Changes in the Second Ventilatory Threshold Following Individualised versus Standardised Exercise Prescription among Physically Inactive Adults: A Randomised Trial
Abstract
:1. Introduction
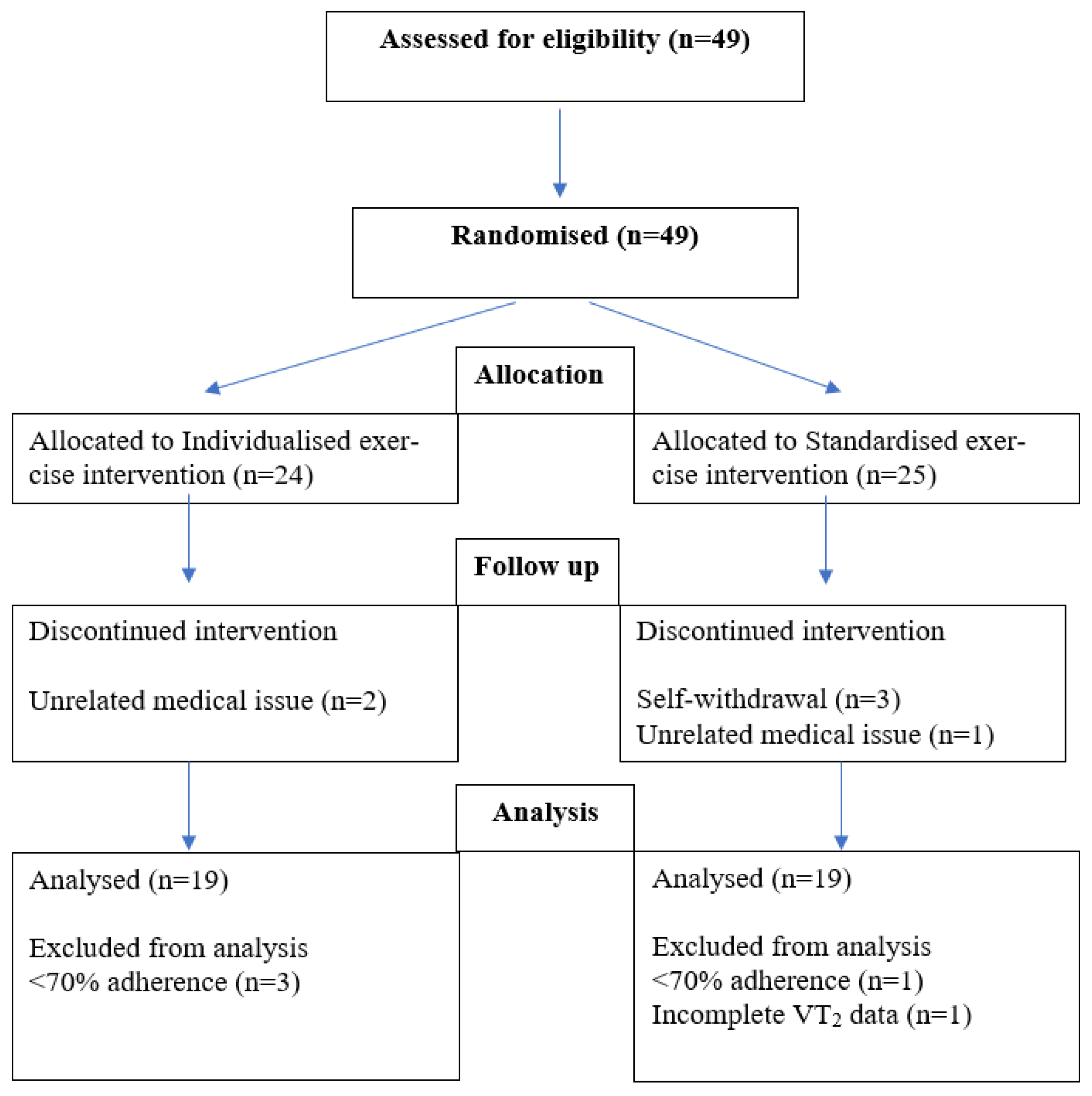
2. Materials and Methods
2.1. Research Design and Methodology
2.2. Ventilatory Threshold
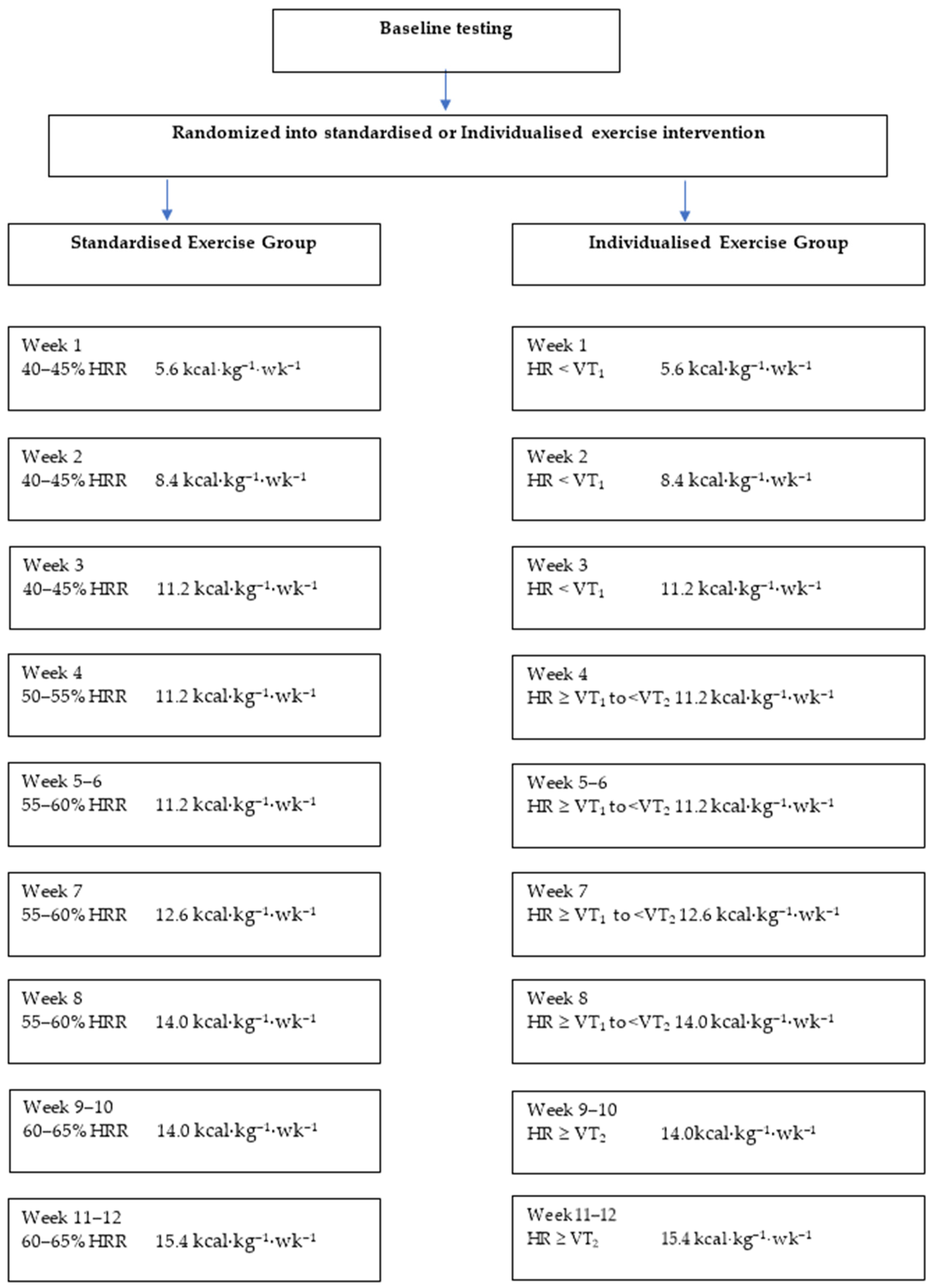
2.3. Exercise Prescription
- Target HR < VT1 = HR range: 10 beats per minute (bpm) below VT1 to the HR at VT1
- Target HR ≥ VT1 to < VT2 = HR range: 15 bpm above VT1 to below VT2
- Target HR ≥ VT2 = HR range: Heart rate at VT2 to 10 bpm above VT2
2.4. Exercise Training
2.5. Data Management and Analysis
3. Results
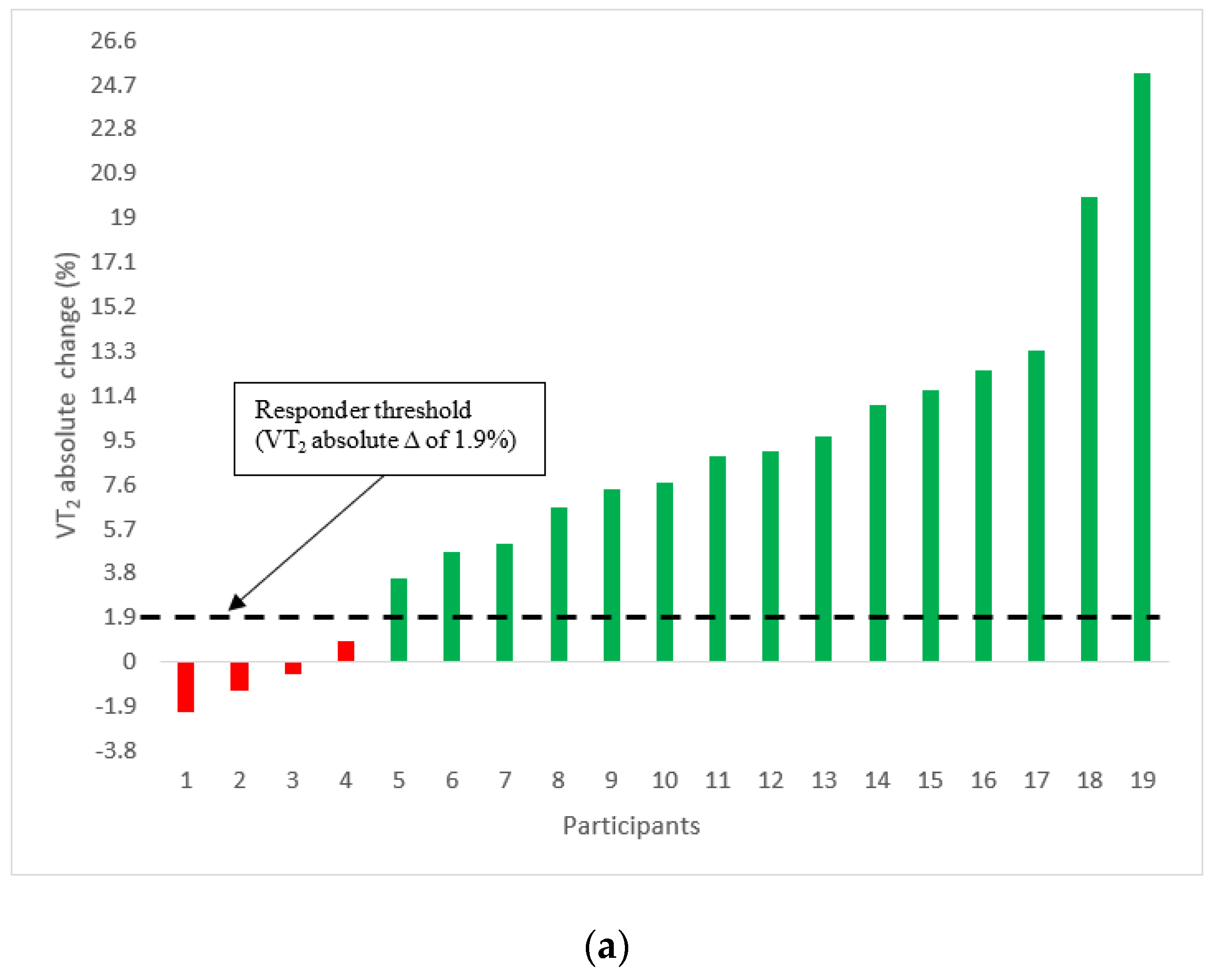
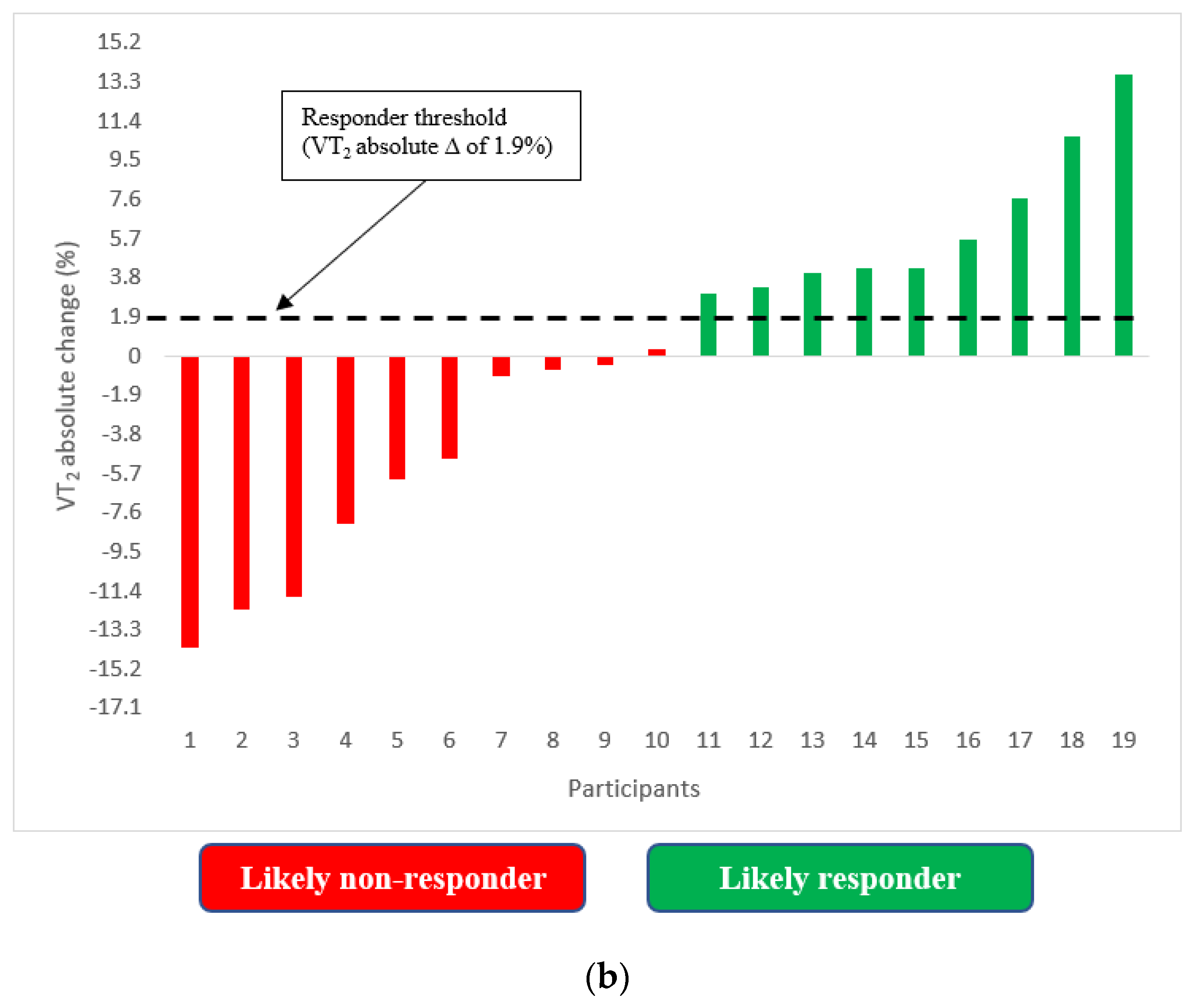
3.1. Changes in VT2
3.2. Incidence of VT2 Responders and Non-Responders
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, A.K. Anaerobic threshold: Its concept and role in endurance sport. Malays. J. Med. Sci. 2004, 11, 24. [Google Scholar] [PubMed]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Slentz, C.A.; Aiken, L.B.; Houmard, J.A.; Bales, C.W.; Johnson, J.L.; Tanner, C.J.; Duscha, B.D.; Kraus, W.E. Inactivity, exercise, and visceral fat. STRRIDE: A randomized, controlled study of exercise intensity and amount. J. Appl. Physiol. 2005, 99, 1613–1618. [Google Scholar] [CrossRef] [Green Version]
- Wisløff, U.; Nilsen, T.I.; Drøyvold, W.B.; Mørkved, S.; Slørdahl, S.A.; Vatten, L.J. A single weekly bout of exercise may reduce cardiovascular mortality: How little pain for cardiac gain? ‘The HUNT study, Norway’. Eur. J. Prev. Cardiol. 2006, 13, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Gao, W.; Zhang, L.; Nyamdorj, R.; Tuomilehto, J. Metabolic syndrome and cardiovascular disease. Ann. Clin. Biochem. 2007, 44, 232–263. [Google Scholar] [CrossRef]
- Serrano-Sánchez, J.A.; Fernández-Rodríguez, M.J.; Sanchis-Moysi, J.; del Cristo Rodríguez-Pérez, M.; Marcelino-Rodríguez, I.; Cabrera de León, A. Domain and intensity of physical activity are associated with metabolic syndrome: A population-based study. PLoS ONE 2019, 14, e0219798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, D.P.; Franklin, B.A. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am. J. Cardiol. 2006, 97, 141–147. [Google Scholar] [CrossRef]
- Gormley, S.E.; Swain, D.P.; High, R.; Spina, R.J.; Dowling, E.A.; Kotipalli, U.S.; Gandrakota, R. Effect of intensity of aerobic training on VO2max. Med. Sci. Sports Exerc. 2008, 40, 1336–1343. [Google Scholar] [CrossRef]
- McKinney, J.; Lithwick, D.J.; Morrison, B.N.; Nazzari, H.; Isserow, S.H.; Heilbron, B.; Krahn, A.D. The health benefits of physical activity and cardiorespiratory fitness. Br. Columbia Med. J. 2016, 58, 131–137. [Google Scholar]
- DiPietro, L.; Dziura, J.; Yeckel, C.W.; Neufer, P.D. Exercise and improved insulin sensitivity in older women: Evidence of the enduring benefits of higher intensity training. J. Appl. Physiol. 2006, 100, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela-Espejo, V.; Courel-Ibáñez, J.; Morán-Navarro, R.; Martínez-Cava, A.; Pallarés, J.G. The relationship between lactate and ventilatory thresholds in runners: Validity and reliability of exercise test performance parameters. Front. Physiol. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Farah, B.Q.; Ritti-Dias, R.M.; Cucato, G.G.; Meneses, A.L.; Gardner, A.W. Clinical Predictors of Ventilatory Threshold Achievement in Claudicants. Med. Sci. Sports Exerc. 2015, 47, 493. [Google Scholar] [CrossRef] [Green Version]
- Lanferdini, F.J.; Silva, E.S.; Machado, E.; Fischer, G.; Peyré-Tartaruga, L.A. Physiological predictors of maximal incremental running performance. Front. Physiol. 2020, 11, 979. [Google Scholar] [CrossRef]
- Gaskill, S.; Walker, A.; Serfass, R.; Bouchard, C.; Gagnon, J.; Rao, D.; Skinner, J.; Wilmore, J.; Leon, A.S. Changes in ventilatory threshold with exercise training in a sedentary population: The HERITAGE Family Study. Int. J. Sports Med. 2001, 22, 586–592. [Google Scholar] [CrossRef]
- Swain, D.P.; Brawner, C.A.; American College of Sports Medicine. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Wolpern, A.E.; Burgos, D.J.; Janot, J.M.; Dalleck, L.C. Is a threshold-based model a superior method to the relative percent concept for establishing individual exercise intensity? A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2015, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Weatherwax, R.M.; Ramos, J.S.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Changes in metabolic syndrome severity following individualized versus standardized exercise prescription: A feasibility study. Int. J. Environ. Res. Public Health 2018, 15, 2594. [Google Scholar] [CrossRef] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. The incidence of training responsiveness to cardiorespiratory fitness and cardiometabolic measurements following individualized and standardized exercise prescription: Study protocol for a randomized controlled trial. Trials 2016, 17, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, V.A.; Midgley, A.W.; Carroll, S.; Astorino, T.A.; de Paula, T.; Farinatti, P.; Cunha, F.A. Is a verification phase useful for confirming maximal oxygen uptake in apparently healthy adults? A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247057. [Google Scholar] [CrossRef]
- Weatherwax, R.; Richardson, T.; Beltz, N.; Nolan, P.; Dalleck, L. Verification testing to confirm VO2max in altitude-residing, endurance-trained runners. Int. J. Sports Med. 2016, 37, 525–530. [Google Scholar] [CrossRef]
- Shimizu, M.; Myers, J.; Buchanan, N.; Walsh, D.; Kraemer, M.; McAuley, P.; Froelicher, V.F. The ventilatory threshold: Method, protocol, and evaluator agreement. Am. Heart J. 1991, 122, 509–516. [Google Scholar] [CrossRef]
- Amann, M.; Subudhi, A.W.; Walker, J.; Eisenman, P.; Shultz, B.; Foster, C. An evaluation of the predictive validity and reliability of ventilatory threshold. Med. Sci. Sports Exerc. 2004, 36, 1716–1722. [Google Scholar] [CrossRef] [Green Version]
- Pfitzinger, P.; Freedson, P. The reliability of lactate measurements during exercise. Int. J. Sports Med. 1998, 19, 349–357. [Google Scholar] [CrossRef]
- Ohuchi, H.; Nakajima, T.; Kawade, M.; Matsuda, M.; Kamiya, T. Measurement and validity of the ventilatory threshold in patients with congenital heart disease. Pediatric Cardiol. 1996, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.E.; Fletcher, B.J.; Rupp, J.C.; Fletcher, G.F.; Lee, J.Y.; Oberman, A. Training level comparison study: Effect of high and low intensity exercise on ventilatory threshold in men with coronary artery disease. J. Cardiopulm. Rehabil. Prev. 1996, 16, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tamai, M.; Kubota, M.; Ikeda, M.; Nagao, K.; Irikura, N.; Sugiyama, M.; Yoshikawa, H.; Kawamori, R.; Kamada, T. Usefulness of anaerobic threshold for evaluating daily life activity and prescribing exercise to the healthy subjects and patients. J. Med. Syst. 1993, 17, 219–225. [Google Scholar] [CrossRef]
- Londeree, B.R. Effect of training on lactate/ventilatory thresholds: A meta-analysis. Med. Sci. Sports Exerc. 1997, 29, 837–843. [Google Scholar] [CrossRef]
- Weatherwax, R.; Harris, N.; Kilding, A.E.; Dalleck, L. Time course changes in confirmed ‘true’VO2max after individualized and standardized training. Sports Med. Int. Open 2019, 3, E32–E39. [Google Scholar]
- Azevedo, L.F.; Perlingeiro, P.S.; Brum, P.C.; Braga, A.M.W.; Negrão, C.E.; de Matos, L.D. Exercise intensity optimization for men with high cardiorespiratory fitness. J. Sports Sci. 2011, 29, 555–561. [Google Scholar] [CrossRef]
- Posner, J.D.; Gorman, K.M.; Klein, H.S.; Cline, C.J. Ventilatory threshold: Measurement and variation with age. J. Appl. Physiol. 1987, 63, 1519–1525. [Google Scholar] [CrossRef]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001, 33 (Suppl. S6), S446–S451. [Google Scholar] [CrossRef] [Green Version]
- Sarzynski, M.A.; Ghosh, S.; Bouchard, C. Genomic and transcriptomic predictors of response levels to endurance exercise training. J. Physiol. 2017, 595, 2931–2939. [Google Scholar] [CrossRef] [Green Version]
- Ito, S. High-intensity interval training for health benefits and care of cardiac diseases-the key to an efficient exercise protocol. World J. Cardiol. 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, D.C.; Gaesser, G.A. Response of ventilatory and lactate thresholds to continuous and interval training. J. Appl. Physiol. 1985, 58, 1115–1121. [Google Scholar] [CrossRef]
- Di Donato, D.M.; West, D.W.; Churchward-Venne, T.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E1025–E1032. [Google Scholar] [CrossRef] [Green Version]
- Laursen, P.B.; Jenkins, D.G. The scientific basis for high-intensity interval training. Sports Med. 2002, 32, 53–73. [Google Scholar] [CrossRef]
- Ahmaidi, S.; Masse-Biron, J.; Adam, B.; Choquet, D.; Freville, M.; Libert, J.-P.; Prefaut, C. Effects of interval training at the ventilatory threshold on clinical and cardiorespiratory responses in elderly humans. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 170–176. [Google Scholar] [CrossRef]
- Marsh, S.A.; Coombes, J.S. Exercise and the endothelial cell. Int. J. Cardiol. 2005, 99, 165–169. [Google Scholar] [CrossRef]
- Ghisi, G.L.d.M.; Durieux, A.; Pinho, R.; Benetti, M. Physical exercise and endothelial dysfunction. Arq. Bras. Cardiol. 2010, 95, e130–e137. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.P.; Yung, L.M.; Laher, I.; Yao, X.; Chen, Z.Y.; Huang, Y. Exercise, vascular wall and cardiovascular diseases. Sports Med. 2008, 38, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Standardised (n = 19; Women, n = 15; Men, n = 4) | Individualised (n = 19; Women, n = 14; Men, n = 5) | Between-Group Difference | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | p-Value | |
| Age | 52.3 ± 11.7 | - | 45.0 ± 11.3 | - | - |
| Height | 168.3 ± 9.6 | - | 172.2 ± 7.0 | - | - |
| Weight | 84.7 ± 21.1 | 84.2 ± 20.2 | 80.2 ± 15.5 | 80.3 ± 15.6 | - |
| VT2 (% VO2max) | 72.5 ± 7.8 | 72.3 ± 5.5 | 70.6 ± 8.5 | 78.7 ± 6.0 * | <0.001 |
| Absolute VO2 at VT2 (L/min) | 1.5 ± 0.5 | 1.6 ± 0.5 | 1.7 ± 0.5 | 2.0 ± 0.7 * | 0.017 |
| Relative VO2 at VT2 (mL/kg/min) | 17.3 ± 3.5 | 18.7 ± 3.1 | 20.8 ± 5.3 | 24.7 ± 7.1 * | 0.016 |
| VO2max (L/min) | 2.0 ± 0.6 | 2.2 ± 0.6 | 2.38 ± 0.8 | 2.5 ± 0.8 | 0.579 |
| VO2max (mL/kg/min) | 24.0 ± 4.7 | 25.9 ± 4.8 | 29.7 ± 7.8 | 31.4 ± 8.5 | 0.635 |
| Daily caloric intake (kcal) | 1519 ± 563 | 1518 ± 500 | 1539 ± 493 | 1555 ± 403 | 0.967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, A.D.; Dalleck, L.C.; Mejuto, G.; Larwood, T.; Weatherwax, R.M.; Ramos, J.S. Changes in the Second Ventilatory Threshold Following Individualised versus Standardised Exercise Prescription among Physically Inactive Adults: A Randomised Trial. Int. J. Environ. Res. Public Health 2022, 19, 3962. https://doi.org/10.3390/ijerph19073962
Martini AD, Dalleck LC, Mejuto G, Larwood T, Weatherwax RM, Ramos JS. Changes in the Second Ventilatory Threshold Following Individualised versus Standardised Exercise Prescription among Physically Inactive Adults: A Randomised Trial. International Journal of Environmental Research and Public Health. 2022; 19(7):3962. https://doi.org/10.3390/ijerph19073962
Chicago/Turabian StyleMartini, Alex D., Lance C. Dalleck, Gaizka Mejuto, Trent Larwood, Ryan M. Weatherwax, and Joyce S. Ramos. 2022. "Changes in the Second Ventilatory Threshold Following Individualised versus Standardised Exercise Prescription among Physically Inactive Adults: A Randomised Trial" International Journal of Environmental Research and Public Health 19, no. 7: 3962. https://doi.org/10.3390/ijerph19073962






