Migration and Removal of Labile Cadmium Contaminants in Paddy Soils by Electrokinetic Remediation without Changing Soil pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Electrokinetic Experiment Setup
2.3. Citric Acid Preacidification Enhancement Experiment
2.4. Analysis and Calculation
2.5. Statistical Analysis
3. Results
3.1. Electric Current, Soil Moisture Content, and Soil pH Variation
3.2. Migration and Redistribution of Cd during EKR process
3.3. Speciation of Heavy Metals in the Soil
3.4. Changes in Total Cd during EKR process
3.5. Citric Acid Preacidification Enhancement EKR Technology
4. Discussion
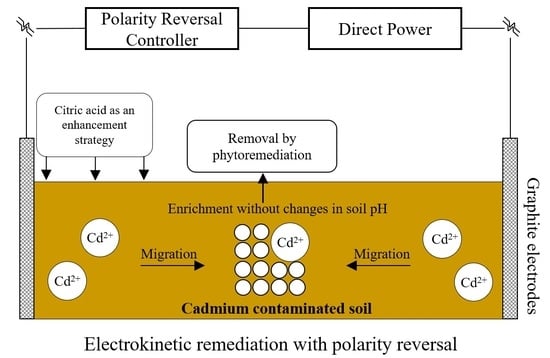
4.1. Mechanisms and Functions of EKR with Polarity Reversal
4.2. Effects of Soil Moisture on Electrokinetic Remediation
4.3. In Situ Electrokinetic-Assisted Phytoremediation Technology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Mopiso, E.; Ito, M.; Hiroyoshi, N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.M.; Xu, Z.C.; Ren, M.Z.; Guo, Q.W.; Hu, X.B.; Hu, G.C.; Wan, H.F.; Peng, P.G. Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotox. Environ. Safe. 2011, 78, 2–8. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.R.; Espinosa, A.F. Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem. J. 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Tefera, W.; Tang, L.; Lu, L.L.; Xie, R.H.; Seifu, W.; Tian, S.K. Rice cultivars significantly mitigate cadmium accumulation in grains and its bioaccessibility and toxicity in human HL-7702 cells. Environ. Pollut. 2020, 272, 116020. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Shafeeq, R.; Abbas, A.; Li, J.M.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Liu, L.W.; Li, W.; Song, W.P.; Guo, M.X. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Tang, J.; Qiu, Z.P.; Tang, H.J.; Wang, H.Y.; Sima, W.P.; Liang, C.; Li, Z.H.; Wan, S.; Dong, J.W. Coupled with EDDS and approaching anode technique enhanced electrokinetic remediation removal heavy metal from sludge. Environ. Pollut. 2020, 272, 115975. [Google Scholar] [CrossRef]
- Wang, Y.C.; Han, Z.J.; Li, A.; Cui, C.W. Enhanced electrokinetic remediation of heavy metals contaminated soil by biodegradable complexing agents. Environ. Pollut. 2021, 283, 117111. [Google Scholar] [CrossRef]
- Guo, F.Y.; Ding, C.F.; Zhou, Z.G.; Han, F.X.; Tang, R.G.; Huang, G.X.; Wang, X.X. Assessment of the immobilization effectiveness of several amendments on a cadmium-contaminated soil using Eisenia fetida. Ecotox. Environ. Saf. 2019, 189, 109948. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, L.F.; Zhou, L.L.; Zhang, S.W. Electrokinetic removal of chromium from chromite ore-processing residue using graphite particle-supported nanoscale zero-valent iron as the three-dimensional electrode. Chem. Eng. J. 2018, 350, 1022–1034. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic Removal Technologies And Future Trends: A Mini Review. J. Clean Prod. 2020, 278, 123805. [Google Scholar] [CrossRef]
- Giannis, A.; Gidarakos, E.; Skouta, A. Transport of cadmium and assessment of phytotoxicity after electrokinetic remediation. J. Environ. Manag. 2008, 86, 535–544. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar] [CrossRef]
- Page, K.L.; Dalal, R.C.; Wehr, J.B.; Dang, Y.P.; Kopittke, P.M.; Kirchhof, G.; Fujinuma, R.; Menzies, N.W. Management of the major chemical soil constraints affecting yields in the grain growing region of Queensland and New South Wales. Australia–a review. Soil Res. 2018, 56, 765–779. [Google Scholar] [CrossRef]
- Batty, L.C.; Dolan, C. The Potential Use of Phytoremediation for Sites with Mixed Organic and Inorganic Contamination. Crit. Rev. Environ. Sci. Technol. 2013, 43, 217–259. [Google Scholar] [CrossRef]
- Saichek, R.E.; Reddy, K.R. Effect of pH control at the anode for the electrokinetic removal of phenanthrene from kaolin soil. Chemosphere 2003, 51, 273–287. [Google Scholar] [CrossRef]
- Zhou, D.M.; Deng, C.F.; Cang, L. Electrokinetic remediation of a Cu contaminated red soil by conditioning catholyte pH with different enhancing chemical reagents. Chemosphere 2004, 56, 265–273. [Google Scholar] [CrossRef]
- Zhou, D.M.; Deng, C.F.; Alshawabkeh, A.N.; Cang, L. Effects of catholyte conditioning on electrokinetic extraction of copper from mine tailings. Environ. Int. 2005, 31, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Souilah, O.; Akretche, D.E.; Cameselle, C. Electroremediation of contaminated soil by heavy metals using ion exchange fibers. Electrochim. Acta 2012, 86, 138–141. [Google Scholar] [CrossRef]
- Huang, W.Y.; Hung, W.; Vu, C.T.; Chen, W.T.; Lai, J.W.; Lin, C. Green and sustainable remediation (GSR) evaluation: Framework, standards, and tool. A case study in Taiwan. Environ. Sci. Pollut. Res. 2016, 23, 21712–21725. [Google Scholar] [CrossRef] [PubMed]
- Gidudu, B.; Chirwa, E.M.N. The combined application of a high voltage, low electrode spacing, and biosurfactants enhances the bio-electrokinetic remediation of petroleum contaminated soil. J. Clean Prod. 2020, 276, 122745. [Google Scholar] [CrossRef]
- Rozas, F.; Castellote, M. Selecting enhancing solutions for electrokinetic remediation of dredged sediments polluted with fuel. J. Environ. Manag. 2015, 151, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Asadollahfardi, G.; Sarmadi, M.S.; Rezaee, M.; Khodadadi-Darban, A.; Yazdani, M.; Paz-Garcia, J.M. Comparison of different extracting agents for the recovery of Pb and Zn through electrokinetic remediation of mine tailings. J. Environ. Manag. 2021, 279, 111728. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, D.H.; Yoo, J.C.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Xiao, C. Focus on factors affecting pH, flow of Cr and transformation between Cr(VI) and Cr(III) in the soil with different electrolytes. Electrochim. Acta 2016, 211, 652–662. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chu, G.H.; Dong, P.; Xiao, J.; Meng, Q.; Baumgartel, M.; Hao, T. Enhanced electrokinetic remediation of lead- and cadmium-contaminated paddy soil by composite electrolyte of sodium chloride and citric acid. J. Soils Sediments 2017, 18, 1915–1924. [Google Scholar] [CrossRef]
- Xie, N.; Chen, Z.; Wang, H.M.; You, C.F. Activated carbon coupled with citric acid in enhancing the remediation of Pb-Contaminated soil by electrokinetic method. J. Clean Prod. 2021, 308, 127433. [Google Scholar] [CrossRef]
- Nasiri, A.; Jamshidi-Zanjani, A.; Darban, A.K. Application of enhanced electrokinetic approach to remediate Cr-contaminated soil: Effect of chelating agents and permeable reactive barrier. Environ. Pollut. 2020, 266, 115197. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Sanroman, M.A.; Cameselle, C. Improvement in electrokinetic remediation of heavy metal spiked kaolin with the polarity exchange technique. Chemosphere 2006, 62, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, J.M.; Zhu, S.F.; Wang, Y.J.; Gao, H. Exchange electrode-electrokinetic remediation of Cr-contaminated soil using solar energy. Sep. Purif. Technol. 2018, 190, 297–306. [Google Scholar] [CrossRef]
- Mao, X.Y.; Shao, X.H.; Zhang, Z.Y. Pilot-scale electro-kinetic remediation of lead polluted field sediments: Model designation, numerical simulation, and feasibility evaluation. Environ. Sci Eur. 2019, 31, 25. [Google Scholar] [CrossRef]
- Xu, Y.F.; Xu, X.J.; Hou, H.T.; Zhang, J.; Zhang, D.Y.; Qian, G.R. Moisture content-affected electrokinetic remediation of Cr(VI)-contaminated clay by a hydrocalumite barrier. Environ. Sci. Pollut. Res. 2015, 23, 6517–6523. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Gouveia, S.; Cabo, A. Analysis and Optimization of Mn Removal from Contaminated Solid Matrixes by Electrokinetic Remediation. Int. J. Environ. Res. Public Health 2020, 17, 1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Y.; Yang, S.H.; Xu, J.Z.; Zhang, J.G.; Liu, J.T. Effects of soil heat storage and phase shift correction on energy balance closure of paddy fields. Atmosfera 2017, 30, 39–52. [Google Scholar] [CrossRef]
- Wu, M.Y.; Cao, X.C.; Guo, X.P.; Xiao, J.F.; Ren, J. Assessment of grey water footprint in paddy rice cultivation: Effects of field water management policies. J. Clean Prod. 2021, 313, 127876. [Google Scholar] [CrossRef]
- Chen, P.; Nie, T.Z.; Chen, S.H.; Zhang, Z.X.; Qi, Z.J.; Liu, W.N. Recovery efficiency and loss of 15N-labelled urea in a rice-soil system under water saving irrigation in the Songnen Plain of Northeast China. Agric. Water Manag. 2019, 222, 139–153. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, J.T.; Peng, Y.H.; Xu, J.Z.; Liao, L.X.; Yang, S.H. Storing and removing nitrogen in drainage from paddy field by using aquatic crops wetland. Paddy Water Environ. 2020, 18, 587–594. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhong, L.B.; Meng, J.; Wang, F.; Zhang, J.J.; Zhi, Y.Y.; Zeng, L.Z.; Tang, X.J.; Xu, J.M. A multi-medium chain modeling approach to estimate the cumulative effects of cadmium pollution on human health. Environ. Pollut. 2018, 239, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guan, Q.; Tian, J.; Lin, J.; Yang, Y.; Yang, L.; Pan, N. Contamination characteristics, source apportionment, and health risk assessment of heavy metals in agricultural soil in the Hexi Corridor. Catena 2020, 191, 104573. [Google Scholar] [CrossRef]
- Hansen, H.K.; Rojo, A. Testing pulsed electric fields in electroremediation of copper mine tailings. Electrochim. Acta 2007, 52, 3399–3405. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, A.; Cui, C.W. Remediation of heavy metal-contaminated soils by electrokinetic technology: Mechanisms and applicability. Chemosphere 2020, 265, 129071. [Google Scholar] [CrossRef]
- He, S.Y.; Yang, X.; He, Z.L.; Baligar, V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Cameselle, C.; Pena, A. Enhanced electromigration and electro-osmosis for the remediation of an agricultural soil contaminated with multiple heavy metals. Process Saf. Environ. Protect. 2016, 104, 209–217. [Google Scholar] [CrossRef]
- Meng, F.S.; Xue, H.; Wang, Y.Y.; Zheng, B.H.; Wang, J.L. Citric-acid preacidification enhanced electrokinetic remediation for removal of chromium from chromium-residue-contaminated soil. Environ. Technol. 2017, 39, 356–362. [Google Scholar] [CrossRef]
- Laurent, J.P.; Ruelle, P.; Delage, L.; Zairi, A.; Nouna, B.B.; Adjmi, T. Monitoring soil water content profiles with a commercial TDR system. Vadose Zone J. 2005, 4, 1030–1036. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.H.; Park, S.W.; Ryu, B.G.; Bajargal, T.; Yang, J.S. Electrolyte conditioning-enhanced electrokinetic remediation of arsenic-contaminated mine tailing. J. Hazard. Mater. 2009, 161, 457–462. [Google Scholar] [CrossRef]
- Iannelli, R.; Masi, M.; Ceccarini, A.; Ostuni, M.B.; Lageman, R.; Muntoni, A.; Spiga, D.; Polettini, A.; Marini, A.; Pomi, R. Electrokinetic remediation of metal-polluted marine sediments: Experimental investigation for plant design. Electrochim. Acta 2015, 181, 146–159. [Google Scholar] [CrossRef]
- Fernández-Ondoño, E.; Bacchetta, G.; Lallena, A.M.; Navarro, F.B.; Ortiz, I.; Jiménez, M.N. Use of BCR sequential extraction procedures for soils and plant metal transfer predictions in contaminated mine tailings in Sardinia. J. Geochem. Explor. 2017, 172, 133–141. [Google Scholar] [CrossRef]
- Janowska, B.; Szymanski, K.; Sidelko, R.; Siebielska, I.; Walendzik, B. Assessment of mobility and bioavailability of mercury compounds in sewage sludge and composts. Environ. Res. 2017, 156, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.S.; Yu, S.; Li, X.D. The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Appl. Geochem. 2012, 27, 995–1004. [Google Scholar] [CrossRef]
- Kirkelund, G.M.; Ottosen, L.M.; Villumsen, A. Investigations of Cu, Pb and Zn partitioning by sequential extraction in harbour sediments after electrodialytic remediation. Chemosphere 2010, 79, 997–1002. [Google Scholar] [CrossRef]
- Krcmar, D.; Varga, N.; Prica, M.; Cveticanin, L.; Zukovic, M.; Dalmacija, B.; Corba, Z. Application of hexagonal two dimensional electrokinetic system on the nickel contaminated sediment and modelling the transport behavior of nickel during electrokinetic treatment. Sep. Purif. Technol. 2018, 192, 253–261. [Google Scholar] [CrossRef]
- Zhuang, Y.F. Large scale soft ground consolidation using electrokinetic geosynthetics. Geotext. Geomembr. 2021, 49, 757–770. [Google Scholar] [CrossRef]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2020, 401, 123345. [Google Scholar] [CrossRef]
- Ho, S.V.; Athmer, C.J.; Sheridan, P.W.; Shapiro, A.P. Scale-up aspects of the Lasagna process for in situ soil decontamination. J. Hazard. Mater. 1997, 55, 39–60. [Google Scholar] [CrossRef]
- Tang, X.; Li, Q.; Wang, Z.; Hu, Y.; Hu, Y.; Li, R. In situ electrokinetic isolation of cadmium from paddy soil through pore water drainage: Effects of voltage gradient and soil moisture. Chem. Eng. J. 2018, 337, 210–219. [Google Scholar] [CrossRef]
- Lukman, S.; Essa, M.H.; Mu’azu, N.D.; Bukhari, A. Coupled electrokinetics-adsorption technique for simultaneous removal of heavy metals and organics from saline-sodic soil. Sci. World J. 2013, 2013, 346910. [Google Scholar] [CrossRef] [Green Version]
- Cherifi, M.; Hazourli, S.; Ziati, M. Initial water content and temperature effects on electrokinetic removal of aluminium in drinking water sludge. Phys. Procedia 2009, 2, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Cai, L.M.; Qi, S.H.; Wu, J.; Gu, X.W.S. The interactive effects between chelator and electric fields on the leaching risk of metals and the phytoremediation efficiency of Eucalyptus globulus. J. Clean Prod. 2018, 202, 830–837. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, W.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Rodríguez, L. Can electrochemistry enhance the removal of organic pollutants by phytoremediation? J. Environ. Manag. 2018, 225, 280–287. [Google Scholar] [CrossRef]
- Wan, Y.A.; Huang, Q.Q.; Wang, Q.; Yu, Y.; Su, D.C.; Qiao, Y.H.; Li, H.F. Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 2020, 384, 121293. [Google Scholar] [CrossRef]
- He, L.P.; Liu, D.; Lin, J.J.; Yu, Z.G.; Yang, X.X.; Fu, C.; Zheng, X.L.; Zhao, Q.H. Total nitrogen and pH-controlled chemical speciation, bioavailability and ecological risk from Cd, Cr, Cu, Pb and Zn in the water level-fluctuating zone sediments of the Three Gorges Reservoir. Chem. Speciation Bioavail. 2017, 29, 89–96. [Google Scholar] [CrossRef]








| Treatment | Voltage Gradient (V cm−1) | Duration (d) | Periodic Power/Day | Polarity Reversal Frequency (h) | Soil Moisture Alternate Wetting and Drying (AWD) Cycles |
|---|---|---|---|---|---|
| EKR 1.0 | 1.0 | 14 | 10 h ON/14 h OFF | 24 | Two AWD cycles |
| EKR 0.8 | 0.8 | ||||
| EKR 0.5 | 0.5 | ||||
| EKRC 1.0 | 1.0 | 6 | 10 h ON/14 h OFF | 24 | One AWD cycle |
| EKRC 0.8 | 0.8 | ||||
| EKRC 0.5 | 0.5 |
| Soil Section | Treatment | Intensity Variation (mA) | Finial pH | Final F1-Cd Concentration (mg kg−1) | Finial Total Cd Concentration (mg kg−1) |
|---|---|---|---|---|---|
| S1 | EKRC 1.0 | 459~807 | 6.25 ± 0.065 ab | 0.195 ± 0.022 a | 0.597 ± 0.015 c |
| EKRC 0.8 | 6.19 ± 0.040 b | 0.183 ± 0.012 a | 0.662 ± 0.015 b | ||
| EKRC 0.5 | 6.33 ± 0.035 a | 0.180 ± 0.014 a | 0.724 ± 0.011 a | ||
| S2 | EKRC 1.0 | 368~647 | 6.50 ± 0.050 a | 0.490 ± 0.019 a | 1.151 ± 0.020 a |
| EKRC 0.8 | 6.51 ± 0.080 a | 0.421 ± 0.019 b | 1.048 ± 0.011 b | ||
| EKRC 0.5 | 6.65 ± 0.140 a | 0.332 ± 0.012 c | 0.960 ± 0.018 c | ||
| S3 | EKRC 1.0 | 241~426 | 6.21 ± 0.087 a | 0.196 ± 0.009 a | 0.582 ± 0.010 b |
| EKRC 0.8 | 6.34 ± 0.087 a | 0.192 ± 0.015 a | 0.643 ± 0.018 a | ||
| EKRC 0.5 | 6.21 ± 0.070 a | 0.194 ± 0.022 a | 0.667 ± 0.016 a |
| Treatment | the Mass of Increased Cd in S2 Section (kg) | |
|---|---|---|
| EKRC 1.0 | 2.51 × 10−3 | 0.400 |
| EKRC 0.8 | 1.79 × 10−3 | 0.360 |
| EKRC 0.5 | 1.17 × 10−3 | 0.230 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Xu, J.; Zhou, J.; Wang, H.; Han, F.; Wang, K.; Lv, Y. Migration and Removal of Labile Cadmium Contaminants in Paddy Soils by Electrokinetic Remediation without Changing Soil pH. Int. J. Environ. Res. Public Health 2022, 19, 3812. https://doi.org/10.3390/ijerph19073812
Luan Y, Xu J, Zhou J, Wang H, Han F, Wang K, Lv Y. Migration and Removal of Labile Cadmium Contaminants in Paddy Soils by Electrokinetic Remediation without Changing Soil pH. International Journal of Environmental Research and Public Health. 2022; 19(7):3812. https://doi.org/10.3390/ijerph19073812
Chicago/Turabian StyleLuan, Yajun, Junzeng Xu, Jing Zhou, Haiyu Wang, Fengxiang Han, Kechun Wang, and Yuping Lv. 2022. "Migration and Removal of Labile Cadmium Contaminants in Paddy Soils by Electrokinetic Remediation without Changing Soil pH" International Journal of Environmental Research and Public Health 19, no. 7: 3812. https://doi.org/10.3390/ijerph19073812