oxidase in mammals and is regarded as an essential trace element in human nutrition [19].
In assessment for several trace elements, the concentrations in serum are often used as an index. The importance of these elements in cancer was reported by Schwartz (1975) [20] which opened the door for new diagnostic and therapeutic endeavors in many areas of medicine and specifically in the areas of oncology. Immunological and biochemical alterations in the serum of such patients can help not only in the early diagnosis and treatment but also in prognosis, as the disease progresses.
Material and Methods
In a randomized clinical trial, thirty patients with precancer (OSMF/L) and 30 patients with OSCC with histopathologically proven lesions were included in this study [3]. For comparison thirty normal subjects were also selected. The age group of these patients ranged from 25–70 years.
The following investigations were carried out in sera obtained from 10 ml blood samples collected from the subjects:
Serum estimation of circulating immune complexes (CICs) using 3.75% Polyethylene Glycol 6000 (PEG) serum precipitation assay [21].
Serum estimation of copper (Cu) using the Colorimetric Oxalyl Dihydrazide method [22].
Serum analysis of iron (Fe) using colorimetric Dipyridyl method [20].
Serum analysis of selenium (Se) using Differential Pulse Cathodic Stripping Voltametry [23].
Results and Discussion
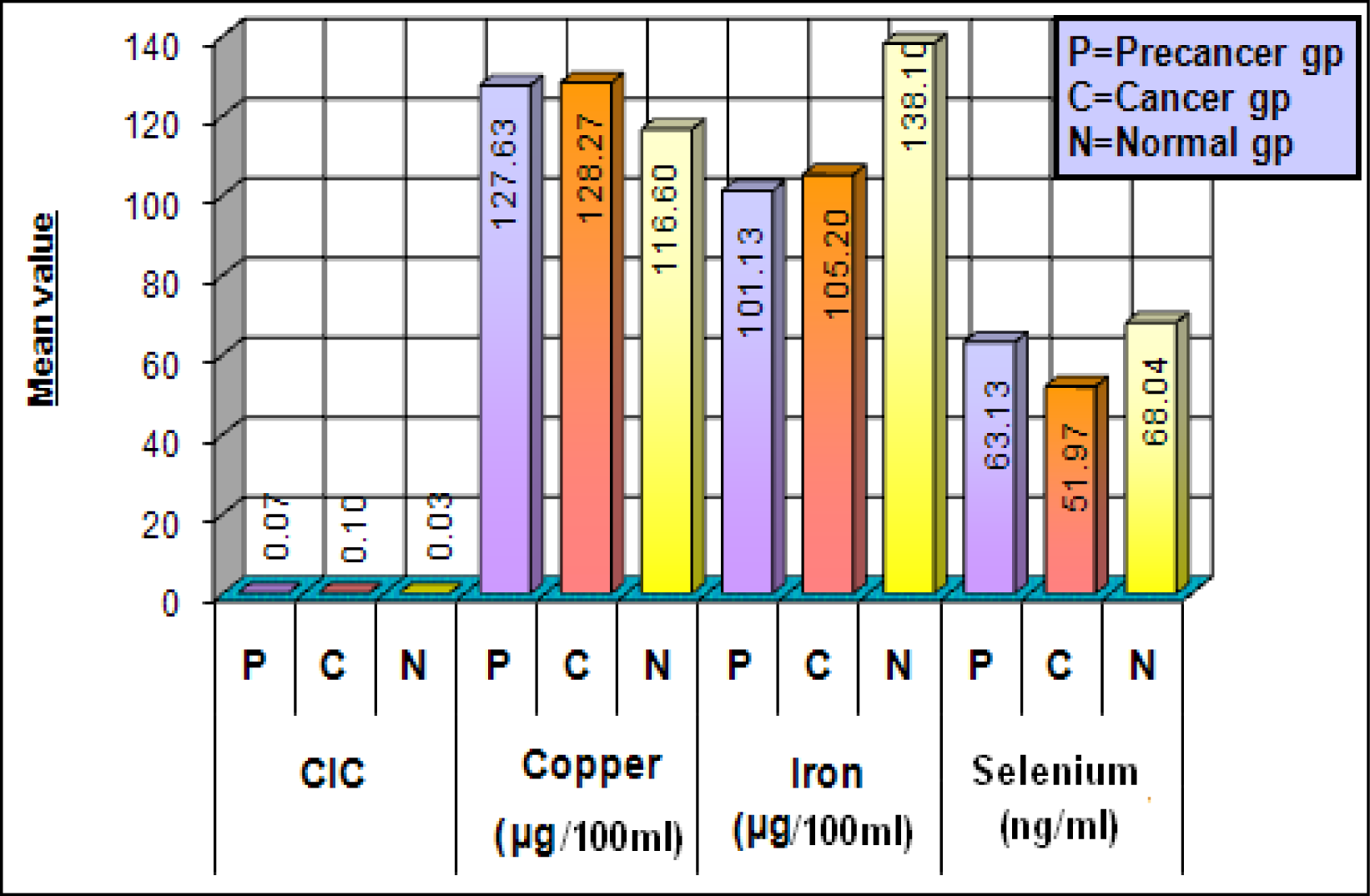
In the present study the levels of circulating immune complexes (CIC) showed a gradual increase in the precancer group and a characteristic marked increase in the cancer group [3] (Figure 1). CIC deposition may lead to inflammation and tissue / cell damage, as well as to suppression of cell mediated immunity and modulates the humoral response.
The immunological abnormalities in patients with cancer in the head and neck appear to be more profound than those associated with cancers of the bronchus, breast, cervix, colon or bladder [24]. The immunoglobulin deposits may represent immune (antigen-antibody) complexes, since circulating immune complexes have been detected in 75% of patients with head and neck carcinoma [25]. Majority of this study group consisted of males (66.67%) who had tobacco, areca nut chewing and associated habits. The mean age was higher in the patients suffering from oral carcinoma.
Gross et al. [26] reported that ageing is associated with a decline in the cell mediated immunity which might predispose to oncogenesis. Hoffken et al. [26] concluded that the elevation of circulating immune complexes was attributed to change in the levels of complement fixing and non-complement fixing of tumor specific antibodies. This implied that it may be possible to monitor the malignant transformation of premalignant lesions. Also, emphasis should be laid on the detection of the antigenic component of the CIC.
Serum levels of copper showed gradual increase from precancer to the cancer group, as compared to the control group showing a statistically significant difference (Figure 1) [3]. High levels of copper in areca nut, a major etiological factor in OSMF plays an initiating role in stimulation of fibrogenesis by up regulation of lysyl oxidase [27] and thereby causing inhibition of degradation of collagen and causing its accumulation. The rise in serum copper can be attributed to increased turnover of ceruloplasmin a copper carrying globulin with essential oxidase activity [28] in the serum of carcinoma patients.
In the present study the serum iron was significantly decreased in the precancer and cancer groups as compared to the control groups (Figure 1). Serum iron levels are considered as biochemical indicators for nutritional assessment. Utilization of iron in collagen synthesis by the hydroxylation of proline and lysine leads to decrease serum iron levels in OSMF patients. In most cases clinical anemia may be a contributing factor. Inadequate intake of food due to burning sensation and vesiculation in the oral cavity might also be an important factor. Reduction in the serum iron level may be due to malnutrition caused by the tumor burden in cancer patients.
Utilization of iron in collagen synthesis by the hydroxylation of proline and lysine leads to decreased serum iron levels in OSMF patients [29]. In most cases clinical anemia may be a contributing factor [30].The initial ulceration in OSMF causes pronounced difficulty in eating and in turn nutritional depletion. There appears to be an association between the serum iron content and oral carcinogenesis. More detailed studies on a large data base should be instituted to elucidate the exact role of iron.
Various epidemiological studies have implicated selenium as a cancer protective agent. In the present study the serum selenium concentration was found to be decreased in the precancer and cancer groups [3] (Figure 1). Studies indicate that higher dietary intake of selenium in humans may be protective. The role of selenium is thus complex which can be attributed to its protective antioxidant role.
Selenium forms the integral part of the enzyme glutathione peroxidase, type 1 iodothyronine deiodinase, metalloprotein, fatty acid binding protein and selenoprotein P. therefore selenium is considered as an antioxidant nutrient and the diseases where low selenium is implicated range from nutritional disorders like protein energy malnutrition to degenerative diseases such as cancer [31].
A significant positive correlation was present between the serum circulating immune complexes levels and copper in the precancer group. Both parameters showed a steady increased (Figure 2). A significant positive correlation was found between age of subjects and circulating immune complexes. Estimation of CIC and trace elements might help in early detection, differential diagnosis and treatment planning of oral premalignant and malignant lesions.
It has been reported that serum concentration of molybdenum, an antioxidant trace element, is decreased in esophageal cancer. Resultantly, further research on the role of this element in the etiopathogenesis of aerodigestive tract and oral carcinomas, has been recommended [32]. Other areas of further research have been identified including the following [33]:
▪ Modifiable common risk factors to oral health and chronic disease, particularly the role of diet, nutrition and tobacco.
▪ Oral health-general health interrelationships.
▪ Psychosocial implications of oral health/illness and quality of life
▪ Inequity in oral health and disease.
▪ Diagnostics and cost-effective intervention strategies in relation to certain conditions such as noma and craniofacial birth defects.
▪ Population studies of oral mucosal lesions, including epidemiological surveys of HIV/AIDS related oral disease in developing countries.
▪ Health systems research on reorientation of oral health services towards prevention and health promotion.
▪ Time-series data for oral health surveillance in developing countries.
It is therefore imperative for developing countries to have stringent laws against abuse from tobacco and associated products. The population dividend which is any growing economy’s asset should be prevented from becoming a liability. Therefore, proactive intervention is the need of the hour. Estimation of CIC and trace elements might help in early detection, differential diagnosis and treatment planning of oral premalignant and malignant lesions.
Conclusions
Findings from the present studies indicate an increase in the concentration levels of circulating immune complexes (CICs) and copper, and a decrease in those of iron and selenium associated with oral carcinogenesis. It can be suggested that immunological and biochemical assessment of oral precancer and cancer patients may help in earlier diagnosis and/or prognosis. This may also serve in predicting malignant potential of the pre malignant lesions. These efforts may be of value for proactive intervention, especially in high risk groups with potentially malignant conditions and lesions. Various studies have highlighted that circulating immune complexes represent the host’s physiological and immunological response in eliciting specific antibodies upon exposure to most antigenic substances. In India, high levels of copper in areca nuts, a major etiological factor in OSMF plays an initiating role in stimulation of fibrinoenesis by the upregulation of lysyl oxidase enzyme thereby causing inhibition of degradation of collagen. The rise in serum copper can be attributed to increased turnover of ceruloplasmin in the serum of carcinoma patients. Utilization of iron in collagen synthesis by the hydroxylation of proline and lysine leads to decrease serum iron levels in OSMF patients. Clinical anemia is also considered an etiological factor for OSMF. Inadequate intake of food due to burning sensation and vesiculation in the oral cavity might also be an important factor. A decrease in the serum selenium level in oral carcinoma patients can be attributed to its protective antioxidant role.
References
- Peterson, PE. Global Research Challenges, Updated on Global. Forum for Health Research 2008, 2, 181–184. [Google Scholar]
- Petersen, PE. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century – the approach of the WHO Global Oral Health Programme. Community. Dent Oral Epidemiology 2003, 31 Suppl 1, 3–24. [Google Scholar]
- Khanna, S; Karjodkar, FR. Immunological & Biochemical markers in oral precancer and cancer. J. of Head & Face Medicine Biomedcentral 2006, 33(2). [Google Scholar]
- Competency Statement for Dental Public Health. American Association of Public Health Dentistry (AAPHD), Available at http://www.aaphd.org, Accessed: August 6, 2007.
- Allukian, M. The Neglected Epidemic and Surgeon General’s Report: A Call to Action for Better Oral Health (editorial). Am J. Public Health 2000, 90(6), 843–45. [Google Scholar]
- Cohen, L; Gift, HC. Socio-Dental sciences in Action. Disease prevention and health promotion; Munksgard, Copenhagen; Federation Dentaire International, 1995; pp. 109–52. [Google Scholar]
- Garcia, GRI. Multicultural issues in oral health. Dental Clinics of North America 2008, 52, 319–332. [Google Scholar]
- Life expectancy in our nation, 2008.
- 2007.
- Khanna, Sunali. Mehta Payal: Geriatric Oral Health: the Indian scenario. Ageing & Society Indian Journal of Gerontology 16(2), 159.
- Agency for health care research and quality. National healthcare disparities report. In USDHHS Pub. No. 04-0035; Rockville (MD); AHRQ, 2003.
- Bouquot, JE; Whitaker, SB. Oral Leukoplakia rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994, 25, 133–140. [Google Scholar]
- Chen, J; Eiserberg, E. Changing trends in oral cancer in United States 1935–1985. A Connecticut Study. J. Oral Maxillofac Surg 1991, 49, 1152–1158. [Google Scholar]
- Ostman, J; Anneroth, E. Malignant oral tumours in Sweden 1962–1989. An Epidemiological Study. Eu. J. Cancer & Oral Oncology 1995, 8, 106–112. [Google Scholar]
- Gupta, PC. Leukoplakia and the incidence of oral cancer. J. Oral Pathol Med. 1989, 18, 11. [Google Scholar]
- Babu, S; Bhat, RV; Kumar, PV; Sesikaran, B; Rao, KV; Aruna, P; Reddy, PR. A comparative clinopathological study of OSMF in habitual chewers of pan masala and betel quid. Clin. Toxicology 1996, 34(3). [Google Scholar]
- Spermulli, VN; Dexter, DL. Human tumour cell heterogenecity and metastasis. J. Clin Oncology 1983, 1, 496. [Google Scholar]
- Salinas, FA; Kian, Wee; Silver, HK. Tumour burden and its relationship to antigen, size and composition of IC. In Protides of the biological fluids; Peters, H, Ed.; Pergamon Press: Oxford, 1984; Volume 31, pp. 749–752. [Google Scholar]
- Mills, CF; Davis, GK. Molybdenum. Mertz, W, Ed.; In Trace elements in human and animal nutrition, 5th ed; Academic Press: New York, 1987; pp. 429–463. [Google Scholar]
- Schwartz, MF. Role of Trace elements in Cancer. Cancer Research 1975, 35, 3481–87. [Google Scholar]
- Riha, I. The use of polyethyleneglycol for immune complex detection in human sera. Immunol 1979, 16, 489. [Google Scholar]
- Gowenlock, AH; Wells, FE. Estimationof iron and copper. In Varley’s Practical Clinical Biochemistry, 6th ed; 1988; pp. 622–634. [Google Scholar]
- Raghunath, R. Studies on environmental levels of toxic metals and their exposure assessment in Greater Bombay PhD Thesis, Envt. Assessment Divs BARC,. 1996.
- Lichenstein: Comparison of immune derangements in patients with different malignancies. Cancer 1980, 45, 2090–5.
- Scully, Crispian. Immunologic abnormalities in oral carcinoma and oral keratosis. J. Max Fac Surg 1982, 113–115. [Google Scholar]
- Gross, L. Immunological defect in the aged population and its relationship to cancer. Cancer 1965, 18, 201–6. [Google Scholar]
- Ma, RH. Increased lysyl oxidase activity in fibroblasts cultured from OSMF. J. Oral Pathol Med. 1995, 9, 407–12. [Google Scholar]
- Jaydeep, A. Serum levels of copper, zinc, iron and ceruloplasumin in oral leukoplakia and sq. cell carcinoma. J. Exp. Clin Cancer Res. 1997, 16(3). [Google Scholar]
- Huang, S; Ling, T; Wu, H. Experimental study on aqueous areca nut extracts inducing OSMF in rats. Effect of mast cells on collagen metabolism Hua Xi et al,. 1997, 15(2), 94–96. [Google Scholar]
- Ramanthan, K. OSMF – an alternative hypothesis to its cause. Med J Malay 1981, 36(4), 243–45. [Google Scholar]
- Shamberger, RJ. Antioxidants and Cancer. Arch Environ Health 1976, 31, 231–33. [Google Scholar]
- Yoshida, M; Ota, Sachie; Fukunaga, Kenji; Nishiyama, Toshimasa. Molybdenum concentration in healthy Japanese adults determined by inductively coupled plasma-mass spectrometry. J. of Trace Elements in Biology 20(1), 19–23.
- Petersen, PE; Kwan, S. Evaluation of community-based oral health promotion and oral disease prevention – WHO recommendations for improved evidence in public health practices. Community Dent Health 2004, 21 Suppl 1, 319–20. [Google Scholar]

© 2008 MDPI All rights reserved.
Share and Cite
Khanna, S. Immunological and Biochemical Markers in Oral Carcinogenesis: The Public Health Perspective. Int. J. Environ. Res. Public Health 2008, 5, 418-422. https://doi.org/10.3390/ijerph5050418
Khanna S. Immunological and Biochemical Markers in Oral Carcinogenesis: The Public Health Perspective. International Journal of Environmental Research and Public Health. 2008; 5(5):418-422. https://doi.org/10.3390/ijerph5050418
Chicago/Turabian StyleKhanna, Sunali. 2008. "Immunological and Biochemical Markers in Oral Carcinogenesis: The Public Health Perspective" International Journal of Environmental Research and Public Health 5, no. 5: 418-422. https://doi.org/10.3390/ijerph5050418



