Cytogenetic Effects of 1,1-Dichloroethane in Mice Bone Marrow Cells
Abstract
:Introduction
Materials and Methods
Chemicals
Animal Maintenance
Dose
Chromosome Aberration Assay
Mitotic Index Determination
Micronucleus Test
Statistical Analysis
Results
Induction of Chromosomal Aberrations in Mice Bone Marrow Cells by 1,1-Dichloroethane
Mitotic Index
Induction of Micronuclei in Mice Bone Marrow Cells Exposed to 1,1-Dichloroethane
Discussion
Conclusions
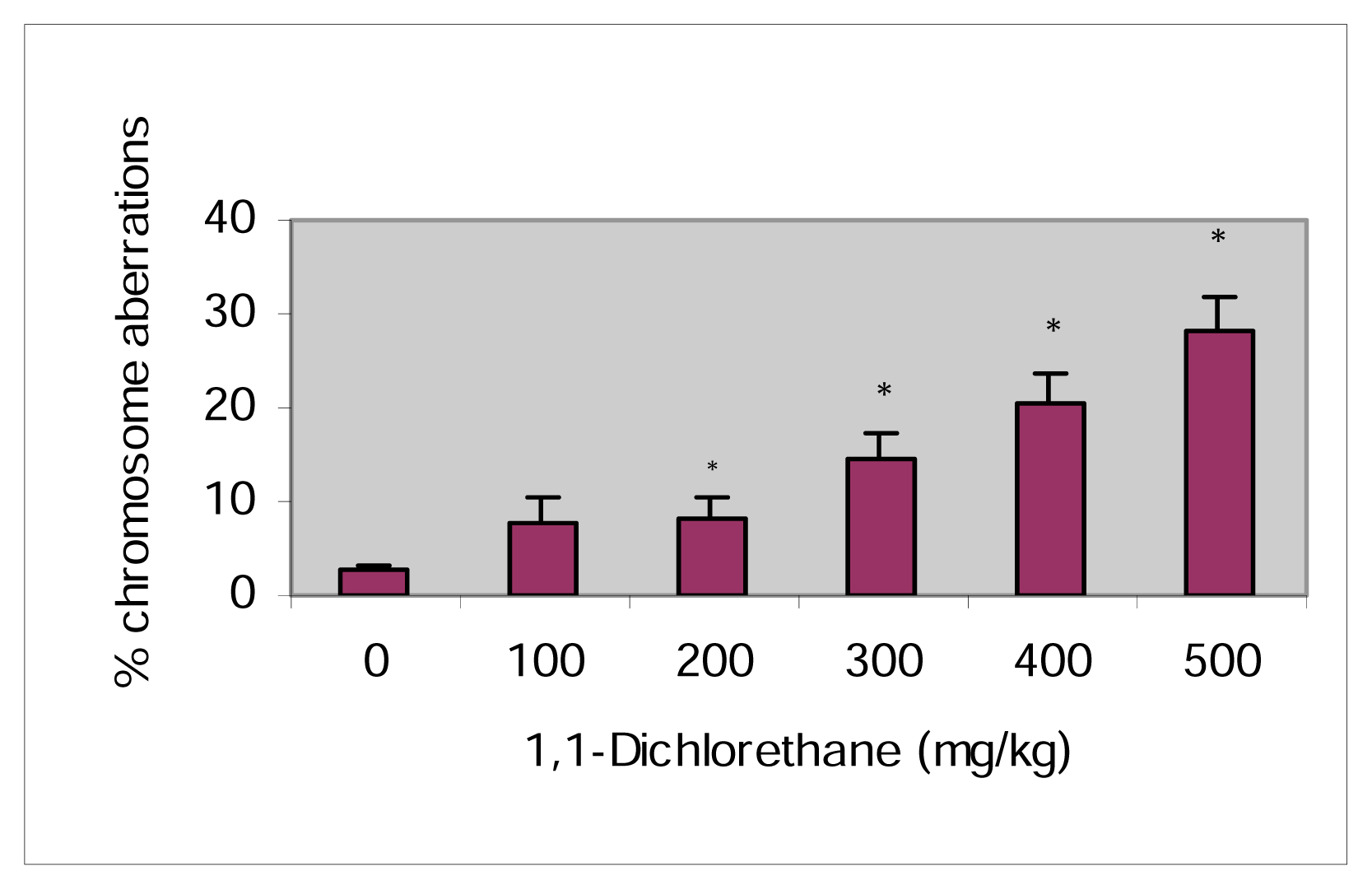
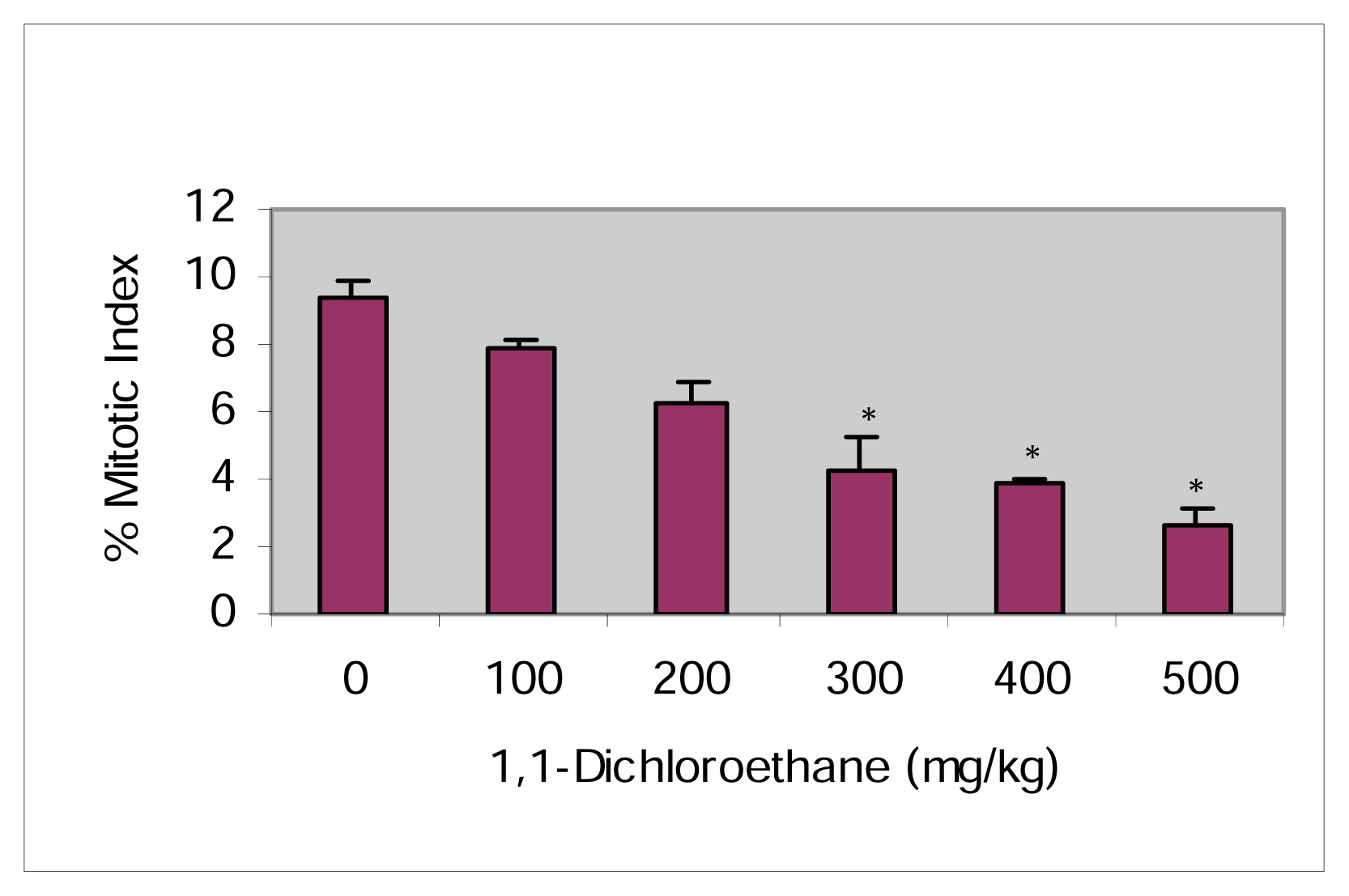
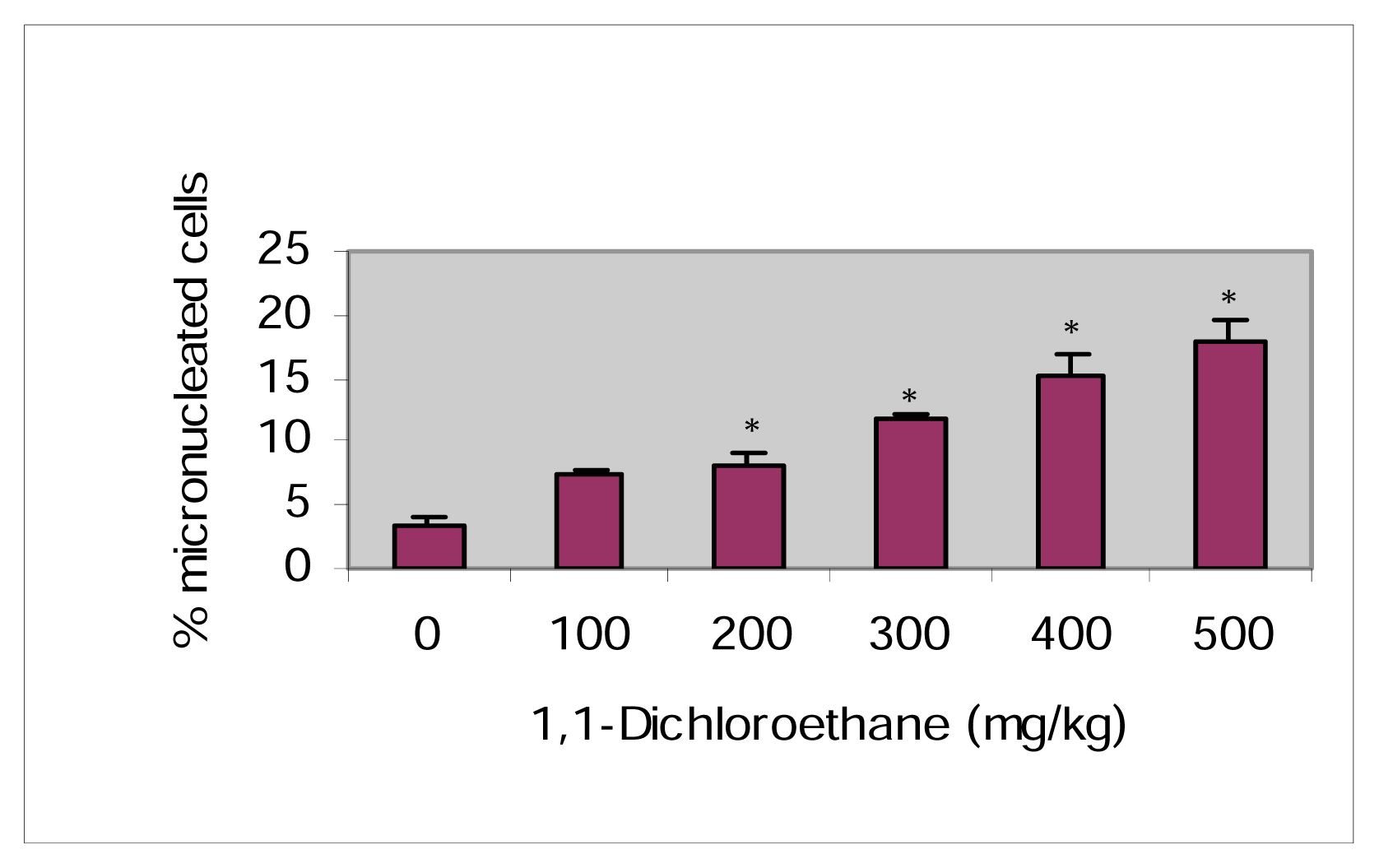
| Dose (mg/kg) | Total Metaphases Examined (n) | MI | Chromatid | Chromosome | Total SCA | Mean % SCA ±SEM | ||
|---|---|---|---|---|---|---|---|---|
| Gap | Break | Gap | Break | |||||
| Ethanol | 300/3 | 9.4 | 2 | 1 | 3 | 2 | 8 | 2.67 ± 0.58 |
| 100 | 300/3 | 7.9 | 4 | 6 | 7 | 6 | 23 | 7.67 ± 2.88 |
| 200 | 300/3 | 6.2 | 7 | 3 | 8 | 7 | 25 | 8.33 ± 2.08 |
| 300 | 300/3 | 4.3 | 6 | 5 | 16 | 17 | 44 | 14.67 ± 2.51 |
| 400 | 300/3 | 3.0 | 15 | 10 | 19 | 17 | 61 | 20.3 ± 3.21 |
| 500 | 300/3 | 2.6 | 20 | 19 | 24 | 21 | 84 | 28.0 ± 3.61 |
| Dose | Rat Number | MN*per 1000 Cells | Fixation Time (h) |
|---|---|---|---|
| Ethanol (1%) | 1 | 3 | |
| 2 | 4 | ||
| 3 | 3 | ||
| Mean ± S.E. | 3.33 ± 0.58a | 30 | |
| 1.1 DCE (mg/kg BW, i.p) | |||
| 100 | 1 | 7 | |
| 2 | 7 | ||
| 3 | 8 | ||
| Mean ± S.E. | 7.33 ± 0.57b | 30 | |
| 200 | 1 | 9 | |
| 2 | 7 | ||
| 3 | 8 | ||
| Mean ± S.E. | 8.0 ± 1.0b | 30 | |
| 300 | 1 | 11 | |
| 2 | 12 | ||
| 3 | 12 | ||
| Mean ± S.E. | 11.67 ± 0.58c | 30 | |
| 400 | 1 | 15 | |
| 2 | 14 | ||
| 3 | 17 | ||
| Mean ± S.E. | 15.3 ± 1.52d | 30 | |
| 500 | 1 | 17 | |
| 2 | 17 | ||
| 3 | 20 | ||
| Mean ± S.E. | 18.0 ± 1.73d | 30 |
References
- ATSDR, Toxicological Profile for 1,1-dichloroethane. In Agency for Toxic Substances and Disease Registry; U.S. Department of Health and Human Services: Atlanta, GA, 1990.
- U.S. EPA. Integrated Risk Information System; Environmental Protection Agency, 1990. Available at: http://www.epa.gov/ir Accessed January 18, 2001.
- Roldan-Arjona, T.; Garcia-Pedrajas, D.; Luque-Romero, F.; Hera, C.; Pueyo, C. An association between mutagenicity of the Ara test of Salmonella typhimurium and carcinogenicity in rodents for 16 halogenated aliphatic hydrocarbons. Mutagenesis 1991, 6(3), 199–205. [Google Scholar]
- Tafazoli, M.; Baeten, A.; Geerlings, P.; Kirsch-Volders, M. In vitro mutagenicity and genotoxicity study of a number of short-chain chlorinated hydrocarbons using the micronucleus test and the alkaline single cell gel electrophoresis technique (comet assay) in human lymphocytes: a structure-activity relationship (QSAR) analysis of the genotoxic and cytotoxic potential. Mutagenesis 1998, 13(2), 115–126. [Google Scholar]
- Rosenkranz, H. S.; Klopman, G. A study of the structural basis of the ability of chlorinated alkanes and alkenes to induce aneuploidy and toxicity in the mold. Aspergillus nidulans. Mutat. Res 1996, 354, 183–193. [Google Scholar]
- Gerhard, I.; Daniel, V.; Link, S.; Monga, B.; Runnebaum, B. Chlorinated hydrocarbons in women with repeated miscarriages. Environ. Health. Perspect 1998, 106, 675–681. [Google Scholar]
- Evans, E. B.; Blaster, R. L. CNS depressant effects of volatile organic solvents. Neurosci. Biobehav. Rev 1991, 15, 233–241. [Google Scholar]
- Wilson, C. Chemical exposure and human health: A reference to 314 chemicals with a guide to symptoms and a directory of organizations; McFarland & Company: North Carolina, 1993. [Google Scholar]
- Preston, R. J.; Dean, B. J.; Galloway, S.; Holden, H.; McFee, A. F.; Shelby, M. Mammalian in vivo cytogenetic assays: Analysis of chromosome aberrations in bone marrow cells. Mutat.Res 1987, 189, 157–165. [Google Scholar]
- Romanga, F.; Staniforth, C. D. The automated bone marrow micronucleus test. Mutat. Res 1989, 213, 91–104. [Google Scholar]
- Schmid, W. the micronuclei test for cytogenetic analysis. Hollaender, A., Ed.; In Chemical mutagens, Principles and Methods for their detection; Volume 4, Plenum Press: New York, 1976; pp. 31–53. [Google Scholar]
- Agarwal, D. K.; Chauhan, L. K. S. An Improved chemical substitute for fetal calf serum for the micronucleus test. Biotechnol. Histochem 1993, 68, 187–188. [Google Scholar]
- Matsuoka, A.; Yamada, K.; Kusakable, H.; Wakuri, S.; et al. Re-evaluation of chromosomal aberration induction on nine mouse lymphoma assay “unique positive” NTP carcinogens. Mutat.Res 1996, 369, 243–252. [Google Scholar]
- Whittaker, S. G.; Zimmermann, F. K.; Dicus, B.; Piegorsch, W. W.; Resnick, M. A.; Fogel, S. Detection of induced mitotic chromosome loss in Saccharomyces cerevisiae – an interlaboratory assessment of 12 chemicals. Mutat. Res. 1990, 241(3), 225–42. [Google Scholar]
- Rank, J.; Nielsen, M. H. Evaluation of the Allium anaphase-telophase test in relation to genotoxicity screening of industrial wastewater. Mutat. Res 1994, 312(1), 17–24. [Google Scholar]
- Sasaki, F.Y.; Yamada, H.; Sugiyama, C.; Kinae, N. Increasing effect of tri-n-butyltins and triphenyltins on the frequency of chemically induced aberrations in cultured Chinese hamster cells. Mutat. Res 1993, 300, 5–14. [Google Scholar]
- Topaktas, M.; Rencuzogullar, E. Chromosomal aberrations in cultured human lymphocytes treated with Marshal and its effective ingredient Carbosulfan. Mutat. Res. 1993, 319(2), 103–11. [Google Scholar]
- Kihlman, B. A.; Hanssan, K.; Andersson, H. C. The effects of post-treatments with caffeine during S and G2 on the frequencies of chromosomal aberrations induced by thiotepa in root-tips of vicia faba and in human lymphocytes in vitro. Mutat. Res 1982, 104(4–5), 323–330. [Google Scholar]
- McFee, A. F.; Tice, R. R. Influence of treatment to sacrifice time and the presence of BrdUrd on chemically-induced aberration rates in mouse marrow cells. Mutat. Res 1990, 241(1), 95–108. [Google Scholar]
- Duma, D.; Raicu, P.; Hamar, M.; Tuta, A. Cytogenetic effects of some pesticides on rodents. Rev. Roum. Biol 1977, 22, 93–96. [Google Scholar]
- Rank, J.; Jensen, A. G.; Skov, B.; Pedersen, L. H.; Jensen, K. Genotoxicity testing of the herbicide Roundup and its active ingredient glyphosate isopropylamine using the mouse bone marrow micronucleus test, Salmonella mutagenicity test, allium anaphase-telophase test. Mutat. Res 1993, 300, 29–36. [Google Scholar]
- Gudi, R. D.; Sandhu, S. S.; Athwal, S. R. Kinetochore identification in micronuclei in mouse bone marrow erythrocytes: An assay for the detection of aneuploidy-inducing agents. Mutat. Res 1990, 234, 263–268. [Google Scholar]
- Tafazoli, M.; Kirsch-Volders, M. In vitro mutagenicity and genotoxicity study of 1,2-dichloroethylene, 1,1,2-trichloroethane, 1,3-dichloropropane, 1,2,3-trichloropropane and 1,1,3-trichloropropene, using the micronucleus test and the alkaline single cell gel electrophoresis technique (comet assay) in human lymphocytes. Mutat. Res 1996, 371(3–4), 185–202. [Google Scholar]
- Jagetia, G. C.; Jyothi, P. Enhancement of micronuclei frequency by vindesine in mouse bone marrow. Mutat. Res 1997, 388, 1–5. [Google Scholar]
- Gutierrez, S.; Carbonell, E.; Galofre, P.; Creus, A.; Marcos, R. Micronuclei induction by 131I exposure: Study in hyperthyroidism patients. Mutat. Res 1997, 373, 39–45. [Google Scholar]
- Anwar, W.; Khalil, M. M.; Wild, C. P. Micronuclei, chromosomal aberrations and aflatoxin-albumin adducts in experimental animals after exposure to aflatoxinB1. Mutat. Res 1994, 322, 61–67. [Google Scholar]
- Jayashree, I. V.; Vijayalaxmi, K. K.; Abdul Rahiman, M. The genotoxicity of Hinosan, an organophosphorus pesticide in the vivo mouse. Mutat. Res 1994, 322(2), 77–85. [Google Scholar]
- Grover, I. S.; Malhi, P. K. Genotoxic effects of some organophosphorous pesticides. I. Induction of micronuclei in bone marrow cells in rat. Mutat. Res 1985, 155(3), 131–4. [Google Scholar]
- Sun, J. T.; Armstrong, M. J.; Galloway, S. M. Rapid method for improving slide quality in the bone marrow micronucleus assay; an adapted cellulose column procedure. Mutat. Res 1999, 439, 121–126. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Patlolla, B.P.; Patlolla, A.K.; Tchounwou, P.B. Cytogenetic Effects of 1,1-Dichloroethane in Mice Bone Marrow Cells. Int. J. Environ. Res. Public Health 2005, 2, 101-106. https://doi.org/10.3390/ijerph2005010101
Patlolla BP, Patlolla AK, Tchounwou PB. Cytogenetic Effects of 1,1-Dichloroethane in Mice Bone Marrow Cells. International Journal of Environmental Research and Public Health. 2005; 2(1):101-106. https://doi.org/10.3390/ijerph2005010101
Chicago/Turabian StylePatlolla, Babu P., Anita K. Patlolla, and Paul B. Tchounwou. 2005. "Cytogenetic Effects of 1,1-Dichloroethane in Mice Bone Marrow Cells" International Journal of Environmental Research and Public Health 2, no. 1: 101-106. https://doi.org/10.3390/ijerph2005010101




