Uterine Fibroid Prevalence in a Predominantly Black, Chicago-Based Cohort
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Individual-Level Variables
2.4. Neighborhood Variables
2.5. Environmental Variables
2.6. Statistical Analysis
3. Results
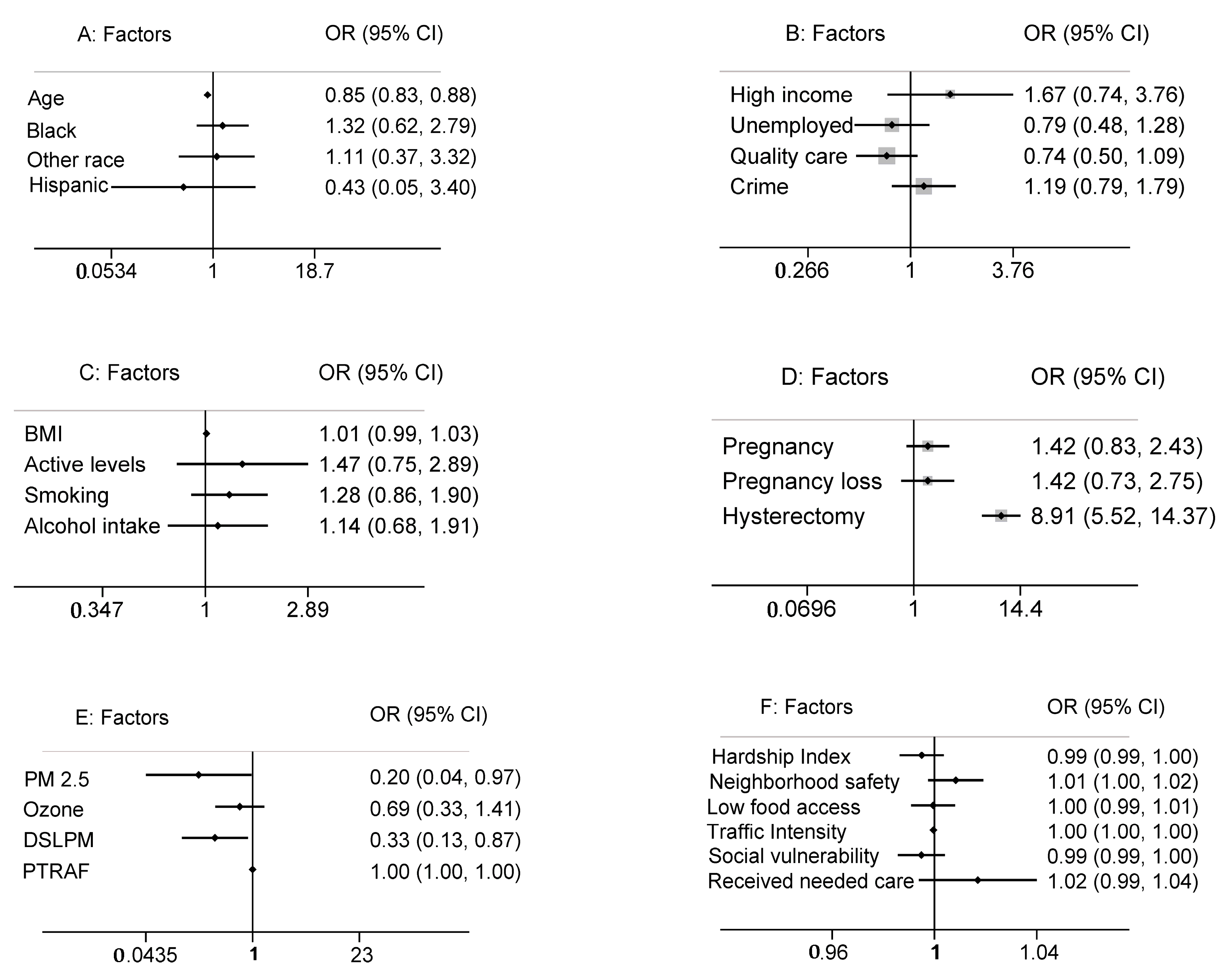
3.1. Individual-Level Variables
3.1.1. Demographic Factors
3.1.2. Socioeconomic Factors
3.1.3. Lifestyle and Behavioral Factors
3.1.4. Reproductive History
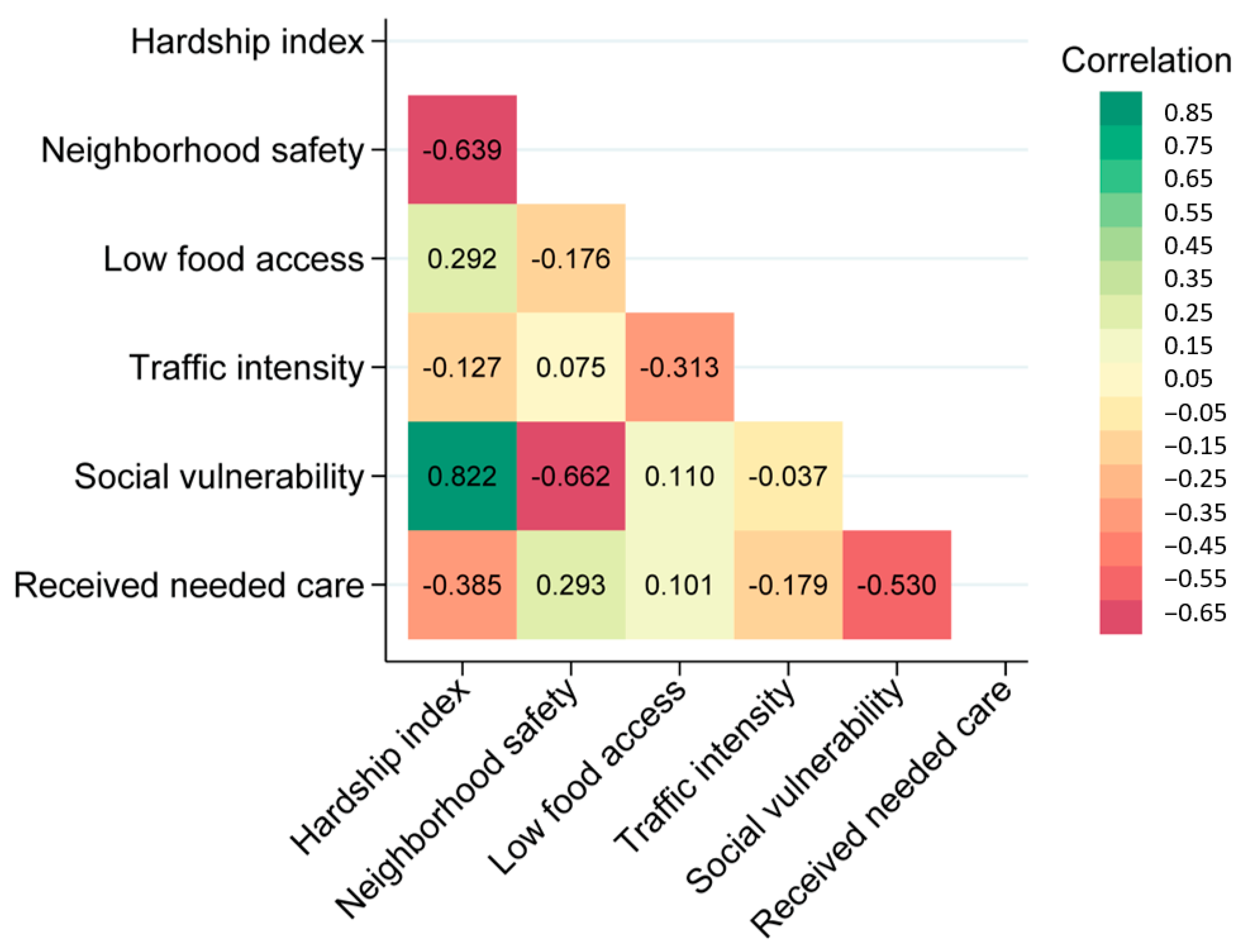
3.2. Neighborhood Variables
3.3. Environmental Variables
4. Discussion
4.1. Strengths and Limitations
4.2. Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UF | Uterine Fibroid |
| COMPASS | Chicago Multiethnic Prevention and Surveillance Study |
| OR | Odds Ratio |
| CI | Confidence Interval |
| BMI | Body Mass Index |
| PM | 2.5 Particulate Matter 2.5 |
| NIH | National Institute of Health |
| NCI | National Cancer Institute |
| CHA | Chicago Health Atlas |
| EJSCREEN | Environmental Justice Screening |
| FIPS | Federal Information Processing Standards |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| WQS | Weighted Quantile Sum |
| DSLPM | Diesel Particulate Matter |
| PTRAF | Proximity to Traffic |
References
- Al-Hendy, A.; Myers, E.; Stewart, E. Uterine Fibroids: Burden and Unmet Medical Need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.Y.; Anachebe, N.F.; Lee, R.; Skanes, H. Racial and Ethnic Disparities in Reproductive Health Services and Outcomes, 2020. Obstet. Gynecol. 2021, 137, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Cozier, Y.C.; Hunt, M.O.; Stewart, E.A.; Rosenberg, L. Perceived Racial Discrimination and Risk of Uterine Leiomyomata. Epidemiology 2007, 18, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Igboeli, P.; Walker, W.; McHugh, A.; Sultan, A.; Al-Hendy, A. Burden of Uterine Fibroids: An African Perspective, A Call for Action and Opportunity for Intervention. Curr. Opin. Gynecol. Obstet. 2019, 2, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2021, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Millien, C.; Manzi, A.; Katz, A.M.; Gilbert, H.; Smith Fawzi, M.C.; Farmer, P.E.; Mukherjee, J. Assessing Burden, Risk Factors, and Perceived Impact of Uterine Fibroids on Women’s Lives in Rural Haiti: Implications for Advancing a Health Equity Agenda, a Mixed Methods Study. Int. J. Equity Health 2021, 20, 1. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Lopez, R. The Riskscape and the Color Line: Examining the Role of Segregation in Environmental Health Disparities. Environ. Res. 2006, 102, 181–196. [Google Scholar] [CrossRef]
- Tinelli, A.; Vinciguerra, M.; Malvasi, A.; Andjić, M.; Babović, I.; Sparić, R. Uterine Fibroids and Diet. Int. J. Environ. Res. Public Health 2021, 18, 1066. [Google Scholar] [CrossRef]
- Tung, E.L.; Johnson, T.A.; O’Neal, Y.; Steenes, A.M.; Caraballo, G.; Peek, M.E. Experiences of Community Violence among Adults with Chronic Conditions: Qualitative Findings from Chicago. J. Gen. Intern. Med. 2018, 33, 1913–1920. [Google Scholar] [CrossRef]
- Kalra, G.L.; Watson, K.E. Health Consequences of Segregation and Disenfranchisement. J. Am. Heart Assoc. 2022, 11, e024772. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, C.-M.; Chen, M.-L.; Hwang, B.-F. The Effects of Exposure to Air Pollution on the Development of Uterine Fibroids. Int. J. Hyg. Environ. Health 2019, 222, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Rosenberg, L.; Wise, L.A.; Jerrett, M.; Coogan, P.F. A Prospective Cohort Study of Ambient Air Pollution Exposure and Risk of Uterine Leiomyomata. Hum. Reprod. 2021, 36, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Mahalingaiah, S.; Hart, J.E.; Laden, F.; Terry, K.L.; Boynton-Jarrett, R.; Aschengrau, A.; Missmer, S.A. Air Pollution and Risk of Uterine Leiomyomata. Epidemiology 2014, 25, 682–688. [Google Scholar] [CrossRef] [PubMed]
- King, K.E. Chicago Residents’ Perceptions of Air Quality: Objective Pollution, the Built Environment, and Neighborhood Stigma Theory. Popul. Environ. 2014, 37, 1–21. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Kibriya, M.G.; Jasmine, F.; Stepniak, L.; Gopalakrishnan, R.; Craver, A.; Zakin, P.; Tasmin, S.; Kim, K.E.; Goss, K.H.; et al. Cohort Profile: The ChicagO Multiethnic Prevention and Surveillance Study (COMPASS). BMJ Open 2020, 10, e038481. [Google Scholar] [CrossRef]
- Chicago Health Atlas. Available online: https://chicagohealthatlas.org/ (accessed on 16 May 2023).
- Carrico, C.K.; Gennings, C.; Wheeler, D.A.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef]
- Luo, J.; Kibriya, M.G.; Shah, S.; Craver, A.; De La Cruz, S.; King, J.; Olopade, C.O.; Kim, K.; Ahsan, H.; Pinto, J.; et al. The Impact of Neighborhood Disadvantage on Asthma Prevalence in a Predominantly African-American, Chicago-Based Cohort. Am. J. Epidemiol. 2023, 192, 549–559. [Google Scholar] [CrossRef]
- Islam, M.M. Social Determinants of Health and Related Inequalities: Confusion and Implications. Front. Public Health 2019, 7, 11. [Google Scholar] [CrossRef]
- Watkinson, C.; van Sluijs, E.M.; Sutton, S.; Hardeman, W.; Corder, K.; Griffin, S.J. Overestimation of Physical Activity Level Is Associated with Lower BMI: A Cross-Sectional Analysis. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 68. [Google Scholar] [CrossRef]
- McEwen, B.S. Protective and Damaging Effects of Stress Mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Noorhasan, D.; Schott, L.L.; Powell, L.; Randolph, J.F.; Johnston, J.M. Racial Differences in Women Who Have a Hysterectomy for Benign Conditions. Women’s Health Issues 2009, 19, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, S.K.; Herring, A.H.; Savitz, D.A.; Olshan, A.F.; Fielding, J.R.; Hartmann, K.E.; Baird, D.D. Pregnancy-Related Fibroid Reduction. Fertil. Steril. 2010, 94, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Columb, M.; Atkinson, M. Statistical Analysis: Sample Size and Power Estimations. BJA Educ. 2016, 16, 159–161. [Google Scholar] [CrossRef]
- Schmier, J.K.; Halpern, M.T. Patient Recall and Recall Bias of Health State and Health Status. Expert Rev. Pharmacoecon. Outcomes Res. 2004, 4, 159–163. [Google Scholar] [CrossRef]
- Marshall, L.M.; Spiegelman, D.; Barbieri, R.L.; Goldman, M.B.; Manson, J.E.; Colditz, G.A.; Willett, W.C.; Hunter, D.J. Variation in the Incidence of Uterine Leiomyoma among Premenopausal Women by Age and Race. Obstet. Gynecol. 1997, 90, 967–973. [Google Scholar] [CrossRef]
- Wise, L.A.; Palmer, J.R.; Stewart, E.A.; Rosenberg, L. Age-Specific Incidence Rates for Self-Reported Uterine Leiomyomata in the Black Women’s Health Study. Obstet. Gynecol. 2005, 105, 563–568. [Google Scholar] [CrossRef]
| Entire Sample (n = 602) | Fibroid Diagnosis (n = 127) | No Fibroid Diagnosis (n = 475) | p-Value | |
|---|---|---|---|---|
| Age (year), mean ± SD | 50.3 ± 12.3 | 37.1 ± 10.5 | 53.8 ± 10.1 | <0.001 |
| BMI, mean ± SD | 31.4 ± 9.0 | 32.2 ± 7.8 | 31.2 ± 9.3 | 0.265 |
| Race, n (%) | ||||
| Black | 513 (85.2) | 111 (87.4) | 402 (84.6) | 0.792 |
| White | 52 (8.6) | 9 (7.1) | 43 (9.1) | |
| Other | 37 (6.2) | 7 (5.5) | 30 (6.3) | |
| Ethnicity, n (%) | ||||
| Non-Hispanic | 546 (90.7) | 113 (89.0) | 433 (91.2) | 0.391 |
| Hispanic | 10 (1.7) | 1 (0.8) | 9 (1.9) | |
| Unknown | 46 (7.6) | 13 (10.2) | 33 (7.0) | |
| Socioeconomic Status, n (%) | ||||
| Employment Status | ||||
| Employment | 127 (21.1) | 30 (23.6) | 97 (20.4) | 0.360 |
| Unemployed | 368 (61.1) | 72 (56.7) | 296 (62.3) | |
| Retired | 65 (10.8) | 18 (14.2) | 47 (9.9) | |
| Unknown | 42 (7.0) | 7 (5.5) | 35 (7.4) | |
| Income Status a | ||||
| Low income | 422 (70.1) | 83 (65.4) | 339 (71.4) | 0.337 |
| Middle income | 44 (7.3) | 8 (6.3) | 36 (7.6) | |
| High Income | 31 (5.2) | 9 (7.1) | 22 (4.6) | |
| Unknown | 105 (17.4) | 27 (21.3) | 78 (16.4) | |
| Behavioral Lifestyle, n (%) | ||||
| Alcohol/Smoking Status | ||||
| Smoking Status | ||||
| Smoker | 312 (51.8) | 72 (56.7) | 240 (50.5) | 0.231 |
| Non-Smoker | 290 (48.2) | 55 (43.3) | 235 (49.5) | |
| Alcohol Consumption | ||||
| Consumer | 105 (17.4) | 29 (22.8) | 76 (16.0) | 0.623 |
| Non-Consumer | 243 (40.4) | 61 (48.0) | 182 (38.3) | |
| Unknown | 254 (42.2) | 37 (29.1) | 217 (46.7) | |
| Reproductive History, n (%) | ||||
| Pregnancy outcome | ||||
| Live birth | 375 (62.3) | 79 (62.2) | 296 (62.3) | 0.385 |
| Pregnancy loss | 51 (8.47) | 14 (11) | 37 (7.8) | |
| Abortion | 62 (10.3) | 15 (11.8) | 47 (9.9) | |
| Not reported | 114 (18.9) | 19 (15) | 95 (20) | |
| Hysterectomy | 97 (16.4) | 57 (45.6) | 40 (8.6) | <0.001 |
| Neighborhood Characteristic, Median (Interquartile Range) | ||||
|---|---|---|---|---|
| Entire Sample (n = 602) | Fibroid Diagnosis (n = 127) | No Fibroid Diagnosis (n = 475) | p-Value | |
| Hardship Index | 83.1 (75.3–89.3) | 83.1 (57.3–86.9) | 83.1 (75.3–89.8) | 0.338 |
| Neighborhood safety | 47.1 (33.6–58.2) | 47.5 (36.5–59.2) | 46.3 (33.6–58.2) | 0.130 |
| Low food access | 36.9 (22.3–63.5) | 36.9 (22.3–63.5) | 36.9 (22.3–63.5) | 0.768 |
| Traffic Intensity | 615.3 (411.5–1630.2) | 563.1 (411.5–1013.0) | 619.2 (411.5–1630.2) | 0.031 |
| Social vulnerability | 81.5 (72.7–83.5) | 81.5 (69.4–82.6) | 81.5 (74.0–83.5) | 0.284 |
| Received needed care | 77.2 (72.7–86.5) | 79.3 (74.6–87.6) | 77.2 (72.7–86.2) | 0.143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndebele, S.; Turner, T.; Liao, C.; Aschebrook-Kilfoy, B.; Randorf, N.; Ahsan, H.; Odunsi, K.; Madueke-Laveaux, O.S. Uterine Fibroid Prevalence in a Predominantly Black, Chicago-Based Cohort. Int. J. Environ. Res. Public Health 2024, 21, 222. https://doi.org/10.3390/ijerph21020222
Ndebele S, Turner T, Liao C, Aschebrook-Kilfoy B, Randorf N, Ahsan H, Odunsi K, Madueke-Laveaux OS. Uterine Fibroid Prevalence in a Predominantly Black, Chicago-Based Cohort. International Journal of Environmental Research and Public Health. 2024; 21(2):222. https://doi.org/10.3390/ijerph21020222
Chicago/Turabian StyleNdebele, Sithembinkosi, Tecora Turner, Chuanhong Liao, Briseis Aschebrook-Kilfoy, Nina Randorf, Habibul Ahsan, Kunle Odunsi, and Obianuju Sandra Madueke-Laveaux. 2024. "Uterine Fibroid Prevalence in a Predominantly Black, Chicago-Based Cohort" International Journal of Environmental Research and Public Health 21, no. 2: 222. https://doi.org/10.3390/ijerph21020222






